我国是水产养殖最发达的国家,为了追求更高的养殖效率,水产养殖逐渐走向集约化和规模化的养殖模式。高密度的养殖环境使水产品更易产生病害,抗生素因其成本低、使用便捷和疗效显著等优点被大量应用于防治水产品疾病[1]。然而,投入的抗生素仅有20%~30%被鱼类等水产品吸收,大部分以原药的形式排入环境,从而造成养殖塘中抗生素的残留和积累,抗生素在环境中的长期赋存可能会产生一系列生态风险,如对非靶生物的毒害、细菌耐药性的产生等[2-3]。
已有大量研究表明,沉积物是抗生素的主要蓄积地,沉积物中抗生素的浓度水平普遍高出水体3~4个数量级[4]。其中,氟喹诺酮类(fluoroquinolones, FQs)是水产养殖中的常用抗生素,也是水产养殖塘沉积物中的主要抗生素种类,有报道发现上海市水产养殖区沉积物中有高达1 279.7 ng·g-1的FQs检出[5]。FQs在沉积物环境中的毒性、浸出、生物降解和积累等行为都与它们在沉积物中的吸附-解吸过程密切相关[6]。FQs的吸附/解吸机理取决于沉积物的理化性质,包括有机质含量、黏土组分、金属氧化物含量和阳离子交换量等,此外,抗生素的亲脂性、水溶性及化学基团在吸附中也起着重要的作用[7]。
受养殖活动的影响,养殖塘沉积物与河流或湖泊沉积物的理化性质差异较大。因此,本研究以养殖塘沉积物作为研究对象,通过吸附/解吸试验研究养殖塘沉积物对恩诺沙星(enrofloxacin, ENR)和诺氟沙星(norfloxacin, NOR)的吸附特性。在此基础上,对沉积物组成及多种环境条件下FQs的吸附/解吸行为进行进一步分析,以深入了解FQs在水产养殖环境中的迁移、归趋,为评价此类抗生素的环境风险提供科学依据。
1 材料与方法(Materials and methods)
1.1 供试沉积物
沉积物A(Sed A)采自上海市青浦区某蟹塘表层沉积物(0~20 cm),沉积物B(Sed B)采自上海市青浦区某鱼塘表层沉积物(0~20 cm)。沉积物样品冻干、除去杂质后研磨并过80目筛网,处理后的沉积物样品储存于4 ℃的冰箱中待用。2种沉积物的理化性质如表1所示。
表1 供试沉积理化性质
Table 1 Physicochemical properties of sediments
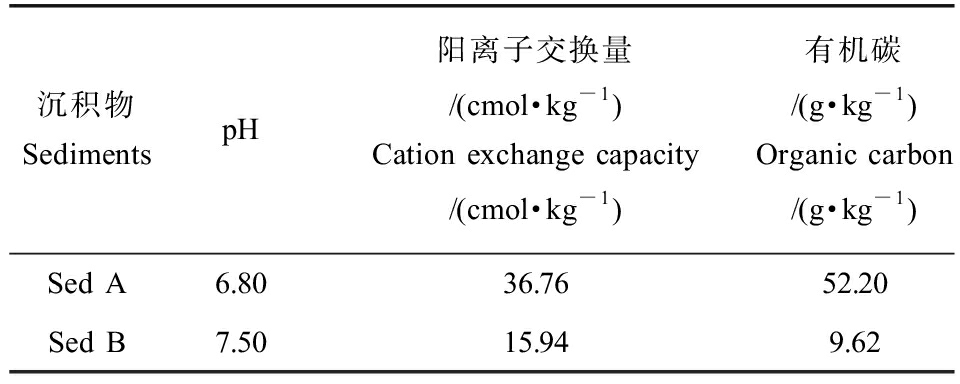
沉积物SedimentspH阳离子交换量/(cmol·kg-1)Cation exchange capacity/(cmol·kg-1)有机碳/(g·kg-1)Organic carbon/(g·kg-1)Sed A6.8036.7652.20Sed B7.5015.949.62
1.2 实验仪器与试剂
低温真空冷冻干燥器(LDG-0.3C,上海昊博有限公司,中国)、旋转培养器(WH-60,常州市顶新实验仪器有限公司,中国)、pH计(S220,梅特勒托利多仪器有限公司,中国)、高速离心机(H1850,上海湘仪仪器有限公司,中国)、高效液相色谱(LC 2695-2998,美国Waters公司)。
诺氟沙星(NOR,纯度97.29%)和恩诺沙星(ENR,纯度99.9%)购自德国Dr. Ehrenstorfer公司,HPLC级甲醇和甲酸购自德国Merck公司,其他试剂均为分析纯购自上海国药化学试剂有限公司(中国)。将NOR溶解在含0.8% 0.5 mol·L-1盐酸的甲醇中,ENR溶解于甲醇中,配制浓度为1 000 mg·L-1的标准储备液,-20 ℃避光保存。ENR和NOR理化性质如表2所示。
表2 恩诺沙星(ENR)和诺氟沙星(NOR)的理化性质
Table 2 Physicochemical properties of enrofloxacin (ENR) and norfloxacin (NOR)
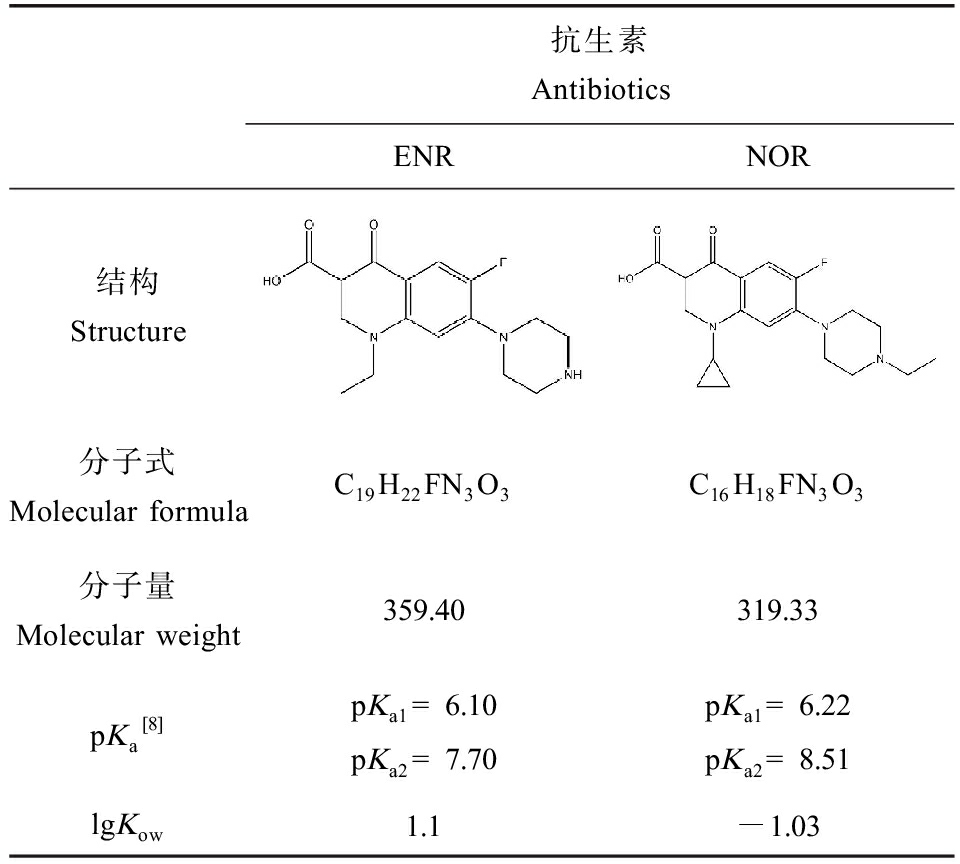
抗生素AntibioticsENRNOR结构Structure分子式Molecular formulaC19H22FN3O3C16H18FN3O3分子量Molecular weight359.40319.33pKa[8]pKa1=6.10pKa2=7.70pKa1=6.22pKa2=8.51lgKow1.1-1.03
注:Kow为辛醇水分配系数;pKa为解离常数。
Note: Kow is octanol water partition coefficient; pKa is the dissociation constant.
