镉(cadmium,Cd)是环境常见污染物,可通过工业废水进入水生生态,在食物链中传递并在更高营养级生物体内蓄积[1-3],导致生物生理机能改变并影响生物行为[2]。国际癌症研究机构(IARC)将Cd归为Ⅰ类致癌物,美国毒物和疾病登记署(ATSDR)将Cd列为第7位危害人体健康的有毒物质[4]。此外,Cd还是脂质过氧化的诱导剂,能够诱导体内产生过量的活性氧(ROS),降低抗氧化能力,引起氧化应激损伤。已有研究表明,Cd对斑马鱼具有不同的毒性效应,包括生殖毒性[5]、生长抑制[6]、组织损伤[7]、异常行为[8]、氧化应激[9]和免疫毒性[10]。最新研究发现,Cd能通过血-脑屏障在生物大脑中积聚并影响中枢神经系统(central nervous system, CNS)[11]。在发育学和解剖学上,视网膜被认为是CNS的一个部分[12],斑马鱼视网膜对重金属高度敏感[13]。据报道铁会导致斑马鱼视网膜损伤,甚至可诱导视网膜细胞死亡[14];甲基汞能够穿过血液-视网膜屏障并积聚在视网膜细胞中,导致光感受器层损伤,影响视觉功能[15];铅暴露损伤视网膜杆状细胞和双极细胞,最终导致视觉反应缺陷[16]。
视网膜能将电信号从视觉系统传递到大脑的CNS[17]。斑马鱼视觉系统与人类视觉系统结构相似[18],且在分子和基因组水平上与人类具有同源性[17],是研究视觉毒理学常用模型之一[19]。斑马鱼视网膜是一个层状结构,由6种神经细胞和一种Müller胶质细胞(Müller glia cells, MGC)构成了3个核层:外核层(outer nuclear layer, ONL)、内核层(inner nuclear layer, INL)、神经节细胞层(ganglion cell layer, GCL),3个核层之间具有2个突触层:外丛状层(outer plexiform layer, OPL)和内丛状层(inner plexiform layer, IPL),主要分布着视网膜细胞的突触[20]。外核层分布着感光细胞(photoreceptors, PR),包括视锥PR(cone photoreceptors, CP)和视杆PR(rod photoreceptors, RP);水平细胞(horizontal cells, HC)、双极细胞(bipolar cell, BC)、无长突细胞(amacrine cells, AC)和MGC主要分布在内核层;视网膜神经节细胞(retinal ganglion cells, RGC)主要分布在神经节细胞层。目前对斑马鱼视网膜发育毒性潜在机制研究仍然有限,因此,本实验通过观察Cd暴露对斑马鱼幼鱼眼睛的形态、视觉行为、视网膜组织结构、检测视网膜发育相关基因(包括ONL感光细胞发育标记基因opn1sw1(UV)、opn1sw2(blue)、opn1mw1(green)、opn1lw1(red)、rhodopsin[21];INL标记基因vsx1[22];GCL神经细胞分化标记基因brn3b[23];调控视网膜视囊形成基因pax6[24],光传导通路相关基因gnat2、grk7a、grk1b;脊椎动物眼睛发育基因pax6、rx1)的表达水平以及氧化应激相关酶的影响,探讨急性镉对斑马鱼视网膜发育的毒性及机理。
1 材料与方法(Materials and methods)
1.1 实验生物
成年AB种类野生型雌雄斑马鱼(Danio rerio,中国科学院水生生物研究所)分开饲养于循环水养殖系统(上海海圣生物实验设备有限公司)中,水温控制在(28±0.5) ℃,光暗比为14 L∶10 D,pH=7.2±1.0,电导率为450~500 μS·cm-1。使用新鲜孵化的丰年虫(天津丰年水产有限公司)喂养,一日3次。
1.2 仪器试剂
1.2.1 实验仪器
恒温光照培养箱(GTOP-310Y,浙江托普云农科技股份有限公司,中国);行为仪(View Point Life Science,ZebraLab,法国);冷冻离心机(ST16R,赛默飞世尔科技,美国);电动组织匀浆机(OSE-Y30,北京天根,中国);体式显微镜(SZ680,重庆奥特光学仪器有限责任公司,中国);超微量分光光度计(Nanodrop 2000c,赛默飞世尔公司,美国);梯度PCR仪(TP350,Takara公司,日本);荧光定量PCR仪(ABI Step One Plus,ABI公司,美国)。
1.2.2 实验试剂
氯化镉(纯度≥99.99%,上海阿拉丁生化科技股份有限公司);无水乙醇(分析纯,≥99.7%,广州化学试剂科技有限公司);苏木精-伊红(广州化学试剂科技有限公司);反转录试剂盒(Evo M-MLV RT Premix for qPCR,广州瑞真生物技术有限公司);荧光定量PCR试剂盒(SYBR ®Green Premix Pro Taq HS qPCR Kit,广州瑞真生物技术有限公司);生工引物(生工生物工程(上海)股份有限公司);活性氧(ROS)试剂盒、丙二醛(MDA)试剂盒、超氧化物歧化酶(SOD)试剂盒、谷胱甘肽S-转移酶(GST)试剂盒(南京建成生物工程研究所)。
1.3 实验方法
1.3.1 斑马鱼幼鱼氯化镉暴露浓度
