塑料的广泛使用带来了极为严重的环境问题,环境中的塑料垃圾降解可生成更小的微塑料(microplastics, MPs; 1 μm~5 mm)和纳米塑料(nanoplastics, NPs; <1 μm)。多项针对环境的调查研究指出微塑料在海洋、土壤、湖泊、河流和水库等环境中均有分布,水体中塑料浓度从24.8 个·m-3到10 200 个·m-3不等,而土壤中含量更高,平均为18 760 个·kg-1[1-5]。环境中塑料丰度整体呈现随粒径变小而指数增长的趋势,预测50 nm的塑料颗粒浓度是5 μm的3倍以上[6]。环境中塑料成分主要为聚乙烯(polyethylene, PE)、聚丙烯(polypropylene, PP)、聚苯乙烯(polystyrene, PS)、聚对苯二甲酸乙二酯(PE terephthalate, PET)和聚甲基丙烯酸甲酯(polymethyl methacrylate, PMMA),其中聚苯乙烯常用于制作一次性餐盒、保温材料等,且在自然环境中较难降解,也因此成为研究的热点[7]。
环境中的微/纳米塑料可以通过饮食、饮水等途径被动物摄入,在鱼、虾和双壳类动物的肠道、肌肉和腮中均检测到微塑料颗粒[8]。哺乳动物进而通过饮水、食用海产品、海盐等方式摄入微/纳米塑料。近年在人体粪便和肠道中检出微塑料,给出了人体摄入塑料颗粒的直接证据[9-10];Leslie等[11]的最新研究证实人体血液中存在塑料颗粒,表明塑料颗粒可进入血液循环系统,造成多器官暴露。小鼠动物模型表明纳米塑料较微米尺度塑料具有更强生物富集性,因其小粒径更易被肠道上皮细胞吸收并通过循环系统,在肝脏中积累,甚至穿过血脑屏障和生殖屏障富集于大脑、睾丸等组织[12]。但是不同研究之间的塑料颗粒积累规律及毒性效应有较大差异[13]。Meng等[14]用50 nm的纳米聚苯乙烯颗粒(PS-NPs)灌胃小鼠(5 mg·d-1)4周,发现PS-NPs在肠道和肾脏蓄积并造成组织结构损伤,诱导血清超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽(GSH)等抗氧化酶活性上升;然而Nikolic等[15]使用40 nm的PS-NPs暴露小鼠(0.1 mg·d-1)5周却未在肾脏中发现PS-NPs的积累,主要分布在胃、肠道和睾丸生精小管,并导致睾丸激素水平下降和IL-23等炎症因子表达上升。因此不同暴露剂量和暴露周期可能影响塑料颗粒的代谢动力学过程,需进一步动态监测纳米塑料摄入后的组织乃至细胞水平分布规律,以明确其主要作用靶器官和致毒机制。
纳米塑料通过饮食摄入后在胃肠道主要被巨噬细胞吞噬,上调炎症因子表达,诱导炎症细胞的浸润,引起肠道炎症反应[16]。巨噬细胞被称为肠道免疫系统的“守卫”,通过识别有害病原菌及异物,维持肠道免疫系统平衡。Kuhn等[17]证明纳米塑料可通过大胞饮、吞噬作用及网格蛋白介导的内吞作用进入巨噬细胞。体外研究发现,小鼠巨噬细胞RAW264.7暴露于100 μg·mL-1的PS-NPs 4 h便会导致活性氧(ROS)大量产生,诱导细胞氧化应激损伤[18-19]。但纳米塑料在胞内的动态累积分布规律及毒性动态响应仍不明确。