1.3 检测方法
使用高效液相色谱(HPLC)对目标物进行检测。高效液相色谱的型号为Waters 2695,检测器为2998二级管阵列检测器,2种目标物优化后的液相检测参数如表3所示。
表3 NOR和ENR的液相检测条件
Table 3 Detection conditions of NOR and ENR by liquid chromatography
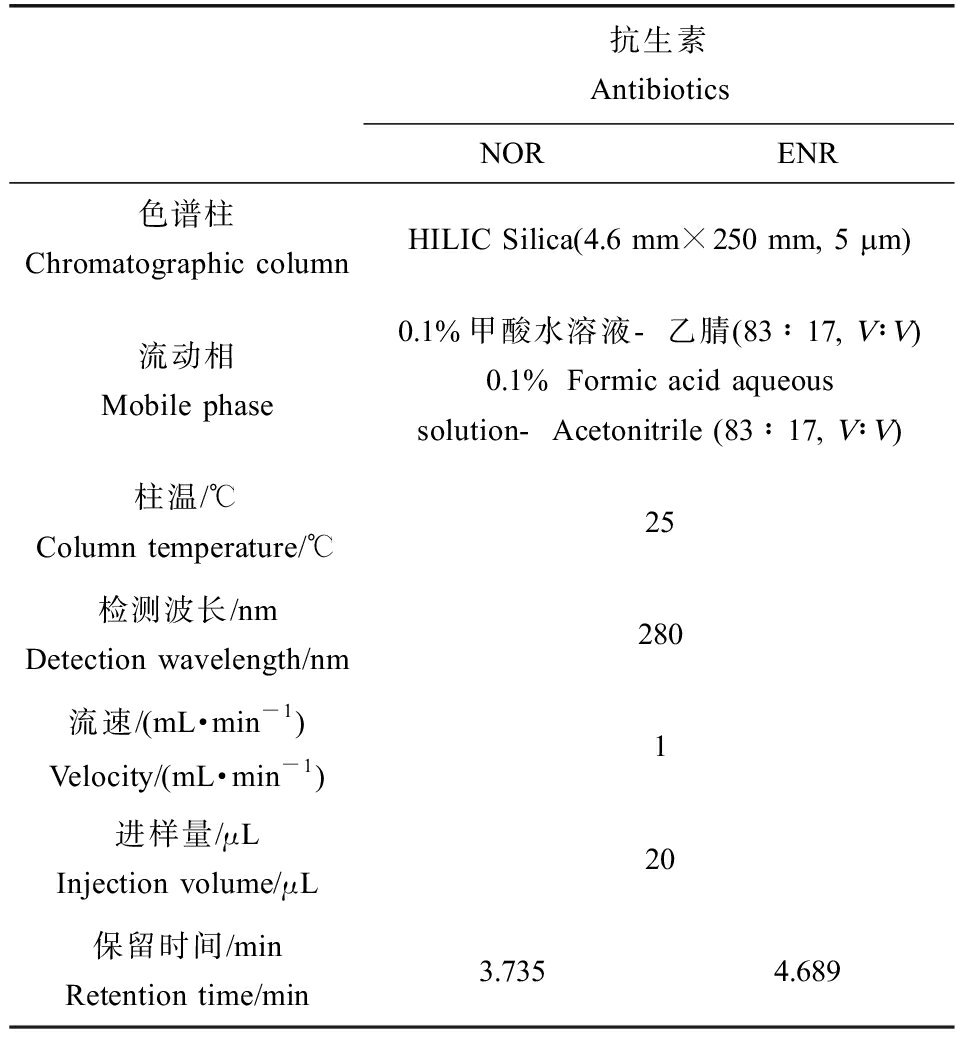
配制浓度范围在0.03~100 mg·L-1的工作液并采用液相进行分析,得到2种抗生素的标准曲线,通过方法的检出限、定量限、回收率及精密度评估方法的有效性。采用3倍信噪比(S/N)计算检出限(LOD),10倍信噪比(S/N)计算定量限(LOQ);将稀释的样品连续进样7次,计算相对标准偏差(RSD)得到仪器精密度;回收率实验的浓度设定分别为1、10、50和100 mg·L-1,每组做3个平行,通过实测浓度平均数与理论浓度的比值计算。ENR和NOR的标准曲线、仪器检出限和定量限、仪器精密度和回收率如表4所示。
表4 ENR和NOR的回归方程、仪器检出限和定量限、仪器精密度和回收率
Table 4 Standard curve, limit of detection (LOD) and limit of quantitation (LOQ), instrument precision and recovery rate of ENR and NOR

抗生素Antibiotic标准曲线Standard curveR2仪器检出限/(μg·L-1)LOD/(μg·L-1)仪器定量限/(μg·L-1)LOQ/(μg·L-1)仪器精密度/(μg·L-1)Precision/(μg·L-1)回收率/%Recovery/%NORY=132 587x-37 3500.99988.729.00.23599.35~100.23ENRY=129 635x-39 7690.99999.933.10.42398.90~100.45
1.4 实验方法
1.4.1 吸附动力学实验
吸附动力学实验参照OECD Guideline 106批平衡方法进行。准确称取2种沉积物样品50.00 mg于30 mL玻璃离心管内,铝箔纸裹于玻璃离心管外部进行避光。加入25.0 mL浓度为0.01 mol·L-1的CaCl2溶液,置于旋转培养器(20 r·min-1)平衡24 h后,添加一定浓度的ENR和NOR混合液,使其在水相中的初始浓度分别为20、40、60和80 mg·L-1,再次置于旋转培养器上。依次在1 min、5 min、10 min、15 min、20 min、30 min、45 min、1 h、2 h、4 h、8 h、16 h和24 h取样。为了保证数据的准确性,前1 h直接取0.2 mL液体,之后经离心(3 000 r·min-1,10 min)后取上清液,经0.22 μm水系滤膜过滤后用HPLC分析。
1.4.2 吸附/解吸热力学实验
热力学实验条件与动力学保持一致。准确称取2种沉积物样品50.00 mg于30 mL玻璃离心管内,铝箔纸裹于玻璃离心管外部进行避光。加入25.0 mL浓度为0.01 mol·L-1的CaCl2溶液,置于旋转培养器(20 r·min-1)平衡24 h后,添加ENR和NOR混合液,使其在水相中的初始浓度分别为10、20、40、60、80和100 mg·L-1,旋转培养24 h后取样测定。离心弃去上清液,重新加入25 mL浓度为0.01 mol·L-1的CaCl2溶液平衡24 h后取样测定。在本部分实验中,同时考察了多种因子对吸附/解吸的影响。
1.4.2.1 溶液pH对吸附/解吸的影响
采用0.01 mol·L-1的NaOH和HCl调节CaCl2溶液pH到4、5、6、7、8和9。
1.4.2.2 沉积物组分对吸附/解吸的影响
通过沉积物组分萃取,分别去除沉积物中的有机质、锰氧化物以及锰铁氧化物。具体方法如下。
(1)去除有机质:称取一定量的沉积物,持续加入30%的H2O2溶液,直至无气泡产生,在70 ℃水浴中将剩余H2O2去除,用超纯水洗至pH=7,自然干燥。
(2)去除猛氧化物:称取0.50 g沉积物于30 mL玻璃离心管中,加入20 mL 0.1 mol·L-1 NH2OH·HCl和0.1 mol·L-1 HNO3混合溶液,置于旋转培养器(30 r·min-1)旋转30 min,离心过滤后,水洗10次,自然干燥。
(3)去除锰/铁氧化物:称取0.50 g沉积物于30 mL玻璃离心管中,加入20 mL 0.2 mol·L-1 (NH4)2C2O4溶液,用H2C2O4调节pH=3.0,避光震荡4 h,离心过滤后,水洗10次,自然干燥。
1.4.2.3 不同离子和离子强度对吸附/解吸的影响
采用浓度为0.01、0.03、0.1和0.3 mol·L-1的NaCl、KCl、CaCl2和MgCl2溶液和5、10、20和50 μmol·L-1的ZnCl2和PbCl2溶液。
以上所有处理均做3次平行,同时设置不含抗生素的处理作为空白,不含沉积物的处理作为对照。
1.5 数据处理
数据为3次平行实验的算术平均值。
被沉积物吸附的抗生素浓度(Qt,mg·g-1)计算如下:
式中:C0是加到水相的初始浓度(mg·L-1);Ct是t时刻水相中目标物的浓度(mg·L-1);V为溶液的体积(L);m为沉积物干质量(g)。
吸附动力学数据用准一级动力学模型、准二级动力学模型和颗粒内扩散模型进行拟合。
准一级吸附动力学模型:
ln(Qe-Qt)=lnQe-k1t
准二级吸附动力学模型:
颗粒内扩散吸附动力学模型:
Qt=k3t1/2
式中:Qt和Qe分别为t时刻和平衡时的吸附量(mg·g-1);k1为一级吸附速率常数(min-1);k2为二级吸附速率常数(kg·mg-1·h-1);k3为颗粒内扩散速率常数(mg·g-1·min-1/2)。
吸附和解吸热力学数据用Langmuir模型和Freundlich模型进行拟合。
Langmuir模型:
Freundlich模型:
式中:Qt和Qe分别为t时刻和平衡状态时的吸附量(mg·g-1);Qm为最大吸附量(mg·g-1);Ce为水相平衡浓度(mg·L-1);Kl为Langmuir常数;Kf为Freundlich常数;n为特征常数,表征吸附剂的吸附性能。
2 结果与讨论(Results and discussion)
2.1 ENR和NOR在沉积物中的吸附动力学
空白组在ENR和NOR的出峰时间没有检出色谱峰,说明这2种沉积物没有被污染且其中的杂质对目标物的检测没有干扰。对照组中ENR和NOR的浓度在实验期间内稳定,未发生降解转化,同时表明实验容器对ENR和NOR没有吸附。