将性成熟的斑马鱼按雌雄比例2∶3置于产卵盒,隔板避光过夜。次日取出隔板,在光照刺激下交配产卵。参照《水质急性毒性的测定斑马鱼卵法》(HJ 1069—2019)的方法和镉对斑马鱼早期胚胎发育的毒性研究[25-26];通过预实验3次生物性重复后确定0、0.2、0.4、0.8、1.6 mg·L-15个氯化镉暴露浓度组。将受精后6 h发育正常的斑马鱼胚胎置于150 mm细胞培养皿中(200 卵·皿-1),分别加入不同浓度氯化镉后加盖封闭,于28.5 ℃恒温光照培养箱中培养,每24 h更换一次液体。
1.3.2 NAC干预实验
胚胎染毒方法参照1.3.1,以及参考文献[27],将胚胎分别暴露于0、0.8 mg·L-1 CdCl2、0.8 mg·L-1 CdCl2+0.4 mg·L-1 NAC、0.4 mg·L-1 NAC这4个浓度组。观察暴露120 hpf斑马鱼幼鱼生长发育情况,并统计存活率、畸形率和孵化率。
1.3.3 斑马鱼幼鱼眼长与身长比值
为比较各组斑马鱼幼鱼眼睛形态之间的差异。各组取24条120 hpf幼鱼用0.03% MS-222麻醉后,通过体式显微镜拍照并测量幼鱼眼睛与身体长度(数码成像系统软件OPTPro3000)。身体长度为从头部顶端到躯干末端(不包括尾鳍)的长度;眼睛长度为眼睛长轴前部至后部[18]的长度。分别计算不同暴露组幼鱼的眼睛长度和身体长度的比值。
1.3.4 斑马鱼幼鱼眼睛组织病理学石蜡切片
72 hpf斑马鱼视网膜分化基本完成,至120 hpf斑马鱼视觉系统发育完善[28],所以取各组120hpf幼鱼用10%中性缓冲福尔马林固定石蜡包埋,纵向切片,厚度5 μm,苏木精-伊红(hematoxylin-eosin, H&E)染色,光学显微镜观察[29]。
1.3.5 斑马鱼幼鱼运动行为实验
各组取120 hpf幼鱼置于24孔板中,每孔一条幼鱼,温度控制在(28.0±1.0) ℃。将24孔板放入View Point Life Science斑马鱼行为仪内适应30 min,通过Zebrafish Video Tracking系统在光暗环境交替下监测幼鱼的运动行为活动。光暗交替环境为:10min光照-10min黑暗-10min光照-10min黑暗。
1.3.6 实时荧光定量聚合酶链式反应
各组取30条120 hpf幼鱼,采用Trizol法裂解组织提取总RNA,选取A260/A280在1.9~2.1之间的RNA。按照反转录试剂盒(Evo M-MLV RT Premix for qPCR)说明书操作,利用TaKaRa梯度PCR仪将总RNA反转录成cDNA。引物序列如表1所示。使用荧光定量PCR试剂盒(SYBR® Green Premix Pro Taq HS qPCR Kit)对cDNA进行视网膜发育相关基因表达量分析。核糖体蛋白L8(ribosomal protein L8, rpl8)作为内参[30]。采用相对Ct法(2-△△Ct)确定mRNA的相对表达量。
表1 视网膜发育相关的目的基因与引物序列
Table 1 Target genes and primer sequences related to retinal development
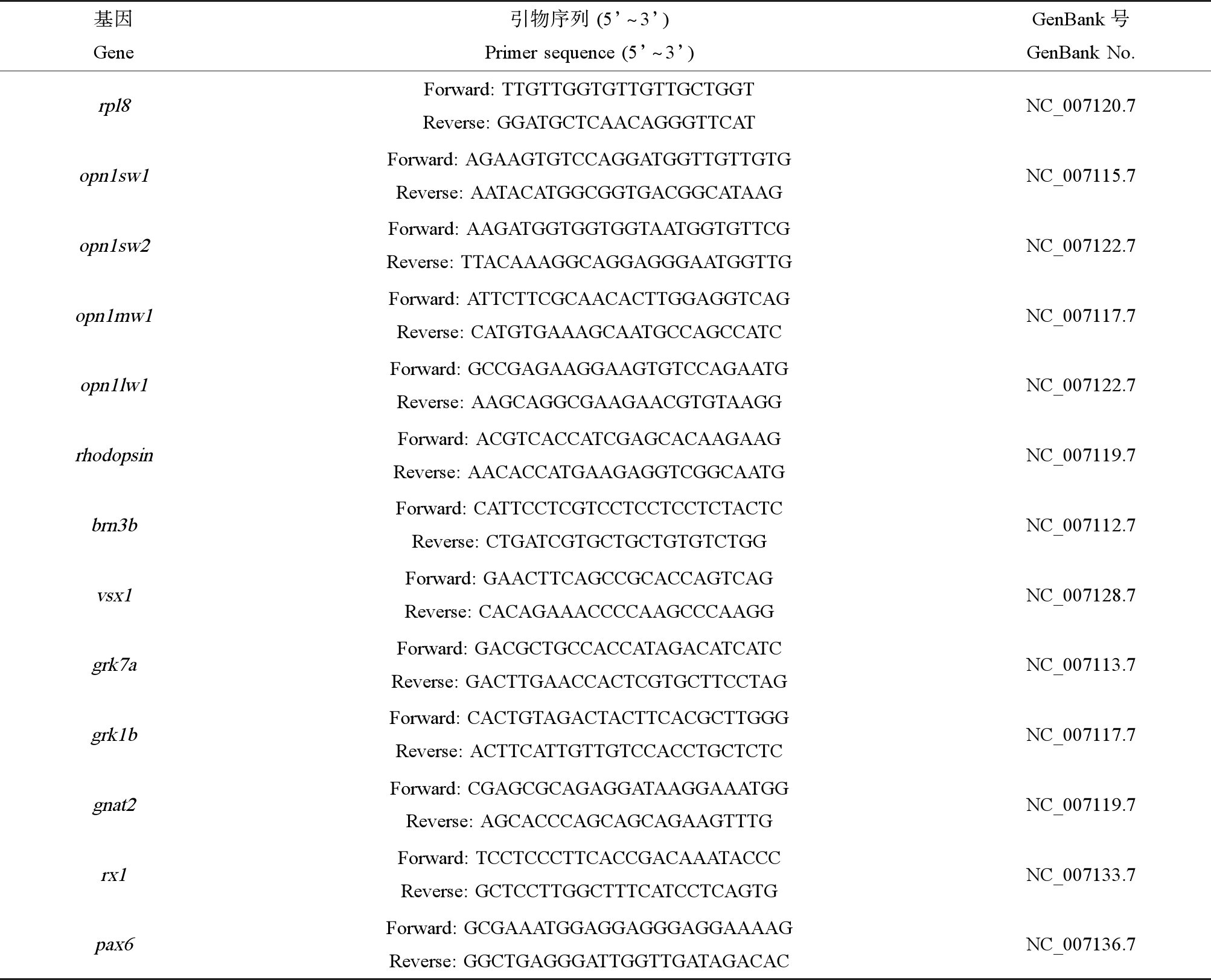
基因Gene引物序列 (5’^3’)Primer sequence (5’^3’)GenBank号GenBank No.rpl8Forward: TTGTTGGTGTTGTTGCTGGTReverse: GGATGCTCAACAGGGTTCATNC_007120.7opn1sw1Forward: AGAAGTGTCCAGGATGGTTGTTGTGReverse: AATACATGGCGGTGACGGCATAAGNC_007115.7opn1sw2Forward: AAGATGGTGGTGGTAATGGTGTTCGReverse: TTACAAAGGCAGGAGGGAATGGTTGNC_007122.7opn1mw1Forward: ATTCTTCGCAACACTTGGAGGTCAGReverse: CATGTGAAAGCAATGCCAGCCATCNC_007117.7opn1lw1Forward: GCCGAGAAGGAAGTGTCCAGAATGReverse: AAGCAGGCGAAGAACGTGTAAGGNC_007122.7rhodopsinForward: ACGTCACCATCGAGCACAAGAAGReverse: AACACCATGAAGAGGTCGGCAATGNC_007119.7brn3bForward: CATTCCTCGTCCTCCTCCTCTACTCReverse: CTGATCGTGCTGCTGTGTCTGGNC_007112.7vsx1Forward: GAACTTCAGCCGCACCAGTCAGReverse: CACAGAAACCCCAAGCCCAAGGNC_007128.7 grk7aForward: GACGCTGCCACCATAGACATCATCReverse: GACTTGAACCACTCGTGCTTCCTAGNC_007113.7grk1bForward: CACTGTAGACTACTTCACGCTTGGGReverse: ACTTCATTGTTGTCCACCTGCTCTCNC_007117.7gnat2Forward: CGAGCGCAGAGGATAAGGAAATGGReverse: AGCACCCAGCAGCAGAAGTTTGNC_007119.7rx1Forward: TCCTCCCTTCACCGACAAATACCCReverse: GCTCCTTGGCTTTCATCCTCAGTGNC_007133.7pax6Forward: GCGAAATGGAGGAGGGAGGAAAAGReverse: GGCTGAGGGATTGGTTGATAGACACNC_007136.7