本研究通过对小鼠进行PS-NPs灌胃处理,研究塑料在小鼠胃肠器官中的动态积累规律及造成的病理损伤;进一步用小鼠巨噬细胞RAW264.7为模型,在细胞水平探讨PS-NPs细胞内暴露剂量及细胞应激响应,揭示纳米塑料细胞毒性的时间效应规律,为后续对环境塑料污染健康风险评估提供依据。
1 材料与方法(Materials and methods)
1.1 试剂
本研究采用的纳米聚苯乙烯(绿色、红色荧光标记和无荧光标记,平均粒径80 nm,货号分别为7-3-0008、7-1-0008、6-1-0006,成分为90%苯乙烯+10%甲基丙烯酸甲酯(MMA),纯度90%)购自天津市倍思乐色谱技术开发中心;ROS试剂盒购自碧云天生物技术有限公司(货号:S0033S);IL-6(货号:EK206/3-96)、TNF-α(货号:EK282HS-96)ELISA试剂盒购自杭州联科生物技术股份有限公司;胎牛血清(FBS)购自浙江天杭生物科技股份有限公司(货号:11011-8611);DMEM培养基购自Gibco(货号:C11995500BT)。
1.2 实验动物和细胞
BALB/c小鼠购自上海BK公司,饲养在浙江中医药大学动物中心,环境室温(23±2)℃,相对湿度45%~60%,昼夜交替周期为12 h,在环境中适应一周,期间自由饮水、摄食。实验方案经浙江中医药大学实验动物伦理要求批准。RAW264.7细胞购自中国科学院上海细胞库,使用含10% FBS的DMEM培养。
1.3 实验方法
动态光散射(DLS)测定。用含有10% FBS的DMEM培养液配制浓度为1 mg·mL-1的PS-NPs,并将悬液在60 W功率下(开4 s关1 s)超声处理10 min。稳定20 min后,通过动态光散射仪(Zetasizer Nano Series, Malvern)测量它们的团聚性,包括流体动力学尺寸和Zeta电位。
体内分布检测。提前一天剔除小鼠体毛,第2天灌胃1.5 mg红色荧光标记的PS-NPs,分别在10 min、30 min、1 h和24 h使用IVIS® Lumina Ⅲ小动物活体成像系统的cy5通道检测小鼠体内红色荧光分布和强度。
病理学检测。将20只小鼠随机分为4组,每组5只,分别灌胃无菌水、0.015、0.15和1.5 mg·d-1PS-NPs连续21 d,处死后取胃肠组织,经4%多聚甲醛固定、梯度酒精脱水、二甲苯透明、浸蜡、包埋、切片、展片和黏片后,进行苏木素-伊红(HE)染色,中性树脂封片,镜下观察。
细胞摄取测定。参考Liu等[20]方法借助塑料颗粒标记荧光强度定量PS-NPs在胞内的含量,首先将绿色荧光标记的PS-NPs用含10% FBS的DMEM培养液,按10倍梯度进行稀释,配制成0.001、0.01、0.1、1、10、100、1 000 μg·mL-1的PS-NPs悬液,并利用流式细胞仪(CytoFLEX, Bechman Coulter)吸取200 μL悬液检测荧光强度,制作PS-NPs荧光强度-颗粒含量的标准曲线。