ENR和NOR的吸附动力学曲线如图1所示,它们在不同沉积物中表现出相似的吸附特征。第1阶段(0~1 h),吸附曲线斜率很大,吸附量迅速增加,可达到平衡吸附量的90%左右。吸附的初始阶段,抗生素主要以扩散的方式吸附在沉积物颗粒表面,沉积物表面有大量的吸附位点,抗生素可通过范德华力、氢键和偶极力迅速吸附,然后以最小的扩散阻力沿径向移动至空隙中,优先占据能量低的吸附位点[9];第2阶段(1~16 h),曲线斜率减缓,沉积物表面的活性位点趋于饱和,溶质分子从大孔径向小孔径中扩散,它们在狭窄的空隙中受到很大阻力,推动力逐渐降低,吸附速率变慢[10];第3阶段(16~24 h)吸附达到平衡,随时间的增加吸附量基本不变。
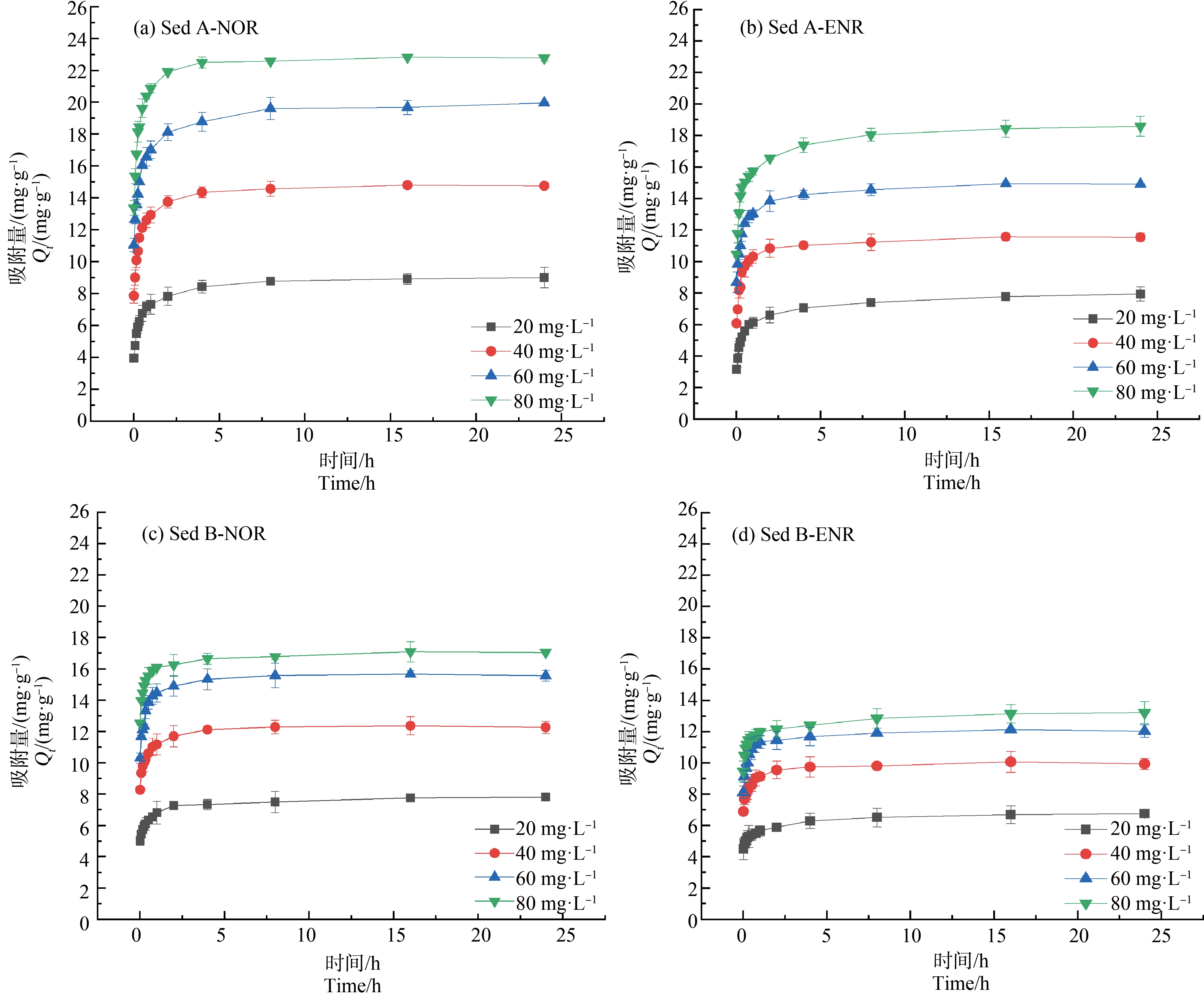
图1 不同初始浓度下ENR和NOR在2种沉积物中的吸附动力学曲线
Fig. 1 Adsorption kinetics of ENR and NOR in 2 kinds of sediments at different initial concentrations
比较2种沉积物的吸附情况发现,不同沉积物的吸附能力、速率和行为均不同,2种抗生素在Sed A上的吸附量和吸附速率均大于Sed B。如表1所示,2种沉积物的pH、阳离子交换量和有机碳含量均有显著差异。有研究表明,沉积物的理化性质,如pH、有机质、阳离子交换量、机械组成和比表面积都会影响抗生素的吸附过程、吸附速率和最终吸附量[11]。
用准一级动力学、准二级动力学和颗粒内扩散模型对吸附过程进行拟合。结果如表5所示,吸附的初始阶段(1 h),一级动力学方程的拟合效果好(R2=0.800~0.956),说明沉积物吸附ENR和NOR的初始阶段,主要以物理吸附为主。二级动力学模型能够更好地拟合整个吸附过程(R2>0.99),物理吸附进行一段时间后,ENR和NOR的有机官能团如羧基、羰基等与黏土矿物、金属氧化物或有机质形成化学键,发生化学吸附,控制吸附速率从而影响整个吸附过程。颗粒内扩散模型未过原点,说明内扩散不是吸附第一步的唯一限速步骤,吸附第一步同时受到边界层和颗粒内扩散的影响。
表5 ENR和NOR在2种沉积物中的吸附/解吸动力学
Table 5 Adsorption/desorption kinetics of ENR and NOR in 2 kinds of sediments
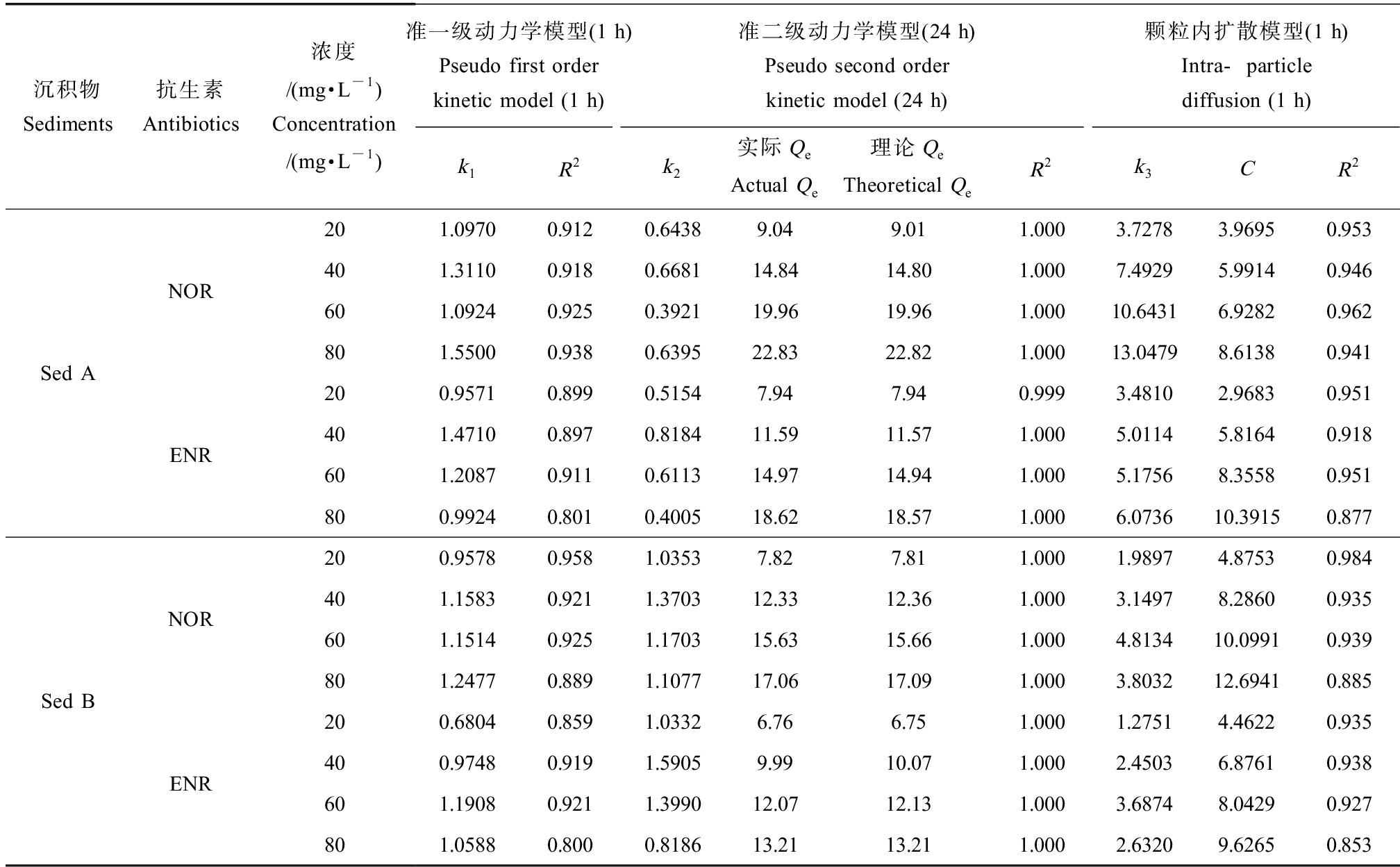
沉积物Sediments抗生素Antibiotics浓度/(mg·L-1)Concentration/(mg·L-1)准一级动力学模型(1 h)Pseudo first order kinetic model (1 h)准二级动力学模型(24 h)Pseudo second order kinetic model (24 h)颗粒内扩散模型(1 h)Intra-particle diffusion (1 h)k1R2k2实际QeActual Qe理论QeTheoretical QeR2k3CR2Sed ANORENR201.09700.9120.64389.049.011.0003.72783.96950.953401.31100.9180.668114.8414.801.0007.49295.99140.946601.09240.9250.392119.9619.961.00010.64316.92820.962801.55000.9380.639522.8322.821.00013.04798.61380.941200.95710.8990.51547.947.940.9993.48102.96830.951401.47100.8970.818411.5911.571.0005.01145.81640.918601.20870.9110.611314.9714.941.0005.17568.35580.951800.99240.8010.400518.6218.571.0006.073610.39150.877Sed BNORENR200.95780.9581.03537.827.811.0001.98974.87530.984401.15830.9211.370312.3312.361.0003.14978.28600.935601.15140.9251.170315.6315.661.0004.813410.09910.939801.24770.8891.107717.0617.091.0003.803212.69410.885200.68040.8591.03326.766.751.0001.27514.46220.935400.97480.9191.59059.9910.071.0002.45036.87610.938601.19080.9211.399012.0712.131.0003.68748.04290.927801.05880.8000.818613.2113.211.0002.63209.62650.853
随着初始浓度的增加,ENR和NOR的平衡吸附量均有提高。同时,沉积物的吸附速率也受初始浓度的影响,初始浓度从20 mg·L-1增加至80 mg·L-1时,吸附速率先增加后减少。吸附过程中扩散受抗生素浓度梯度的控制,由于较高的浓度增加了ENR和NOR的活化分子数量从而增加了它们与沉积物之间有效碰撞的可能性,这样可以有效克服ENR和NOR与沉积物之间的传质阻力,从而增加吸附能力[12]。ENR和NOR的吸附百分比随浓度的增加而降低,表明较低浓度下沉积物有能力吸附较高比例的抗生素。因此,在环境浓度下沉积物可能吸附>80%的化合物,表明吸附是水体中ENR和NOR去除的主要途径。
2.2 ENR和NOR在沉积物中的吸附/解吸热力学
如图2(a)所示,随着初始浓度的增加,2种抗生素的吸附量均呈现先增加后趋于饱和的趋势。本研究选用Langmuir和Freundlich模型拟合ENR和NOR的吸附/解吸行为,结果表明,2种模型均能很好地拟合ENR和NOR的吸附行为(Langmuir,R2=0.864~0.984;Freundlich,R2=0.887~0.949)(表6),Sed A对ENR和NOR的吸附过程Langmuir能更好地描述,表明吸附过程主要是单层吸附,Sed B的吸附过程Freundlich拟合结果更好,表明发生了多层可逆吸附[9]。不同沉积物的吸附倾向存在差异,说明不同沉积物可能存在不同的吸附机制。

图2 ENR和NOR在2种沉积物中的吸附/解吸等温线
注:(a) ENR和NOR在2种沉积物中的吸附等温线;(b)ENR和NOR在2种沉积物中的解吸等温线。
Fig. 2 Adsorption/desorption isotherms of ENR and NOR in 2 kinds of sediments
Note:(a) Adsorption isotherms of ENR and NOR in 2 kinds of sediments; (b) Desorption isotherms of ENR and NOR in 2 kinds of sediments.