1.3.7 斑马鱼幼鱼氧化应激实验
收集各组100条120 hpf幼鱼,使用1×PBS清洗,加PBS至1 mL进行匀浆并于4 ℃、13 000 g,离心10 min,弃上清。接下来严格按照所购买ROS试剂盒说明书进行。各组取100条120 hpf幼鱼于1.5 mL离心管中,低温静置后称量,按照1∶9比例加入生理盐水进行匀浆并低温冷冻离心(3 500 r·min-1,10 min)。上清液定量测定总蛋白后检测氧化应激指标(SOD活性、MDA及GST含量),方法参照文献[31]。
1.4 统计学分析
采用SPSS 22.0进行统计分析,用Graphpad Prism 8.0绘图。数据之间的差异通过单因素方差分析(one-way analysis of variance, one-way ANOVA)和Tukey多重比较法来确定对照组和暴露组之间的显著性差异。数值均以平均值±标准差(Mean±SD)表示,当P<0.05、P<0.01、P<0.001时认为具有统计学意义。
2 结果(Results)
2.1 不同浓度的氯化镉对幼鱼生长发育的影响
与对照组相比,0.4 mg·L-1氯化镉暴露组的120 hpf幼鱼存活率明显降低(P<0.05)(图1(a)),0.8 mg·L-1和1.6 mg·L-1氯化镉暴露组中的120 hpf幼鱼存活率和孵化率显著降低(P<0.01) (图1(a)和图1(b))。暴露组的斑马鱼畸形率明显高于对照组(图1(c)),其中0.8 mg·L-1和1.6 mg·L-1氯化镉暴露组的畸形率分别为18.33%、26.33%(P<0.001)。

图1 不同浓度氯化镉对斑马鱼幼鱼存活率(a)、孵化率(b)、畸形率(c)的影响
注:(a)、(b)、(c)设置3个生物性重复,每个浓度3个平行,每个平行200颗胚胎;与对照组相比,*P<0.05,**P<0.01,***P<0.001。
Fig. 1 Effects of different concentrations of cadmium chloride on the survival rate (a), hatching rate (b)and deformity rate (c) of zebrafish larvae
Note: (a), (b), (c) Three biological replicates were set up with three parallels per concentration, 200 embryos per parallel; compare with the control, *represents P<0.05, **represents P<0.01, and ***represents P<0.001.
2.2 NAC干预氯化镉对斑马鱼幼鱼生长发育的影响
与对照组相比,0.8 mg·L-1氯化镉暴露组斑马鱼存活率、孵化率明显降低,分别降低了23% (P<0.01) (图2(a))和13.67% (P<0.01) (图2(b)),畸形率明显增高了13.33% (P<0.01) (图2(c));与0.8 mg·L-1氯化镉暴露组相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组斑马鱼存活率、孵化率显著上升,分别上升了10.34%(P<0.05) (图2(a))和9.67%(P<0.05) (图2(b)),畸形率显著下降了9% (P<0.01) (图2(c))。0.8 mg·L-1氯化镉暴露组对120 hpf幼鱼发育产生明显影响,主要表现为脊柱弯曲(e)、卵黄肿大、心包水肿(f)、眼睛畸形(g)。
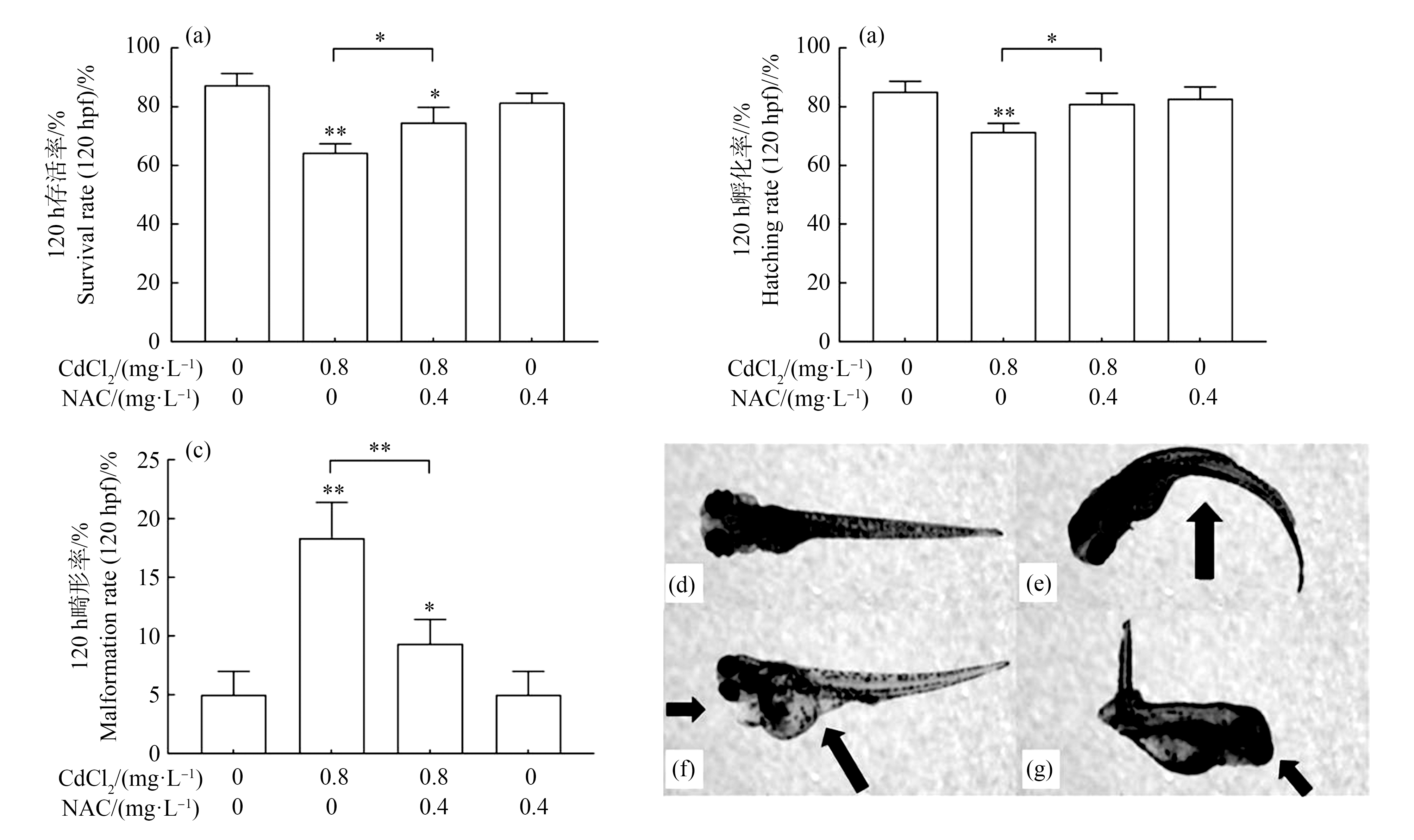
图2 N-乙酰半胱氨酸(NAC)干预对斑马鱼幼鱼存活率(a)、孵化率(b)、畸形率(c)的影响
注:(a)、(b)、(c)设置3个生物性重复,每个浓度3个平行,每个平行200颗胚胎;(d)正常幼鱼,(e)脊柱弯曲,(f)卵黄肿大、心包水肿,(g)眼睛畸形;与对照组相比,*P<0.05,**P<0.01。
Fig. 2 Effects of N-acetylcysteine (NAC) intervention on zebrafish larvae survival (a), hatching rate (b), and deformity rate (c)
Note: (a), (b), (c) Three biological replicates were set up with three parallels per concentration, 200 embryos per parallel; (d) Normal juvenile fish, (e) Spinal column curvature, (f) Yolk cyst, pericardial edema, (g) Eye deformity; compare with the control, *represents P<0.05, **represents P<0.01.