将RAW264.7细胞以5×104个·mL-1密度种于12孔培养板上,用100 μg·mL-1的绿色荧光标记的PS-NPs处理1~24 h后,用不含EDTA的胰酶消化后离心再用PBS重悬离心清洗2次后,以200 μL的PBS重悬,过200目筛网,用流式细胞仪检测荧光强度。
活性氧测定。将细胞以5×104个·mL-1密度种于培养板上,用100 μg·mL-1的无荧光标记的PS-NPs处理1~24 h后,以不含血清的DMEM清洗2次,加入以不含血清的DMEM稀释1 000倍的DCFH-DA探针孵育30 min,再用不含血清的DMEM清洗2次,用不含EDTA的胰酶消化后离心再用PBS重悬离心清洗2次后,以200 μL的PBS重悬,过200目筛网,用流式细胞仪检测ROS水平。
ELISA测定。将细胞以5×104个·mL-1密度种于培养板上,以100 μg·mL-1浓度的无荧光标记的PS-NPs刺激细胞1~24 h后,收集上清液,300 g离心10 min取上清用ELISA试剂盒检测IL-6和TNF-α。
1.4 数据处理
使用GraphPad Prism 8和SPSS 26进行数据分析,所有试验设置3组平行。实验结果以平均值±标准差呈现,采用单因素方差分析(One-way ANOVA)处理组与对照组的显著性差异,以P<0.05为差异有统计学意义。
2 结果(Results)
2.1 PS-NPs的流体动力学分析
通过DLS测量在培养液环境中,PS-NPs粒子的颗粒大小和团聚情况。结果表明,55.2%的PS-NPs颗粒在培养液中的粒径集中在100~200 nm,团聚粒径为131.3 nm,Zeta为-13.8 mV,未发生明显团聚(图1和表1)。
表1 PS-NPs在细胞培养液中的水动力学特征分析
Table 1 Hydrodynamic analysis of PS-NPs in cell culture medium

原始粒径/nmOriginal particle size/nm团聚粒径/nmAgglomerated particle size/nmZeta电位/mVZeta potential/mV60131.3-13.8
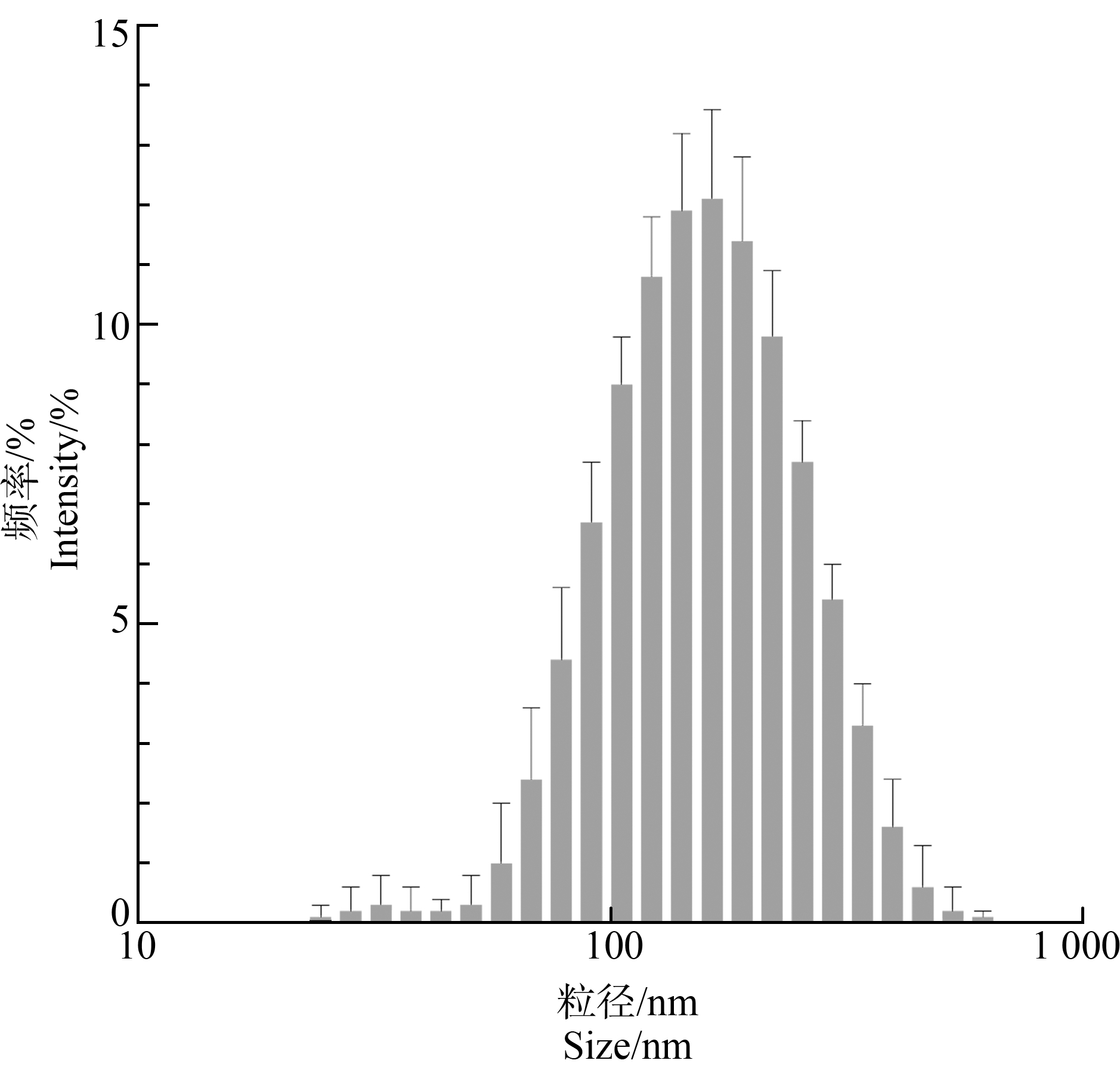
图1 纳米聚苯乙烯塑料(PS-NPs)在细胞培养液中的粒径分析
Fig. 1 Particle size analysis of polystyrene nanoplastics(PS-NPs)in cell culture medium
2.2 PS-NPs在小鼠体内的分布规律及病理损伤
对BALB/c小鼠灌胃1.5 mg PS-NPs后,小鼠体内的分布和转移结果如图2所示。在灌胃后1 h内,PS-NPs逐步从胃向肠道转移,而在24 h后,仅在肠道有少许PS-NPs残留能检测到荧光信号。

图2 灌胃24 h内PS-NPs在小鼠体内的分布和转运
注:(a)PS-NPs在24 h内的体内转运和分布;(b)小鼠口腔、胃和肠道24 h内PS-NPs的定量分析。
Fig.2 Distribution and transport of PS-NPs in mice within 24 h after gavage