表6 ENR和NOR在2种沉积物上的吸附/解吸热力学参数
Table 6 Adsorption/desorption thermodynamic parameters of ENR and NOR on 2 kinds of sediments
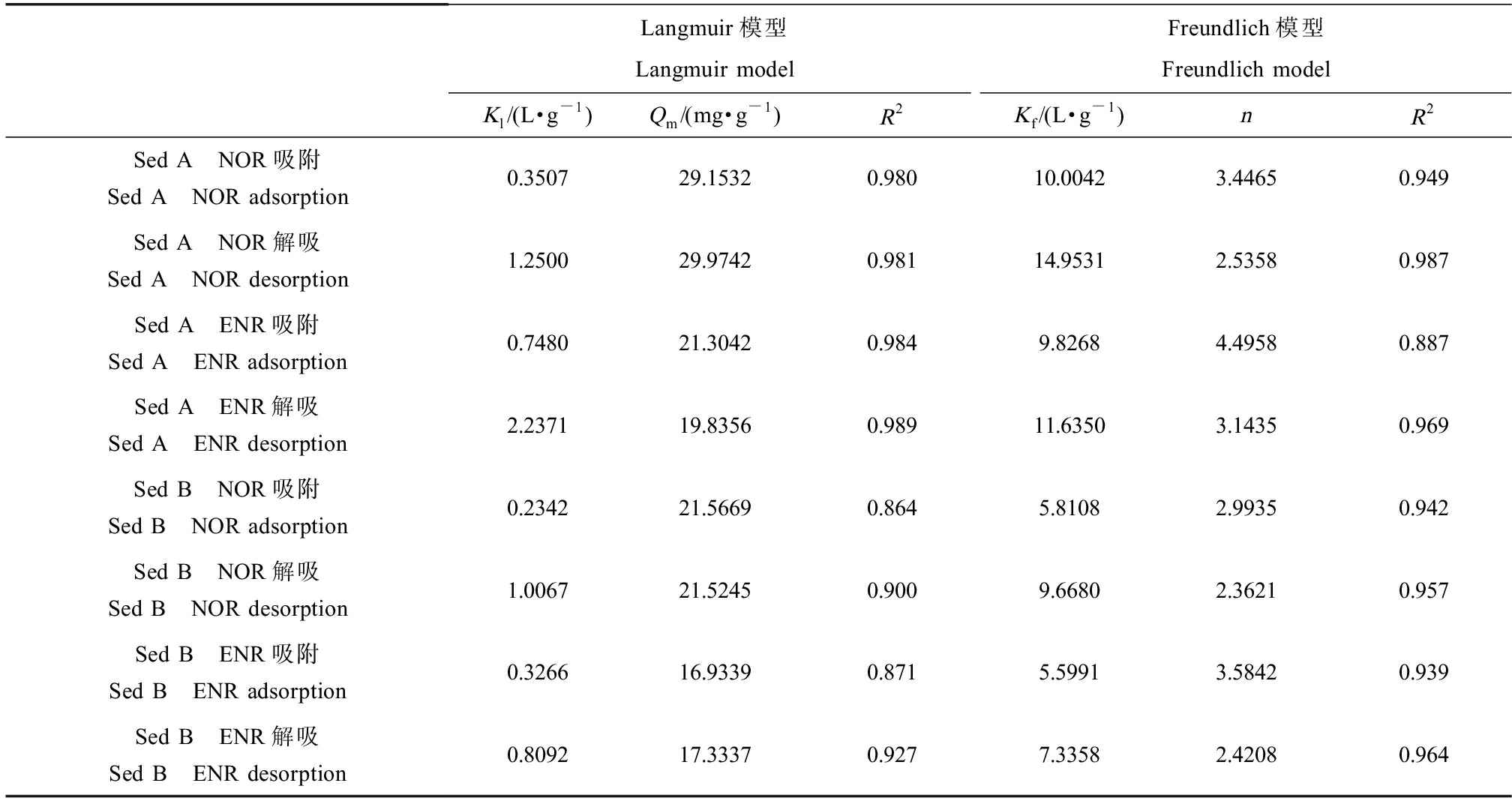
Langmuir模型Langmuir modelFreundlich模型Freundlich modelKl/(L·g-1)Qm/(mg·g-1)R2Kf/(L·g-1)nR2Sed A NOR吸附Sed A NOR adsorption 0.350729.15320.98010.00423.44650.949Sed A NOR解吸Sed A NOR desorption1.250029.97420.98114.95312.53580.987Sed A ENR吸附Sed A ENR adsorption 0.748021.30420.9849.82684.49580.887Sed A ENR解吸Sed A ENR desorption2.237119.83560.98911.63503.14350.969Sed B NOR吸附Sed B NOR adsorption 0.234221.56690.8645.81082.99350.942Sed B NOR解吸Sed B NOR desorption1.006721.52450.9009.66802.36210.957Sed B ENR吸附Sed B ENR adsorption 0.326616.93390.8715.59913.58420.939Sed B ENR解吸Sed B ENR desorption0.809217.33370.9277.33582.42080.964
与其他氟喹诺酮类抗生素一样,ENR和NOR的吸附性能很强,本研究中ENR和NOR的Kd值范围分别为106.72~3 213.72 L·kg-1和165.57~9 231.79 L·kg-1。与其他研究相比,不同的吸附剂(土壤和沉积物)上的吸附系数数量级相似[13]。ENR和NOR的lgKow分别为1.1和-1.03,说明它们的吸附主要不是依靠疏水分配。氟喹诺酮类为两性化合物,可以以不同的带电形式存在[14],Sed A和Sed B的pH值分别为6.8和7.5,ENR和NOR在此pH下主要以中性分子形式存在,部分带正电,极少量带负电,可能的吸附机制为阳离子交换和中性分子的表面络合[15]。
抗生素在沉积物上吸附的本质是在有机质和无机矿物上的吸附[16]。沉积物不同组分对抗生素的吸附机制存在差异,同时抗生素的形态也会影响它们的吸附能力[17]。因此,有必要进一步探究它们在不同pH条件和沉积物的各组成物质对ENR和NOR在沉积物吸附中的影响。
ENR和NOR在2种沉积物上的解吸等温线显示(图2(b)),解吸达到平衡后,仍有大部分留在沉积物中。用Langmuir和Freundlich模型均能很好地拟合ENR和NOR的解吸行为(Langmuir,R2=0.900~0.989;Freundlich,R2=0.957~0.987)(表6)。解吸量明显小于其吸附量,表明解吸过程中发生了“滞后现象”,这种现象主要是由于抗生素与沉积物形成了不可逆的化学键[18]。Sed A的吸附能力高于Sed B,而Sed B的解吸率高于Sed A,这表明Sed A上存在更多的高能结合位点和化学键。随着沉积物中抗生素浓度的增加,2种沉积物的解吸率增加,ENR和NOR在沉积物上的亲和力降低,换言之,较高的解吸量是由于吸附量的增加引起的,这与海洋沉积物中甲氧苄啶和四环素的研究结果一致[12,19]。有文献报道,化合物吸附可能会引起沉积物吸附位点的变形,导致解吸途径不同于吸附途径[20]。
2.3 溶液pH对吸附/解吸的影响
溶液pH会影响抗生素的离子形态,也会影响沉积物的表面电子数和阳离子交换量[18]。本研究考察了不同pH环境(4≤pH≤9)下,2种沉积物对抗生素吸附量的变化,如图3所示。pH与沉积物吸附抗生素的总量整体呈负相关,即ENR和NOR在沉积物上的吸附量及Kd值整体表现为随着pH值的升高而降低。有文献表明,具有更高阳离子交换量的沉积物和较低的pH环境有利于抗生素的吸附,与本研究的结果一致[21]。pH=8时ENR吸附量下降率明显高于NOR,由于pH=8时NOR仍以两性离子为主,这点与前述研究相同。随着pH的升高,吸附量明显下降,但下降后沉积物仍能吸附大量ENR和NOR,说明阴离子形态下,ENR和NOR的去质子化羧基与无机矿物表面络合或通过阳离子键桥与无机矿物中带负电的吸附点位结合等吸附机制对抗生素的吸附仍有较大的贡献[22]。

图3 不同pH下ENR和NOR在2种沉积物上的吸附/解吸等温线
注:(a)为拟合Freundlich模型等温线;(b)为拟合Langmuir模型等温线。
Fig. 3 Adsorption/desorption isotherms of ENR and NOR in 2 kinds of sediments at different pH values
Note: (a) Fitting Freundlich model isotherm; (b) Fitting Langmuir model isotherm.