2.3 NAC干预氯化镉对斑马鱼幼鱼眼长/身长比值的影响
评估NAC干预氯化镉对斑马鱼幼鱼眼睛形态上的变化,结果可知,对照组、0.8 mg·L-1氯化镉暴露组、0.8 mg·L-1氯化镉与0.4 mg·L-1NAC联合暴露组、0.4 mg·L-1NAC暴露组斑马鱼眼长/身长比值分别为(7.35±0.58)%、(6.55±1.01)%、(6.78±0.52)%、(7.04±0.59)%。与对照组相比,0.8 mg·L-1氯化镉暴露组幼鱼的眼长/身长比值显著性降低(P<0.01),0.8 mg·L-1氯化镉与0.4 mg·L-1NAC联合暴露组降低(P<0.05),而NAC暴露组没有出现显著性变化。与0.8 mg·L-1氯化镉暴露组相比,0.8 mg·L-1氯化镉与0.4 mg·L-1NAC联合暴露组幼鱼的眼长/身长比值轻微上升,但不显著(图3)。
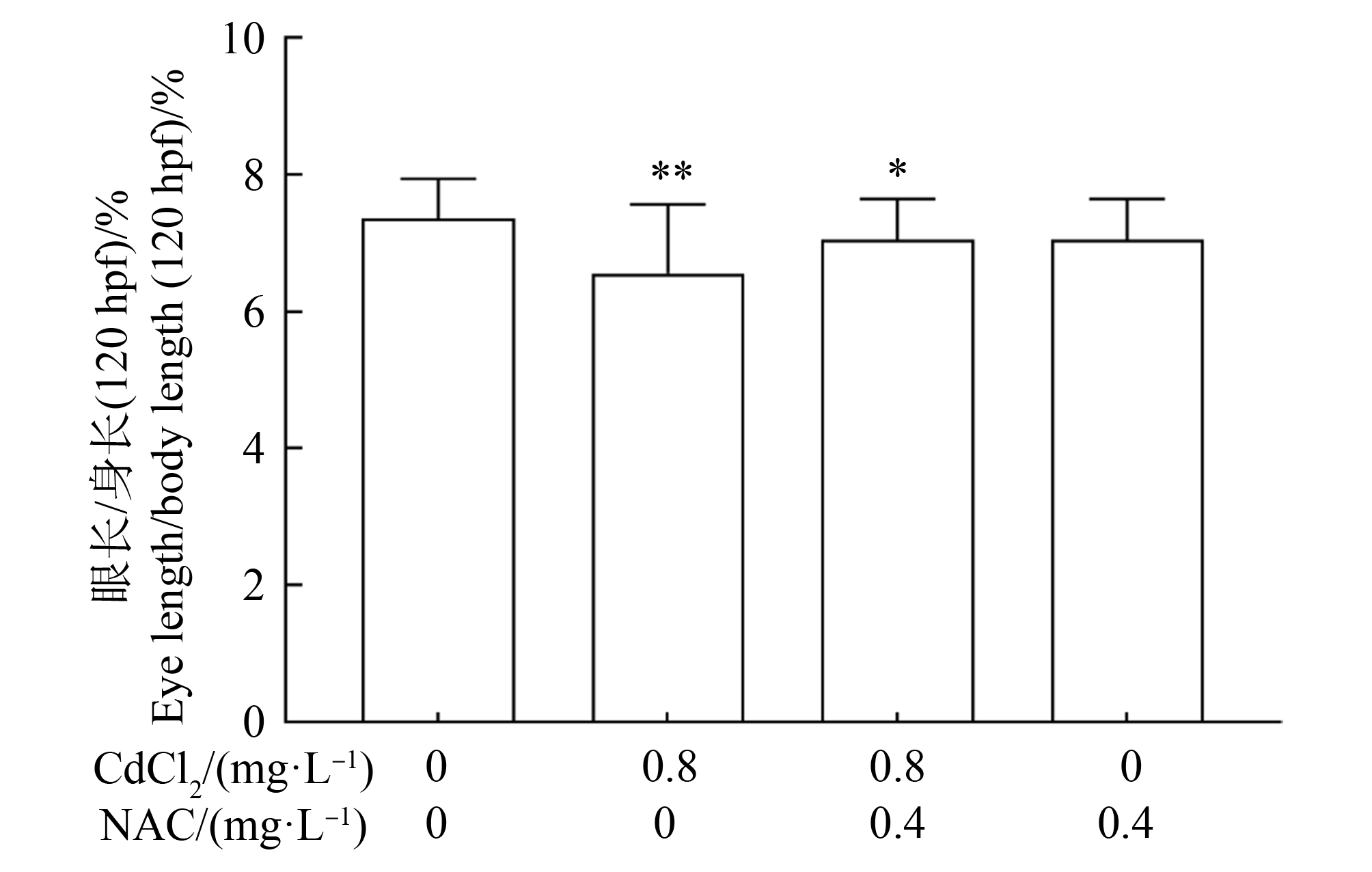
图3 NAC干预120 hpf斑马鱼幼鱼眼长/身长的比值
注:每个实验设置3个平行,每个平行观察24条幼鱼;与对照组相比,*P<0.05,**P<0.01。
Fig. 3 Ratio of eye length to body length in 120 hpf larval zebrafish treated with NAC
Note: Three parallels were set up for each experiment, and 24 juveniles were observed in each parallel; compare with the control, *represents P<0.05, **represents P<0.01.