Note:(a)Transport and distribution of PS-NPs in vivowithin 24 h;(b)Quantitative analysis of PS-NPs in the oral cavity, stomach and intestinal tract of mice within 24 h.
急性暴露后(24 h),本实验剂量PS-NPs并未对胃肠道造成明显病理损伤,但在对BALB/c小鼠灌胃PS-NPs 21 d后(低剂量0.015 mg·d-1,中剂量0.15mg·d-1,高剂量1.5 mg·d-1),发现和对照组相比,经PS-NPs暴露的小鼠消化道存在不同程度的病理性损伤,胃肠道腺体明显减少,炎症细胞大量浸润(箭头所示),且随暴露浓度的增加,组织受损程度也愈发严重,如图3所示。

图3 PS-NPs灌胃后小鼠胃肠道HE染色观察
注:1 000×放大倍数,箭头指示炎症细胞浸润。
Fig.3 HE staining observation of mouse gastrointestinal tract after intragastric administration of PS-NPs
Note: 1 000×magnification, arrows indicate inflammatory cell infiltration.
2.3 PS-NPs在RAW264.7细胞中的动态分布
根据纳米塑料荧光强度与浓度的关系得到标准曲线y=1 337.28+2 133.23x(R2=0.99)(图4),验证准确率bias为-4.152%,回收率108.7%。通过荧光信号对胞内PS-NPs进行动态示踪,并根据荧光强度与颗粒含量的线性关系来定量PS-NPs的胞内剂量。

图4 PS-NPs含量与荧光强度的拟合曲线
Fig. 4 Fitting curve of PS-NPs concentration and fluorescence intensity
观察RAW264.7细胞PS-NPs暴露1~24 h的纳米塑料摄入量,结果表明PS-NPs在细胞暴露的前8 h,胞内的塑料颗粒含量呈线性增加(y=-3.86888+3.4436x, R2=0.99),到8 h到达峰值;随后至24 h,含量下降趋于稳定(图5(a))。

图5 PS-NPs在RAW264.7细胞内随时间的积累情况
注:(a)PS-NPs在RAW264.7细胞中24 h内的累积情况;(b)PS-NPs在RAW264.7细胞中8 h内的累积情况(*P<0.05)。
Fig.5 The accumulation of PS-NPs in RAW264.7 cells over time
Note:(a)Accumulation of PS-NPs in RAW264.7 cells within 24 h;(b)Accumulation of PS-NPs in RAW264.7 cells within 8 h(*P<0.05).