Langmuir和Freundlich模型均能很好地拟合不同pH下ENR和NOR的吸附/解吸过程(表7)。不同初始浓度下,ENR和NOR的解吸量均随pH的升高而增加,可能是阴离子形态下沉积物对抗生素的结合力相对较弱,同时存在与带负电沉积物颗粒表面的静电斥力,使其不能稳定地与吸附位点结合。不同pH条件下,解吸量均小于吸附量,ENR的解吸量始终大于NOR,Sed B的解吸量始终大于Sed A,这与2.2所述结果一致。
表7 不同pH下ENR和NOR在2种沉积物上的吸附/解吸热力学参数
Table 7 Adsorption/desorption thermodynamic parameters of ENR and NOR on 2 kinds of sediments at different pH
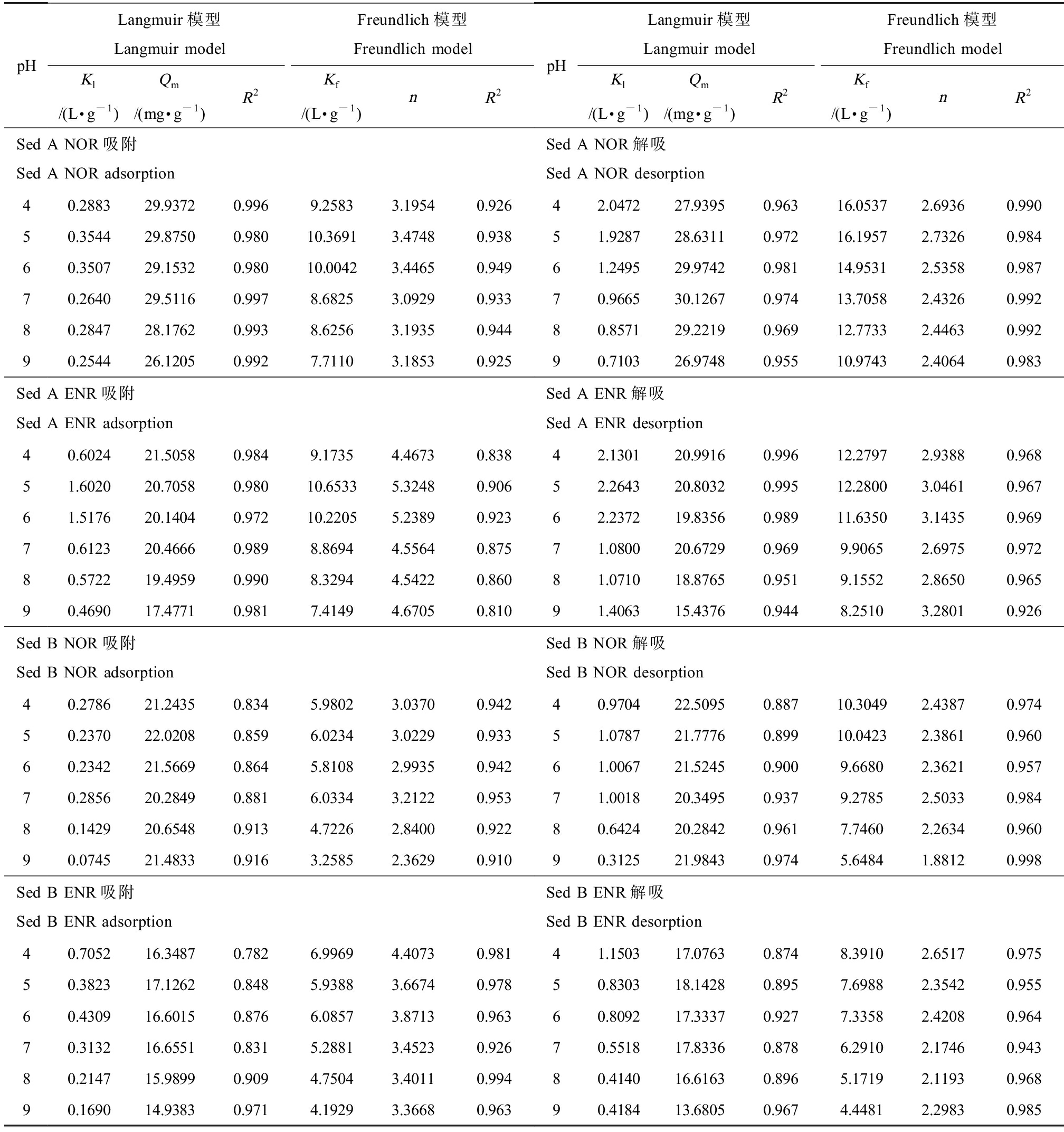
pHLangmuir模型Langmuir modelFreundlich模型Freundlich modelKl/(L·g-1)Qm/(mg·g-1)R2Kf/(L·g-1)nR2pHLangmuir模型Langmuir modelFreundlich模型Freundlich modelKl/(L·g-1)Qm/(mg·g-1)R2Kf/(L·g-1)nR2Sed A NOR吸附Sed A NOR adsorptionSed A NOR解吸Sed A NOR desorption40.288329.93720.9969.25833.19540.92642.047227.93950.96316.05372.69360.99050.354429.87500.98010.36913.47480.93851.928728.63110.97216.19572.73260.98460.350729.15320.98010.00423.44650.94961.249529.97420.98114.95312.53580.98770.264029.51160.9978.68253.09290.93370.966530.12670.97413.70582.43260.99280.284728.17620.9938.62563.19350.94480.857129.22190.96912.77332.44630.99290.254426.12050.9927.71103.18530.92590.710326.97480.95510.97432.40640.983Sed A ENR吸附Sed A ENR adsorptionSed A ENR解吸Sed A ENR desorption40.602421.50580.9849.17354.46730.83842.130120.99160.99612.27972.93880.96851.602020.70580.98010.65335.32480.90652.264320.80320.99512.28003.04610.96761.517620.14040.97210.22055.23890.92362.237219.83560.98911.63503.14350.96970.612320.46660.9898.86944.55640.87571.080020.67290.9699.90652.69750.97280.572219.49590.9908.32944.54220.86081.071018.87650.9519.15522.86500.96590.469017.47710.9817.41494.67050.81091.406315.43760.9448.25103.28010.926Sed B NOR吸附Sed B NOR adsorptionSed B NOR解吸Sed B NOR desorption40.278621.24350.8345.98023.03700.94240.970422.50950.88710.30492.43870.97450.237022.02080.8596.02343.02290.93351.078721.77760.89910.04232.38610.96060.234221.56690.8645.81082.99350.94261.006721.52450.9009.66802.36210.95770.285620.28490.8816.03343.21220.95371.001820.34950.9379.27852.50330.98480.142920.65480.9134.72262.84000.92280.642420.28420.9617.74602.26340.96090.074521.48330.9163.25852.36290.91090.312521.98430.9745.64841.88120.998Sed B ENR吸附Sed B ENR adsorptionSed B ENR解吸Sed B ENR desorption40.705216.34870.7826.99694.40730.98141.150317.07630.8748.39102.65170.97550.382317.12620.8485.93883.66740.97850.830318.14280.8957.69882.35420.95560.430916.60150.8766.08573.87130.96360.809217.33370.9277.33582.42080.96470.313216.65510.8315.28813.45230.92670.551817.83360.8786.29102.17460.94380.214715.98990.9094.75043.40110.99480.414016.61630.8965.17192.11930.96890.169014.93830.9714.19293.36680.96390.418413.68050.9674.44812.29830.985
2.4 沉积物组分对吸附/解吸的影响
本研究采用化学选择性萃取法将沉积物中的主要组分进行分离(包括有机质、铁氧化物和锰氧化物),以考察不同组分对ENR和NOR的吸附/解吸的影响[23]。由图4可知,2种沉积物的组分对ENR和NOR的吸附贡献不同,Sed A中各组分的吸附贡献依次为其他>有机质>铁氧化物>锰氧化物,Sed B中各组分的吸附贡献依次为有机质>铁氧化物>锰氧化物和其他(吸附贡献指去除各组分后减少的吸附量)。