2.4 NAC干预氯化镉对斑马鱼幼鱼视网膜结构的影响
为进一步评估NAC干预氯化镉对视网膜发育的影响,分别对各组120 hpf幼鱼眼球切片进行H&E染色,并在显微镜下观察视网膜的结构变化。对照组视网膜形态正常,有明显的界限,外核层(ONL)、内核层(INL)和神经节细胞层(GCL) 3个细胞层的细胞排列整齐(图4(a)和(e));与对照组相比,0.8 mg·L-1氯化镉暴露组晶状体直径及外核层(ONL)和内丛状层(IPL)厚度显著减小,而内核层(INL)厚度显著增大;神经纤维层明显增厚,呈空泡化;神经节细胞层(GCL)和内核层(INL)中细胞较大,细胞核紊乱,神经节细胞层(GCL)中细胞密度变小(图4(b)和(f));与0.8 mg·L-1氯化镉暴露组相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组视网膜逐渐恢复正常(图4(c)和(g));与对照组相比,0.4 mg·L-1 NAC暴露组视网膜结构没有明显变化(图4(d)和(h))。
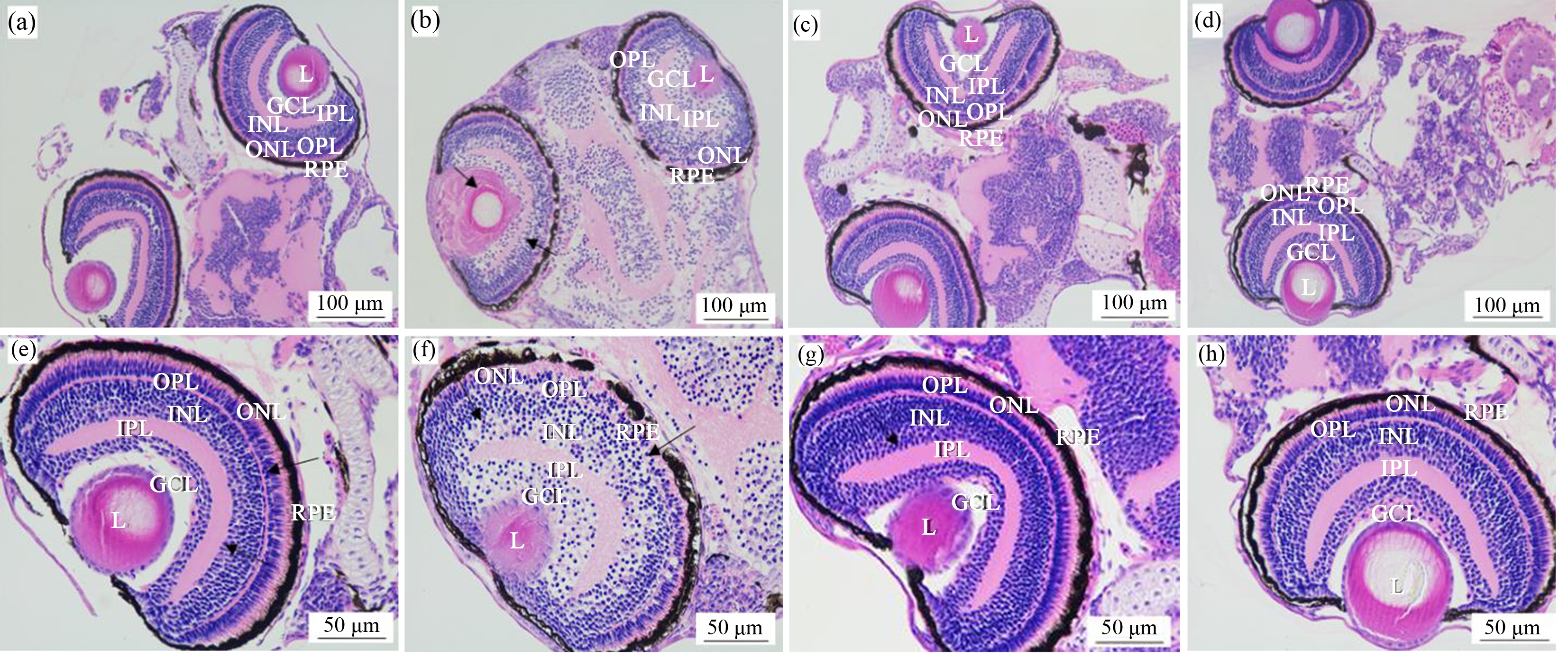
图4 NAC干预对氯化镉暴露120 h斑马鱼幼鱼视网膜分层的影响
注:L,晶状体;GCL,神经节细胞层;IPL,内丛状层;INL,内核层;OPL,外丛状层;ONL,外核层;RPE,色素上皮细胞;每个实验设置3个平行,每个平行3条幼鱼。
Fig. 4 Effects of NAC intervention on retinal stratification in zebrafish larvae exposed to cadmium chloride for 120 h
Note: L, lens; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear cell; REP, retinal pigmented epithelium; three parallels were set up for each experiment, with three juveniles per parallel.
2.5 NAC干预氯化镉暴露对幼鱼运动行为活动的影响
120 hpf幼鱼在行为活动监测过程中的运动模式如图5(a)所示,各组120 hpf幼鱼在每个时间段内的平均游泳速度如5(b)所示。与对照组相比,在第一个光照期(0~10 min),0.8 mg·L-1氯化镉暴露组幼鱼的平均运动速度(16.72 mm·s-1)明显减慢(P<0.05);经历光暗转换后的第一个黑暗期(10~20 min),0.8 mg·L-1氯化镉暴露组幼鱼的平均运动速度(17.56 mm·s-1)低于对照组幼鱼的平均速度(19.37 mm·s-1)(P<0.01),也低于0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组的速度(18.77 mm·s-1)(P<0.05)。第2个光照与第2个黑暗期与上述情况相似,与对照组相比,0.8 mg·L-1氯化镉暴露组幼鱼的平均运动速度显著降低;与0.8 mg·L-1氯化镉暴露组相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露后幼鱼平均运动速度显著提高。
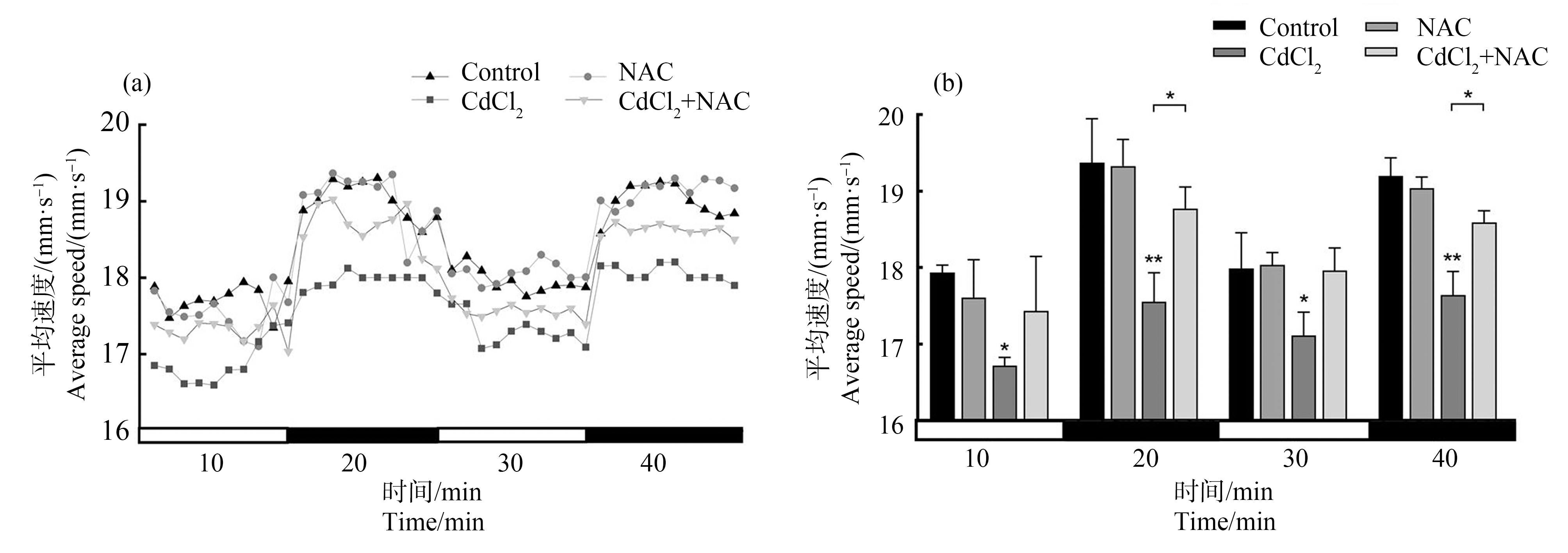
图5 NAC干预氯化镉对斑马鱼幼鱼运动行为活动的影响
注:(a)光暗交替模式下幼鱼的运动方式,(b)光暗交替模式下幼鱼在各时间段的平均运动速度;每组设24个平行样;与对照组相比,*P<0.05,**P<0.01。
Fig. 5 Effects of NAC intervention on locomotor activity in larval zebrafish after cadmium chloride exposure
Note: (a) Locomotor trace of larvae under the light-dark transition stimulation, (b) The average swimming speed of larval zebrafish during the photoperiod stimulation; 24 parallel samples in each group; compare with the control, *represents P<0.05, and **represents P<0.01.