2.4 PS-NPs诱导RAW264.7细胞内氧化应激损伤
PS-NPs被巨噬细胞吞噬后可诱导胞内氧化应激损伤[21],本研究具体探讨其产生活性氧的动态规律,结果表明在24 h内,ROS的产生总体呈现正弦函数波形分布;在前12 h内具有较好的线性递增规律(y=-1 205.77+2 751.24x, R2=0.96)(图6(b)),但在16~20 h ROS大幅下降,而在24 h再次增强(图6(a))。ROS的产生与胞内PS-NPs的累积在8 h内呈显著正相关(皮尔逊相关系数0.9821)(图7(b)),而在8 h后胞内含量变化与ROS的产生并不同步。
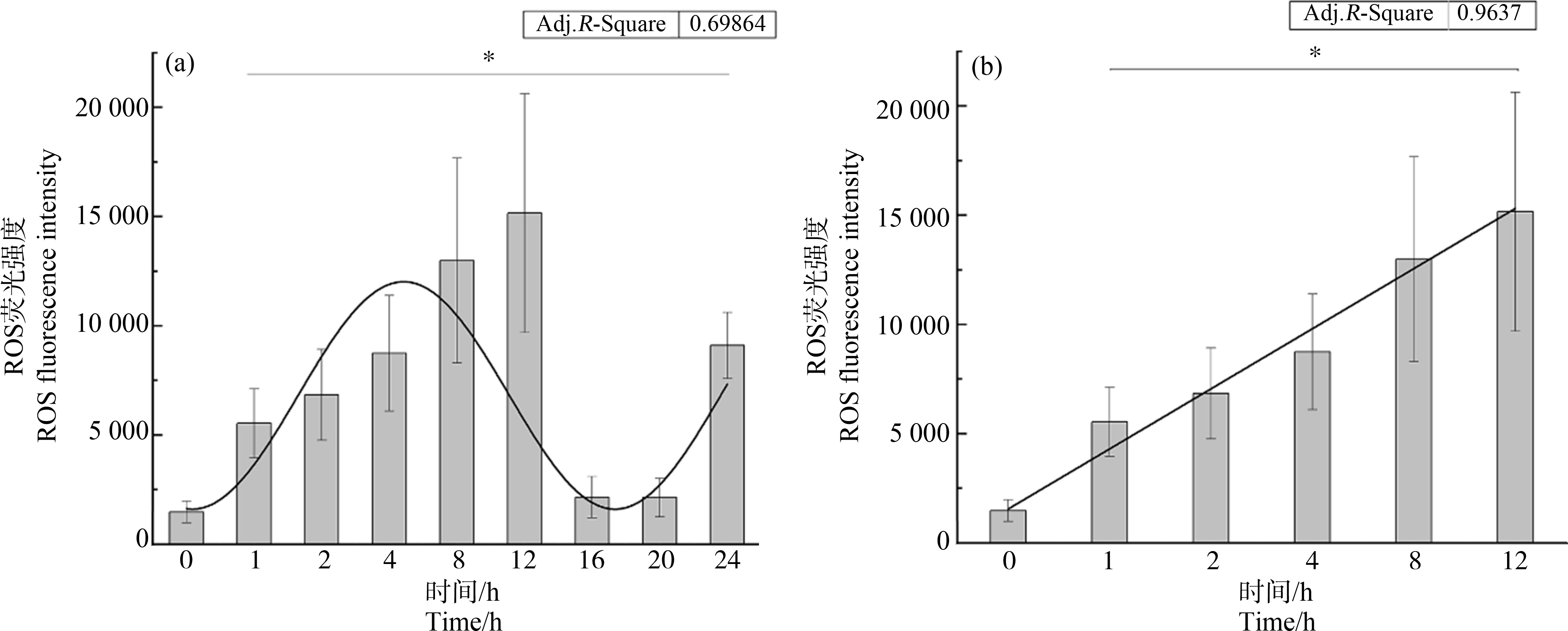
图6 PS-NPs在不同时间点刺激RAW264.7细胞产生的活性氧(ROS)水平
注:(a)PS-NPs在24 h内诱导的ROS水平;(b)PS-NPs在12 h内诱导的ROS水平(*P<0.05)。
Fig.6 The reactive oxygen species(ROS)levels induced by PS-NPs in RAW264.7 cells at different time points
Note:(a)ROS levels induced by PS-NPs within 24 h;(b)ROS levels induced by PS-NPs within 12 h(*P<0.05).