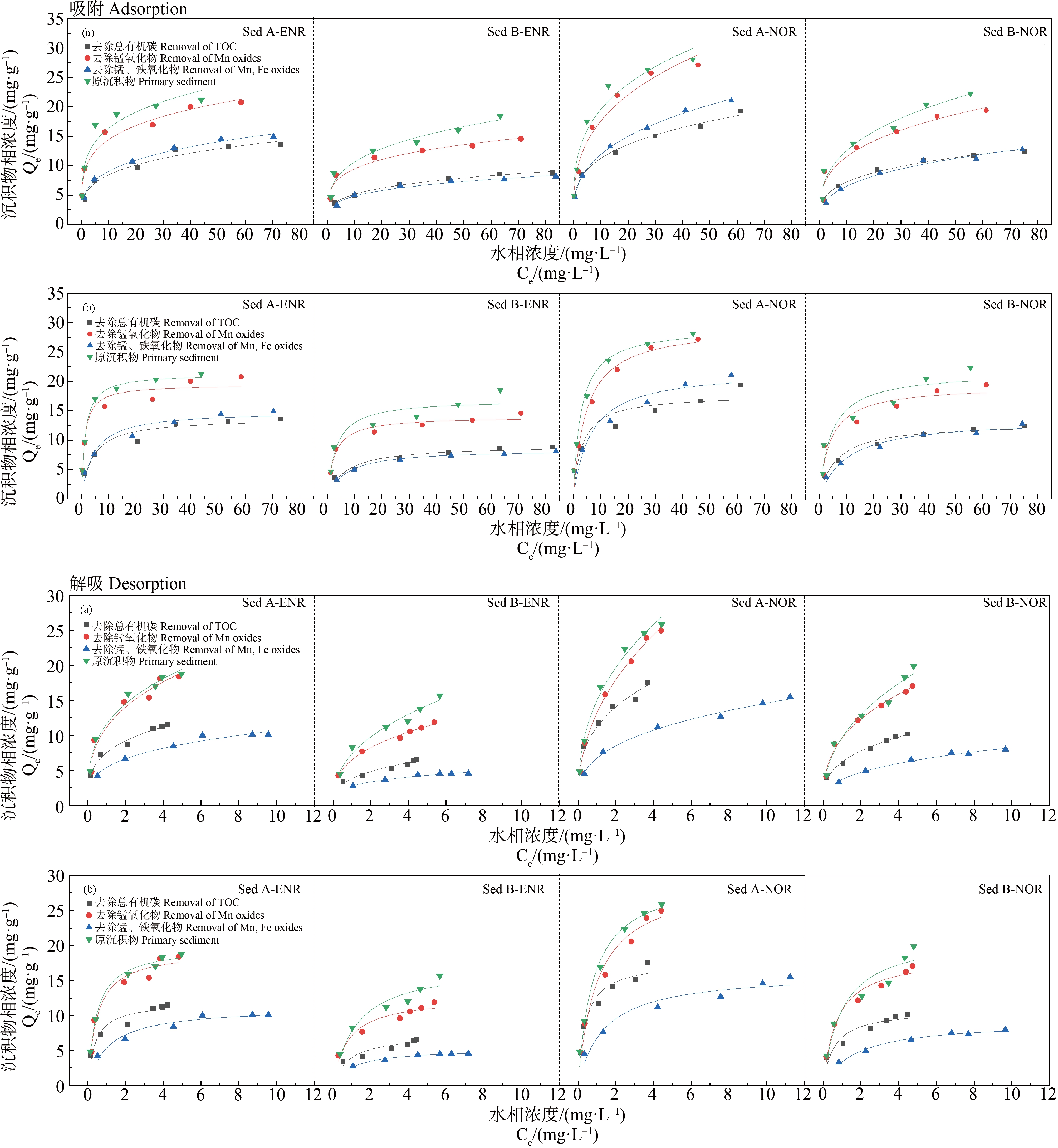
图4 去除各沉积物组分后ENR和NOR在2种沉积物上的吸附/解吸等温线
注:(a) 拟合Freundlich模型等温线;(b) 拟合Langmuir模型等温线。
Fig. 4 Adsorption/desorption isotherms of ENR and NOR in 2 sediments after removal of sediment components
Note: (a) Fitting the Freundlich model isotherm; (b) Fitting the Langmuir model isotherm.
去除有机质后2种沉积物对2种抗生素的吸附量均明显下降。有机质的主要成分为腐殖质,腐殖质含有多种官能团包括羧基、醇羟基、酚羟基和羰基等使其具有离子交换性、配合性、氧化还原性以及生理活性等[24]。FQs带正电(氨基)和负电(羧基)基团,在空间上可以分开,可以与有机质不同带电表面官能团发生静电作用,阳离子氨基可发生阳离子交换并与有机质中去质子化官能团形成离子对,阴离子羧基可通过金属离子在表面发生络合形成表面复合物,FQs不带电区域也可与腐殖酸形成相对较弱的极性相互作用,例如氢键[25-27]。Teixidó等[28]报道了添加腐殖酸可提高ENR的吸附。除腐殖酸外,有机质中还存在一定数量的颗粒有机物[29],它们也会对抗生素的吸附产生影响。Pan等[30]报道了FQs在土壤上的平衡吸附浓度与有机碳含量成正相关。本研究中,Sed B的有机碳含量低于Sed A,但Sed B中有机质对抗生素的吸附贡献大于Sed A,表明有机碳不是唯一的原因。也有相关研究表明,FQs的吸附主要受阳离子交换量的影响,有机碳含量对吸附的影响则相对较小[31]。
金属氧化物中铁氧化物对ENR和NOR的吸附贡献明显高于锰氧化物,通常沉积物中铁元素的含量远高于锰元素,这可以解释为什么铁氧化物的吸附贡献更大。FQs在氧化物上的吸附机制主要为络合作用,金属氧化物在水体中因水解其表面会形成大量的表面羟基,FQs中的羧基可以与这些表面羟基形成络合物而吸附,喹啉环的羰基与氧化物表面形成氢键对吸附也有一定贡献[32]。
用Langmuir和Freundlich模型拟合去除各组分后的吸附等温线发现,n值均有所提高,说明吸附的非均质性提高(表8)。这可能是由于有机质和无机矿物组分中的官能团与抗生素分子结构中的官能团发生了更加复杂的作用,故表现出位点间的差异性更大,异质性更强。从解吸量来看,吸附损失越高,解吸量越大。由于沉积物成分的复杂性和含量的差异性,使沉积物对抗生素的吸附为多种机制的复合作用,可能是交叉或联合作用。
表8 去除沉积物各组分后ENR和NOR在2种沉积物上的吸附/解吸热力学参数
Table 8 Adsorption/desorption thermodynamic parameters of ENR and NOR on 2 kinds of sediments after removal of sediment components
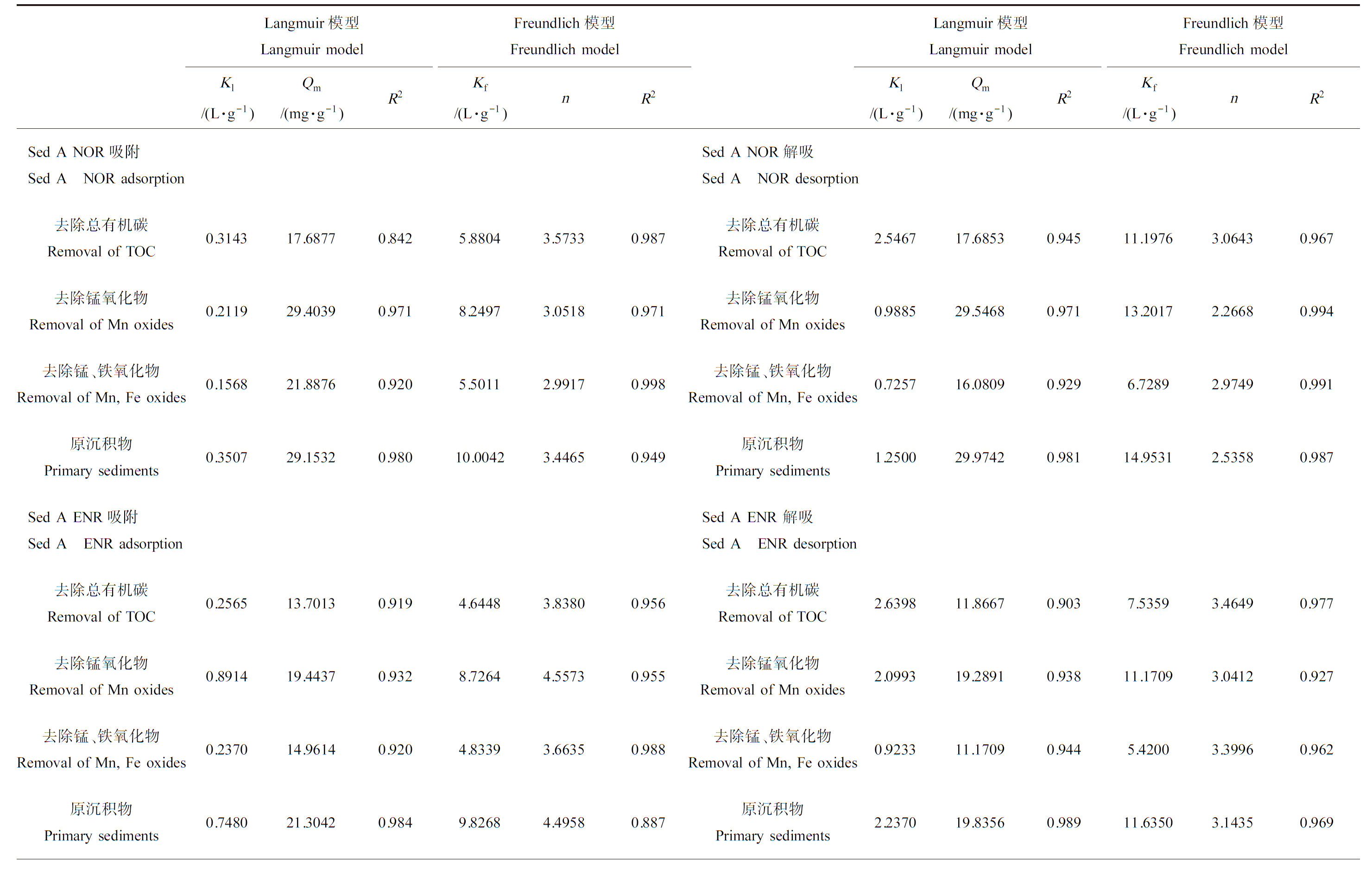
Langmuir模型Langmuir modelFreundlich模型Freundlich modelKl/(L·g-1)Qm/(mg·g-1)R2Kf/(L·g-1)nR2Langmuir模型Langmuir modelFreundlich模型Freundlich modelKl/(L·g-1)Qm/(mg·g-1)R2Kf/(L·g-1)nR2Sed A NOR吸附Sed A NOR adsorptionSed A NOR解吸Sed A NOR desorption去除总有机碳Removal of TOC0.314317.68770.8425.88043.57330.987去除总有机碳Removal of TOC2.546717.68530.94511.19763.06430.967去除锰氧化物Removal of Mn oxides0.211929.40390.9718.24973.05180.971去除锰氧化物Removal of Mn oxides0.988529.54680.97113.20172.26680.994去除锰、铁氧化物Removal of Mn, Fe oxides0.156821.88760.9205.50112.99170.998去除锰、铁氧化物Removal of Mn, Fe oxides0.725716.08090.9296.72892.97490.991原沉积物Primary sediments0.350729.15320.98010.00423.44650.949原沉积物Primary sediments1.250029.97420.98114.95312.53580.987Sed A ENR吸附Sed A ENR adsorptionSed A ENR 解吸Sed A ENR desorption去除总有机碳Removal of TOC0.256513.70130.9194.64483.83800.956去除总有机碳Removal of TOC2.639811.86670.9037.53593.46490.977去除锰氧化物Removal of Mn oxides0.891419.44370.9328.72644.55730.955去除锰氧化物Removal of Mn oxides2.099319.28910.93811.17093.04120.927去除锰、铁氧化物Removal of Mn, Fe oxides0.237014.96140.9204.83393.66350.988去除锰、铁氧化物Removal of Mn, Fe oxides0.923311.17090.9445.42003.39960.962原沉积物Primary sediments0.748021.30420.9849.82684.49580.887原沉积物Primary