2.6 NAC干预氯化镉暴露对斑马鱼视网膜发育相关基因转录水平的影响
检测了120 hpf幼鱼中与视网膜发育有关的12个基因,在0.8 mg·L-1氯化镉暴露组中发现了部分基因的表达水平显著下降,包括opn1sw1(0.85±0.04,P<0.05)、vsx1(0.75±0.06,P<0.05)、brn3b(0.82±0.07,P<0.05)以及pax6(0.70±0.13,P<0.05)(图6)。与0.8 mg·L-1氯化镉暴露组相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组其他基因的表达水平有轻微上调,但不显著。

图6 NAC干预氯化镉暴露对斑马鱼幼鱼视网膜相关基因表达的影响
注:每个组设6个平行,每个平行做2个技术重复;与对照组相比,*P<0.05,**P<0.01。
Fig. 6 Effects of NAC intervention on cadmium chloride exposure on retina-related gene expression in larval zebrafish
Note: Each group has 6 parallels, and each parallel does two technical repetitions; compare with the control, *represents P<0.05, and **represents P<0.01.
2.7 NAC干预氯化镉对斑马鱼幼鱼的氧化应激
与对照组相比,0.8 mg·L-1氯化镉暴露组的斑马鱼幼鱼体内的ROS含量显著升高 (P<0.001);0.8 mg·L-1氯化镉暴露组和0.8 mg·L-1氯化镉与0.4 mg·L-1NAC联合暴露相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组的ROS含量降低(P<0.001)(图7(a))。0.8 mg·L-1氯化镉暴露组的斑马鱼幼鱼体内的MDA含量与对照组相比出现显著升高(P<0.05);0.8 mg·L-1氯化镉暴露组和0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组的MDA含量下降(P<0.01)(图7(b))。0.8 mg·L-1氯化镉暴露组的斑马鱼幼鱼SOD活性与对照组相比显著降低(P<0.05);0.8 mg·L-1氯化镉暴露组和0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组的SOD含量显著升高(P<0.01) (图7(c))。0.8 mg·L-1氯化镉暴露组的斑马鱼幼鱼GST含量与对照组相比有轻微下降,但不显著;0.8 mg·L-1氯化镉暴露组和0.8 mg·L-1氯化镉与0.4 mg·L-1NAC联合暴露组相比,0.8 mg·L-1氯化镉与0.4 mg·L-1 NAC联合暴露组的GST含量出现显著升高(P<0.01) (图7(d))。

图7 NAC干预氯化镉对斑马鱼幼鱼氧化应激的影响
注:每个浓度3个平行,每个平行100条幼鱼;与对照组相比,*P<0.05,**P<0.01,***P<0.001。
Fig. 7 Effects of NAC intervention on cadmium chloride on oxidative stress in larval zebrafish
Note: Three parallels per concentration with 100 juveniles per parallel; compare with the control, *represents P<0.05, **represents P<0.01, ***represents P<0.001.
3 讨论(Discussion)
为探究Cd对斑马鱼视网膜发育的影响,本研究将斑马鱼胚胎暴露于氯化镉、氯化镉与NAC中,观察120 hpf斑马鱼幼鱼生长发育状况,通过形态学、组织病理学切片评估斑马鱼眼睛大小以及视网膜结构变化;通过行为学评估Cd对斑马鱼幼鱼运动的影响;进一步分析斑马鱼幼鱼视网膜发育相关基因的表达水平,揭示Cd影响斑马鱼视网膜发育的机制。
本研究结果显示,氯化镉暴露对斑马鱼的影响具有浓度依赖性,其中0.8 mg·L-1氯化镉暴露组中120 hpf斑马鱼幼鱼致死、致畸效应较强。与对照组相比,氯化镉暴露组斑马鱼幼鱼眼长/身长比值显著降低。因此,笔者推测Cd可导致斑马鱼幼鱼眼睛形态上发生改变,这与Chow等[32]研究发现一致。组织切片显示,相比于对照组,氯化镉单独暴露组和联合暴露组中斑马鱼幼鱼晶状体直径明显减小,外核层(ONL)的厚度明显变小,神经节细胞层(GCL)的细胞密度变小。研究发现环境污染物会造成斑马鱼幼鱼眼部感光细胞外节盘数量明显减少,外网状层及内网状层明显变薄[33]。有研究报道,金属铜可导致斑马鱼胚胎视网膜上的感光细胞分布异常,并出现细胞凋亡现象[34]。故认为斑马鱼晶状体改变是因为Cd暴露导致斑马鱼视网膜上的细胞发生改变。
运动行为是反映生理状态变化的敏感指标[35],运动行为实验可以快速检测各种因子对斑马鱼活动的影响。许多行为依赖于鱼类的视觉功能[36],视觉功能和视网膜结构的改变会影响视觉引导的运动行为[37]结果显示,氯化镉暴露组斑马鱼幼鱼的运动速度明显降低,Tian等[38]研究发现Cd暴露亲本斑马鱼60 d后,子代幼鱼游泳速度降低和距离缩短,体内多巴胺、血清素等水平被破坏,神经发育和神经递质代谢相关基因表达模式改变,从而诱导子代幼鱼的发育神经毒性。这与笔者的研究结果一致,由于Cd暴露损害幼鱼的神经系统,导致其运动速度减慢。Gu等[39]报道了斑马鱼幼鱼行为活性不足可能与视网膜的形态变化有关。因此,与Cd暴露组相比,氯化镉与NAC联合暴露幼鱼平均运动速度显著提高。在组织病理学研究中,联合暴露组的视网膜形态结构,明显要优于Cd暴露组。另外,NAC暴露组幼鱼的运动行为模式与对照组的运动行为模式相近,说明0.4 mg·L-1 NAC暴露对斑马鱼幼鱼运动行为模式无影响。根据研究证明,Cd可诱导氧化应激的活性氧(ROS)生成,其毒性效应能够影响乙酰胆碱酯酶(AchE)活性,引起神经行为障碍[40]以及NAC可以预防氯胺酮诱导的心脏毒性、发育毒性和神经毒性[41]。由此笔者推测,NAC通过消除Cd产生的过量氧自由基,从而使神经递质水平不受干扰,最终恢复幼鱼的运动速度。
在视网膜结构中,感光细胞易受环境污染而发生改变[42]。本研究发现经氯化镉暴露120 h后,与对照组相比,氯化镉暴露组幼鱼视网膜发育基因opn1sw1、brn3b、vsx1、pax6的表达水平显著性下调。据报道,opn1sw1基因是外核层(ONL)层的视锥细胞标记基因,对于斑马鱼视网膜光感受器的发育是必不可少[20];opn1sw1(UV)基因表达水平的下调说明可能会损害斑马鱼的视网膜光感受器分化;vsx1基因是内核层(INL)层细胞向神经元分化所必需的[43],vsx1基因表达水平下调表明氯化镉至少间接地干扰内核层(INL)特异性标志物来影响内核层(INL)的发展;pax6基因是视网膜细胞分化标记基因,发育早期pax6决定了未来的眼睛组织(包括视网膜和视网膜色素上皮),pax6基因表达水平下调可能会使眼睛变小[44]。因此,Cd可通过影响部分斑马鱼视网膜发育相关基因的表达水平从而导致视网膜发生改变。
本次实验还检测了幼鱼体内ROS、MDA、SOD和GST。SOD可清除机体中过量的ROS,是生物体内清除氧自由基保护机体的第一道防线[45];GST可与过氧化物及自由基相结合从而参与到体内的氧化还原反应,对抗自由基对机体的损害[46];当生物体的ROS含量超过抗氧化酶的清除能力时,过量的ROS就会刺激MDA的产生,MDA是反映机体抗氧化潜在能力的重要参数,间接反映组织过氧化损伤程度[19]。研究结果显示,与对照组相比,氯化镉暴露组斑马鱼幼鱼体内ROS含量和MDA含量升高、SOD活性水平明显降低、GST含量略微下降。通过结果可看出,Cd能够刺激斑马鱼幼鱼产生氧化应激反应,这可能导致斑马鱼幼鱼神经毒性,从而影响视觉行为。与氯化镉暴露组相比,氯化镉与NAC联合暴露组GST含量出现显著升高,ROS含量显著下降。NAC具有抗氧化特性,能保护机体免受氧化应激损伤[47]。除此,NAC还能够部分削弱Cd诱导的斑马鱼幼鱼眼部形态和视网膜组织病理学损伤,提高斑马鱼视网膜发育相关基因的表达。
综上所述,Cd暴露可导致斑马鱼胚胎的存活率、孵化率明显降低,畸形率明显升高;还可降低视网膜相关发育基因的表达水平,导致视网膜发育缺陷,从而损害视觉介导行为,最终改变幼鱼的运动行为能力,氧化应激在其中有一定的影响,相关机制还需进一步的研究。
通信作者简介:郭庶(1973—),男,博士,研究员,主要研究方向为劳动卫生与环境卫生。
共同通信作者简介:谷红梅(1970—),女,硕士,副教授,主要研究方向为流行病与卫生统计。
[1] Campoy-Diaz A D, Escobar-Correas S, Canizo B V, et al. A freshwater symbiosis as sensitive bioindicator of cadmium [J]. Environmental Science and Pollution Research International, 2020, 27(3): 2580-2587
[2] Zhou Y F, Yang Y Y, Liu G H, et al. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene [J]. Water Research, 2020, 184: 116209
[3] Yi Y J, Zhang S H. The relationships between fish heavy metal concentrations and fish size in the upper and middle reach of Yangtze River [J]. Procedia Environmental Sciences, 2012, 13: 1699-1707
[4] 李争显, 李伟, Lei Jiajun, 等. 常见金属元素对人体的作用及危害[J]. 中国材料进展, 2020, 39(12): 934-944