sediments2.237019.83560.98911.63503.14350.969Sed B NOR吸附Sed B NOR adsorptionSed B NOR解吸Sed B NOR desorption去除总有机碳Removal of TOC0.166612.84330.9573.74233.50880.985去除总有机碳Removal of TOC1.748110.80120.8566.18323.04540.987去除锰氧化物Removal of Mn oxides0.250519.25150.8805.71403.29320.922去除锰氧化物Removal of Mn oxides1.379018.49430.9639.56192.73500.986去除锰、铁氧化物Removal of Mn, Fe oxides0.103413.60440.9622.97782.94670.978去除锰、铁氧化物Removal of Mn, Fe oxides0.57209.19660.9773.68512.85910.983原沉积物Primary sediments0.234221.56690.8645.81082.99350.942原沉积物Primary sediments1.006721.52450.9009.66802.36210.957Sed B ENR吸附Sed B ENR adsorptionSed B ENR解吸Sed B ENR desorption

续表8Langmuir模型Langmuir modelFreundlich模型Freundlich modelKl/(L·g-1)Qm/(mg·g-1)R2Kf/(L·g-1)nR2Langmuir模型Langmuir modelFreundlich模型Freundlich modelKl/(L·g-1)Qm/(mg·g-1)R2Kf/(L·g-1)nR2去除总有机碳Removal of TOC0.16009.06910.9052.79493.75490.990去除总有机碳Removal of TOC1.20817.27240.8253.80992.96160.940去除锰氧化物Removal of Mn oxides0.412213.99730.9565.64804.45340.926去除锰氧化物Removal of Mn oxides1.300812.59300.9196.47432.88950.991去除锰、铁氧化物Removal of Mn, Fe oxides0.15738.45390.9832.67723.88770.966去除锰、铁氧化物Removal of Mn, Fe oxides0.95815.26240.9752.76143.67420.962原沉积物Primary sediments0.326616.93390.8715.59913.58420.939原沉积物Primary sediments0.809217.33370.9277.33582.42080.964
2.5 离子强度和离子类型对吸附/解吸的影响
实际环境中抗生素的吸附存在与共存离子的相互作用和相互影响,它们通过交叉作用改变抗生素或环境介质的结构和性质,进而影响抗生素的迁移转化和归趋[33]。
由图5可知,除Zn2+外,随着离子强度的增加,吸附/解吸量都呈现逐渐减少的趋势,且二价离子的吸附常数明显低于单价离子。Li和Zhang[34]同样发现,Ca2+和Mg2+的存在显著降低了氟喹诺酮类抗生素在活性污泥上的吸附量。已有研究证明,二价阳离子中Ca2+和Mg2+与FQs形成的络合物最不稳定[35],说明金属离子与ENR和NOR的竞争吸附作用在吸附中占主导,由于二价离子相比单价离子对阳离子吸附位点具有更高的竞争优势,因此抗生素表现出更低的吸附量。金属离子的络合能力与金属离子的电负性呈正相关,重金属Pb和Zn具有更高的核电荷,能够形成更稳定的络合物[36],但结果显示Pb2+的增加抑制了抗生素的吸附,说明存在其他机制使抑制作用明显大于促进作用,有报道指出Pb在水溶液中能够形成较厚的水化膜,阻塞吸附位点,并且能与有机质表面的羟基和羧基等基团结合,从而抑制有机物的吸附[37-38]。相比于Pb2+,Zn2+对ENR和NOR在沉积物上的吸附具有明显的促进作用,Graouer-Bacart等[39]的研究表明,土壤中同时存在Zn2+和ENR时,Kd值显著升高,光谱结果显示由于形成ENR-Zn2+络合物或土壤表面配位基团和ENR配位基团之间键桥形成三元络合物使ENR在土壤中的吸附增强。推测竞争吸附同样存在,但此时络合作用在沉积物对抗生素的吸附中占主导,表观上仅体现了Zn2+对ENR和NOR吸附的促进作用。解吸规律与先前的实验结果一致,即吸附率越高解吸率越低。
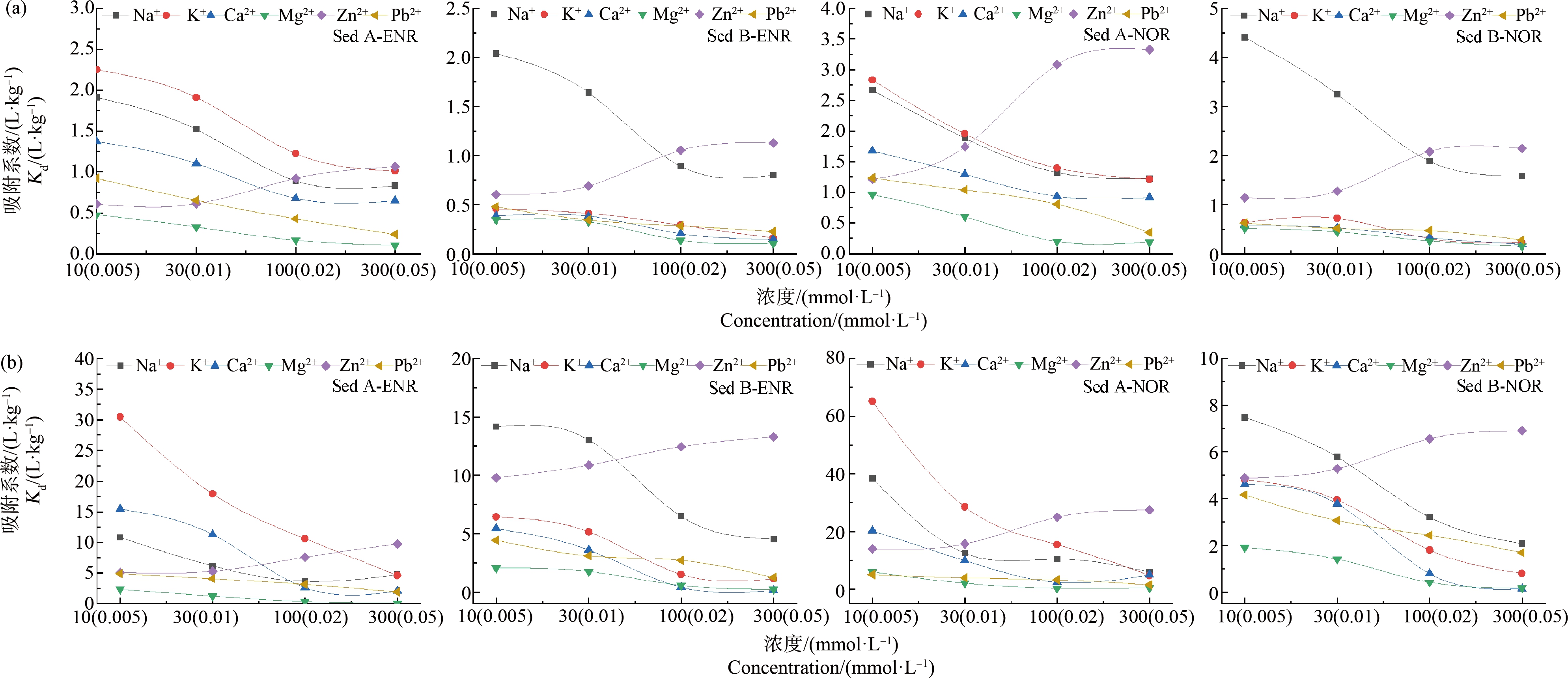
图5 不同离子类型和离子强度对ENR和NOR在2种沉积物上吸附/解吸的影响
注:(a) ENR和NOR在2种沉积物上的吸附常数;(b) ENR和NOR在2种沉积物上的解吸常数;NaCl、KCl、CaCl2和MgCl2的浓度范围为10、30、100和300 mmol·L-1;ZnCl2和PbCl2的浓度范围为0.005、0.01、0.02和0.05 mmol·L-1。
Fig. 5 Effect of different ion types and ionic strengths on the adsorption/desorption of ENR and NOR in 2 sediments
Note: (a) Adsorption constant of ENR and NOR in 2 kinds of sediments; (b) Desorption constant of ENR and NOR in 2 kinds of sediments; the concentration range of NaCl, KCl, CaCl2 and MgCl2 are 10, 30, 100 and 300 mmol·L-1; the concentration range of ZnCl2 and PbCl2 are 0.005, 0.01, 0.02 and 0.05 mmol·L-1.