Li Z X, Li W, Lei J J, et al. Effect and hazard of common metal elements on human body [J]. Materials China, 2020, 39(12): 934-944 (in Chinese)
[5] Chen H, Chen K, Qiu X C, et al. The reproductive toxicity and potential mechanisms of combined exposure to dibutyl phthalate and diisobutyl phthalate in male zebrafish (Danio rerio) [J]. Chemosphere, 2020, 258: 127238
[6] Awoyemi O M, Subbiah S, Velazquez A, et al. Nitrate-N-mediated toxicological responses of Scenedesmus acutus and Daphnia pulex to cadmium, arsenic and their binary mixture (Cd/Asmix) at environmentally relevant concentrations [J]. Journal of Hazardous Materials, 2020, 400: 123189
[7] Zhang Y, Li Z Y, Kholodkevich S, et al. Effects of cadmium on intestinal histology and microbiota in freshwater crayfish (Procambarus clarkii) [J]. Chemosphere, 2020, 242: 125105
[8] Capriello T, Grimaldi M C, Cofone R, et al. Effects of aluminium and cadmium on hatching and swimming ability in developing zebrafish [J]. Chemosphere, 2019, 222: 243-249
[9] Tang S, Doering J A, Sun J X, et al. Linking oxidative stress and magnitude of compensatory responses with life-stage specific differences in sensitivity of white sturgeon (Acipenser transmontanus) to copper or cadmium [J]. Environmental Science &Technology, 2016, 50(17): 9717-9726
[10] Chen J Q, Xu Y M, Han Q, et al. Immunosuppression, oxidative stress, and glycometabolism disorder caused by cadmium in common carp (Cyprinus carpio L.): Application of transcriptome analysis in risk assessment of environmental contaminant cadmium [J]. Journal of Hazardous Materials, 2019, 366: 386-394
[11] Akinyemi A J, Oboh G, Fadaka A O, et al. Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats [J]. Neurotoxicology, 2017, 62: 75-79
[12] Stenkamp D L. Neurogenesis in the fish retina [J]. International Review of Cytology, 2007, 259: 173-224
[13] Naïja A, Kestemont P, Chénais B, et al. Effects of Hg sublethal exposure in the brain of peacock blennies Salariapavo: Molecular, physiological and histopathological analysis [J]. Chemosphere, 2018, 193: 1094-1104
[14] Boyd P, Hyde D R. Iron contributes to photoreceptor degeneration and Müller glia proliferation in the zebrafish light-treated retina [J]. Experimental Eye Research, 2022, 216: 108947
[15] Mela M, Cambier S, Mesmer-Dudons N, et al. Methylmercury localization in Danio rerio retina after trophic and subchronic exposure: A basis for neurotoxicology [J]. Neurotoxicology, 2010, 31(5): 448-453
[16] Chen J F, Chen Y H, Liu W, et al. Developmental lead acetate exposure induces embryonic toxicity and memory deficit in adult zebrafish [J]. Neurotoxicology and Teratology, 2012, 34(6): 581-586
[17] Sakai C, Ijaz S, Hoffman E J. Zebrafish models of neurodevelopmental disorders: Past, present, and future [J]. Frontiers in Molecular Neuroscience, 2018, 11: 294
[18] Avanesov A, Malicki J. Analysis of the retina in the zebrafish model [J]. Methods in Cell Biology, 2010, 100: 153-204
[19] d'Amora M, Giordani S. The utility of zebrafish as a model for screening developmental neurotoxicity [J]. Frontiers in Neuroscience, 2018, 12: 976
[20] Amini R, Rocha-Martins M, Norden C. Neuronal migration and lamination in the vertebrate retina [J]. Frontiers in Neuroscience, 2017, 11: 742
[21] Chen X, Qiu T T, Xiao P, et al. Retinal toxicity of isoflucypram to zebrafish (Danio rerio) [J]. Aquatic Toxicology, 2022, 243: 106073
[22] Xiao P, Li W H, Lu J F, et al. Effects of embryonic exposure to bixafen on zebrafish (Danio rerio) retinal development [J]. Ecotoxicology and Environmental Safety, 2021, 228: 113007
[23] DeCarvalho A C, Cappendijk S L T, Fadool J M. Developmental expression of the POU domain transcription factor Brn-3b (Pou4f2) in the lateral line and visual system of zebrafish [J]. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 2004, 229(4): 869-876
[24] Handa O, Naito Y, Yoshikawa T. Redox biology and gastric carcinogenesis: The role of Helicobacter pylori [J]. Redox Report: Communications in Free Radical Research, 2011, 16(1): 1-7
[25] Zhang T, Zhou X Y, Ma X F, et al. Mechanisms of cadmium-caused eye hypoplasia and hypopigmentation in zebrafish embryos [J]. Aquatic Toxicology, 2015, 167: 68-76
[26] Jin Y X, Liu Z Z, Liu F, et al. Embryonic exposure to cadmium (Ⅱ) and chromium (Ⅵ) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio) [J]. Neurotoxicology and Teratology, 2015, 48: 9-17