[1] Liu X, Steele J C, Meng X Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review [J]. Environmental Pollution, 2017, 223: 161-169
[2] 陈笑雪, 王智源, 管仪庆, 等. 淡水环境中抗生素抗性基因的来源、归趋和风险[J]. 生态毒理学报, 2021, 16(3): 14-27
Chen X X, Wang Z Y, Guan Y Q, et al. Source, fate and risk of antibiotic resistance genes in freshwater environment [J]. Asian Journal of Ecotoxicology, 2021, 16(3): 14-27 (in Chinese)
[3] Kumar M, Jaiswal S, Sodhi K K, et al. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance [J]. Environment International, 2019, 124: 448-461
[4] Zhang R L, Pei J Y, Zhang R J, et al. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood [J]. Ecotoxicology and Environmental Safety, 2018, 154: 27-35
[5] 李贞金, 张洪昌, 沈根祥, 等. 水产养殖水、沉积物中抗生素检测方法优化及残留特征研究[J]. 生态毒理学报, 2020, 15(1): 209-219
Li Z J, Zhang H C, Shen G X, et al. Optimization of antibiotic detection methods and residual characteristics in aquaculture water and sediment [J]. Asian Journal of Ecotoxicology, 2020, 15(1): 209-219 (in Chinese)
[6] Wang S L, Wang H. Adsorption behavior of antibiotic in soil environment: A critical review [J]. Frontiers of Environmental Science &Engineering, 2015, 9(4): 565-574
[7] Picó Y, Andreu V. Fluoroquinolones in soil: Risks and challenges [J]. Analytical and Bioanalytical Chemistry, 2007, 387(4): 1287-1299
[8] 孟磊, 杨兵, 薛南冬. 氟喹诺酮类抗生素环境行为及其生态毒理研究进展[J]. 生态毒理学报, 2015, 10(2): 76-88
Meng L, Yang B, Xue N D. A review on environmental behaviors and ecotoxicology of fluoroquinolone antibiotics [J]. Asian Journal of Ecotoxicology, 2015, 10(2): 76-88 (in Chinese)
[9] Huang Y Q, Wang Y, Huang Y Z, et al. Impact of sediment characteristics on adsorption behavior of typical antibiotics in Lake Taihu, China [J]. The Science of the Total Environment, 2020, 718: 137329
[10] Jiang Y F, Zhang Q, Deng X R, et al. Single and competitive sorption of sulfadiazine and chlortetracycline on loess soil from Northwest China[J]. Environmental Pollution, 2020, 263(Pt A): 114650
[11] 高俊红, 谢晓芸, 张涵瑜, 等. 三种氟喹诺酮类抗生素在黄河沉积物中的吸附行为[J]. 兰州大学学报: 自然科学版, 2016, 52(5): 593-598
Gao J H, Xie X Y, Zhang H Y, et al. Adsorption behaviors of three fluoroquinolone antibiotics onto Yellow River sediments [J]. Journal of Lanzhou University: Natural Sciences, 2016, 52(5): 593-598 (in Chinese)
[12] Li J, Zhang H. Factors influencing adsorption and desorption of trimethoprim on marine sediments: Mechanisms and kinetics [J]. Environmental Science and Pollution Research International, 2017, 24(27): 21929-21937
[13] van Doorslaer X, Dewulf J, van Langenhove H, et al. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants [J]. The Science of the Total Environment, 2014, 500-501: 250-269
[14] Leal R M, Alleoni L R, Tornisielo V L, et al. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils [J]. Chemosphere, 2013, 92(8): 979-985
[15] 李宗宸. 河流沉积物吸附四环素类抗生素的行为规律研究[D]. 上海: 东华大学, 2017: 16
Li Z C. Study on the adsorption behaviors and rules of tetracyclines on river sediment [D]. Shanghai: Donghua University, 2017: 16 (in Chinese)
[16] Li J, Zhang H. Adsorption-desorption of oxytetracycline on marine sediments: Kinetics and influencing factors [J]. Chemosphere, 2016, 164: 156-163
[17] Rath S, Fostier A H, Pereira L A, et al. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils [J]. Chemosphere, 2019, 214: 111-122
[18] Riaz L, Mahmood T, Khalid A, et al. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil [J]. Chemosphere, 2018, 191: 704-720
[19] Xu X R, Li X Y. Sorption and desorption of antibiotic tetracycline on marine sediments [J]. Chemosphere, 2010, 78(4): 430-436
[20] Braida W J, Pignatello J J, Lu Y F, et al. Sorption hysteresis of benzene in charcoal particles [J]. Environmental Science &Technology, 2003, 37(2): 409-417
[21] Vasudevan D, Bruland G L, Torrance B S, et al. pH-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption [J]. Geoderma, 2009, 151(3-4): 68-76
[22] Gu X Y, Tan Y Y, Tong F, et al. Surface complexation modeling of coadsorption of antibiotic ciprofloxacin and Cu(Ⅱ) and onto goethite surfaces [J]. Chemical Engineering Journal, 2015, 269: 113-120
[23] 陈蕾, 郑西来, 王婷, 等. 表层沉积物主要矿化组分对Mn(Ⅱ)的吸附特性与贡献[J]. 环境科学研究, 2014, 27(11): 1345-1350
Chen L, Zheng X L, Wang T, et al. Characteristics and contribution of major mineralized components of the surface sediment to Mn(Ⅱ) adsorption [J]. Research of Environmental Sciences, 2014, 27(11): 1345-1350 (in Chinese)
[24] 提清清, 高增文, 季慧慧, 等. 抗生素在土壤中的吸附行为研究进展[J]. 土壤, 2017, 49(3): 437-445
Ti Q Q, Gao Z W, Ji H H, et al. Adsorption of antibiotics in soils: A review [J]. Soils, 2017, 49(3): 437-445 (in Chinese)
[25] Aristilde L, Sposito G. Binding of ciprofloxacin by humic substances: A molecular dynamics study [J]. Environmental Toxicology and Chemistry, 2010, 29(1): 90-98
[26] Martínez-Mejía M J, Sato I, Rath S. Sorption mechanism of enrofloxacin on humic acids extracted from Brazilian soils [J]. Environmental Science and Pollution Research, 2017, 24(19): 15995-16006
[27] 郭丽, 王淑平, 周志强, 等. 环丙沙星在深浅两层潮土层中吸附-解吸特性研究[J]. 农业环境科学学报, 2014, 33(12): 2359-2367
Guo L, Wang S P, Zhou Z Q, et al. Adsorption and desorption of ciprofloxacin by surface and subsurface soils of ustic cambosols in China [J]. Journal of Agro-Environment Science, 2014, 33(12): 2359-2367 (in Chinese)
[28] Teixidó M, Medeiros J, Beltrán J L, et al. Sorption of enrofloxacin and ciprofloxacin in agricultural soils: Effect of organic matter [J]. Adsorption Science &Technology, 2014, 32(2-3): 153-163
[29] Guo X Y, Luo L, Ma Y B, et al. Sorption of polycyclic aromatic hydrocarbons on particulate organic matters [J]. Journal of Hazardous Materials, 2010, 173(1-3): 130-136
[30] Pan B, Wang P, Wu M, et al. Sorption kinetics of ofloxacin in soils and mineral particles [J]. Environmental Pollution, 2012, 171: 185-190
[31] Figueroa-Diva R A, Vasudevan D, MacKay A A. Trends in soil sorption coefficients within common antimicrobial families [J]. Chemosphere, 2010, 79(8): 786-793
[32] Guo X T, Tu B, Ge J H, et al. Sorption of tylosin and sulfamethazine on solid humic acid [J]. Journal of Environmental Sciences (China), 2016, 43: 208-215
[33] Wang S L, Wang H. Adsorption behavior of antibiotic in soil environment: A critical review [J]. Frontiers of Environmental Science &Engineering, 2015, 9(4): 565-574
[34] Li B, Zhang T. Biodegradation and adsorption of antibiotics in the activated sludge process [J]. Environmental Science &Technology, 2010, 44(9): 3468-3473
[35] Urbaniak B, Kokot Z J. Analysis of the factors that significantly influence the stability of fluoroquinolone-metal complexes [J]. Analytica Chimica Acta, 2009, 647(1): 54-59
[36] Zhao Z L, Wang J, Han Y, et al. Nutrients, heavy metals and microbial communities co-driven distribution of antibiotic resistance genes in adjacent environment of mariculture [J]. Environmental Pollution, 2017, 220(Pt B): 909-918
[37] Chen G C, Shan X Q, Pei Z G, et al. Adsorption of diuron and dichlobenil on multiwalled carbon nanotubes as affected by lead [J]. Journal of Hazardous Materials, 2011, 188(1-3): 156-163
[38] Wang Y S, Pei Z G, Shan X Q, et al. Effects of metal cations on sorption-desorption of p-nitrophenol onto wheat ash [J]. Journal of Environmental Sciences (China), 2011, 23(1): 112-118
[39] Graouer-Bacart M, Sayen S, Guillon E. Adsorption of enrofloxacin in presence of Zn(Ⅱ) on a calcareous soil [J]. Ecotoxicology and Environmental Safety, 2015, 122: 470-476