[27] 鲁疆, 王占洋, 袁玉婷, 等. 氯化镉对斑马鱼胚胎的发育毒性[J]. 生态毒理学报, 2013, 8(3): 381-388
Lu J, Wang Z Y, Yuan Y T, et al. Developmental toxicity of cadmium chloride to zebrafish embryo [J]. Asian Journal of Ecotoxicology, 2013, 8(3): 381-388 (in Chinese)
[28] Bilotta J, Saszik S. The zebrafish as a model visual system [J]. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 2001, 19(7): 621-629
[29] Copper J E, Budgeon L R, Foutz C A, et al. Comparative analysis of fixation and embedding techniques for optimized histological preparation of zebrafish [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2018, 208: 38-46
[30] Yu Y J, Hou Y B, Dang Y, et al. Exposure of adult zebrafish (Danio rerio) to tetrabromobisphenol A causes neurotoxicity in larval offspring, an adverse transgenerational effect [J]. Journal of Hazardous Materials, 2021, 414: 125408
[31] Araujo G F, Soares L O S, Junior S F S, et al. Oxidative stress and metal homeostasis alterations in Danio rerio (zebrafish) under single and combined carbamazepine, acetamiprid and cadmium exposures [J]. Aquatic Toxicology, 2022, 245: 106122
[32] Chow E S, Hui M N, Cheng C W, et al. Cadmium affects retinogenesis during zebrafish embryonic development [J]. Toxicology and Applied Pharmacology, 2009, 235(1): 68-76
[33] 张灵, 刘秘, 王雯雯, 等. 三氯生单独暴露对斑马鱼幼鱼眼部的毒性研究[J]. 生命科学研究, 2019, 23(4): 290-296
Zhang L, Liu M, Wang W W, et al. Toxic effect of triclosan exposure on the eyes of zebrafish larvae [J]. Life Science Research, 2019, 23(4): 290-296 (in Chinese)
[34] Zhao G, Sun H J, Zhang T, et al. Copper induce zebrafish retinal developmental defects via triggering stresses and apoptosis [J]. Cell Communication and Signaling, 2020, 18(1): 45
[35] Xia Y, Zhu J W, Xu Y J, et al. Effects of ecologically relevant concentrations of cadmium on locomotor activity and microbiota in zebrafish [J]. Chemosphere, 2020, 257: 127220
[36] Shi Q P, Wang Z Y, Chen L G, et al. Optical toxicity of triphenyl phosphate in zebrafish larvae [J]. Aquatic Toxicology, 2019, 210: 139-147
[37] Qian L, Qi S Z, Wang Z, et al. Environmentally relevant concentrations of boscalid exposure affects the neurobehavioral response of zebrafish by disrupting visual and nervous systems [J]. Journal of Hazardous Materials, 2021, 404(Pt A): 124083
[38] Tian J J, Hu J, Liu D, et al. Cadmium chloride-induced transgenerational neurotoxicity in zebrafish development [J]. Environmental Toxicology and Pharmacology, 2021, 81: 103545
[39] Gu J, Zhang J Y, Chen Y Y, et al. Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio) [J]. Chemosphere, 2019, 217: 629-635
[40] Hsu T, Huang K M, Tsai H T, et al. Cadmium(Cd)-induced oxidative stress down-regulates the gene expression of DNA mismatch recognition proteins MutS homolog 2 (MSH2) and MSH6 in zebrafish (Danio rerio) embryos [J]. Aquatic Toxicology, 2013, 126: 9-16
[41] Gu Q, Rodgers J, Robinson B, et al. N-acetylcysteine prevents verapamil-induced cardiotoxicity with no effect on the noradrenergic arch-associated neurons in zebrafish [J]. Food and Chemical Toxicology, 2020, 144: 111559
[42] Zhang X, Hong Q, Yang L, et al. PCB1254 exposure contributes to the abnormalities of optomotor responses and influence of the photoreceptor cell development in zebrafish larvae [J]. Ecotoxicology and Environmental Safety, 2015, 118: 133-138
[43] Chow R L, Snow B, Novak J, et al. Vsx1, a rapidly evolving paired-like homeobox gene expressed in cone bipolar cells [J]. Mechanisms of Development, 2001, 109(2): 315-322
[44] Nornes S, Clarkson M, Mikkola I, et al. Zebrafish contains two pax6 genes involved in eye development [J]. Mechanisms of Development, 1998, 77(2): 185-196
[45] 黄惠琳, 刘华钢, 蒙怡, 等. 氯化两面针碱对斑马鱼胚胎SOD和MDA的影响[J]. 毒理学杂志, 2011, 25(4): 243-245
Huang H L, Liu H G, Meng Y, et al. The effect of nitidine chloride on SOD activity and MDA content of zebrafish embryo [J]. Journal of Toxicology, 2011, 25(4): 243-245(in Chinese)
[46] 杨晨, 耿月攀, 田然. 哺乳动物谷胱甘肽转移酶研究进展[J]. 南京师大学报(自然科学版), 2021, 44(1): 91-98
Yang C, Geng Y P, Tian R. Advance in mammalian glutathione transferase research [J]. Journal of Nanjing Normal University (Natural Science Edition), 2021, 44(1): 91-98(in Chinese)
[47] 彭秋雨, 高举, 陈敏. N-乙酰半胱氨酸在血液系统疾病治疗中的研究进展[J]. 中国药房, 2021, 32(1): 115-120
Peng Q Y, Gao J, Chen M. Research progress of N- acetylcysteine in the treatment of hematological diseases [J]. China Pharmacy, 2021, 32(1): 115-120 (in Chinese)