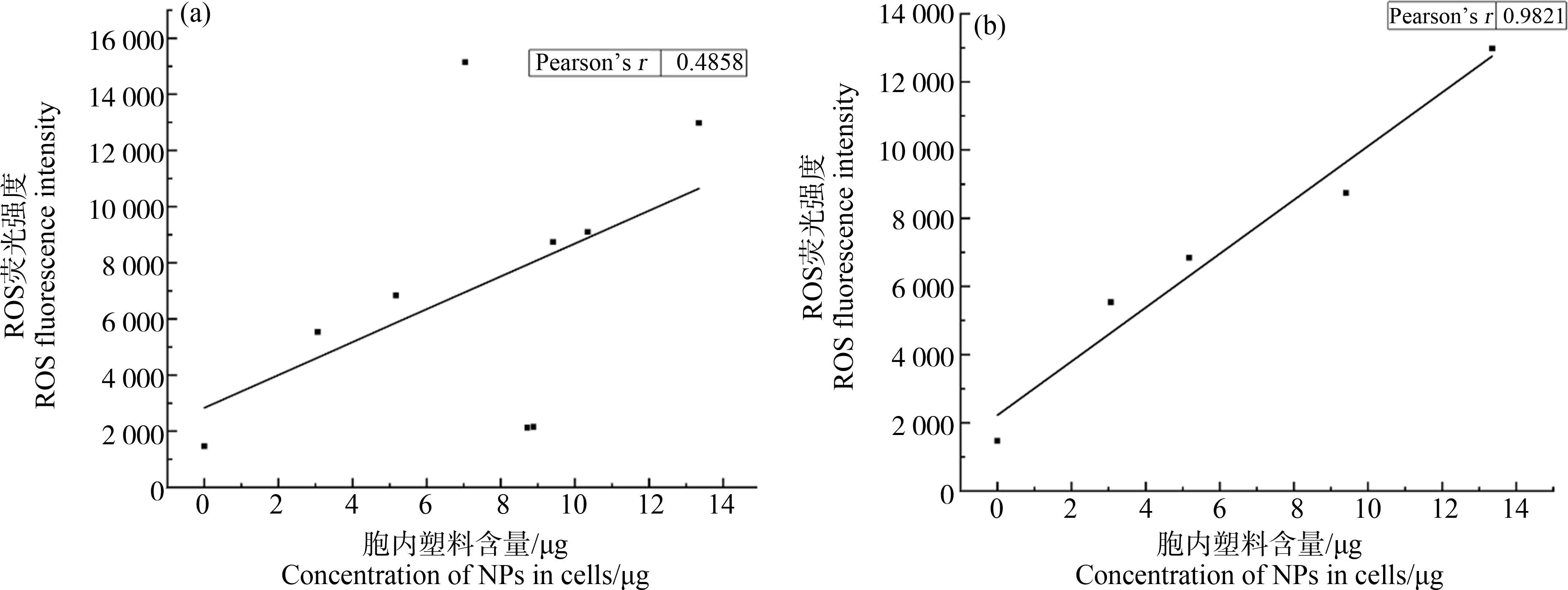
图7 PS-NPs诱导细胞产生的ROS水平与其胞内含量的相关性分析
注:(a)PS-NPs在24 h内诱导的ROS水平与其胞内含量之间的相关性分析;(b)PS-NPs在8 h内诱导的ROS水平与其胞内含量之间的相关性分析。
Fig.7 Correlation analysis of ROS levels induced by PS-NPs in RAW264.7 cells and their intracellular mass
Note:(a)Correlation analysis between the level of ROS induced by PS-NPs within 24 h and its intracellular content;(b)Correlation analysis between the level of ROS induced by PS-NPs within 8 h and its intracellular content.
2.5 PS-NPs诱导RAW264.7细胞内炎性因子表达规律
在动物水平观察到PS-NPs暴露后有明显的炎症细胞浸润,进而在细胞水平分析其诱导炎性因子产生规律。使用ELISA试剂盒检测经PS-NPs暴露后的IL-6和TNF-α的表达,结果显示TNF-α表达量在1 h后便急剧上升,且随时间延长基本趋于稳定;但是PS-NPs诱导IL-6的表达仅在24 h后才有显著的上升(图8)。同时炎症因子的上调与胞内PS-NPs的积累并无显著相关性(TNF-α的皮尔逊相关系数为0.572,IL-6为0.332)。
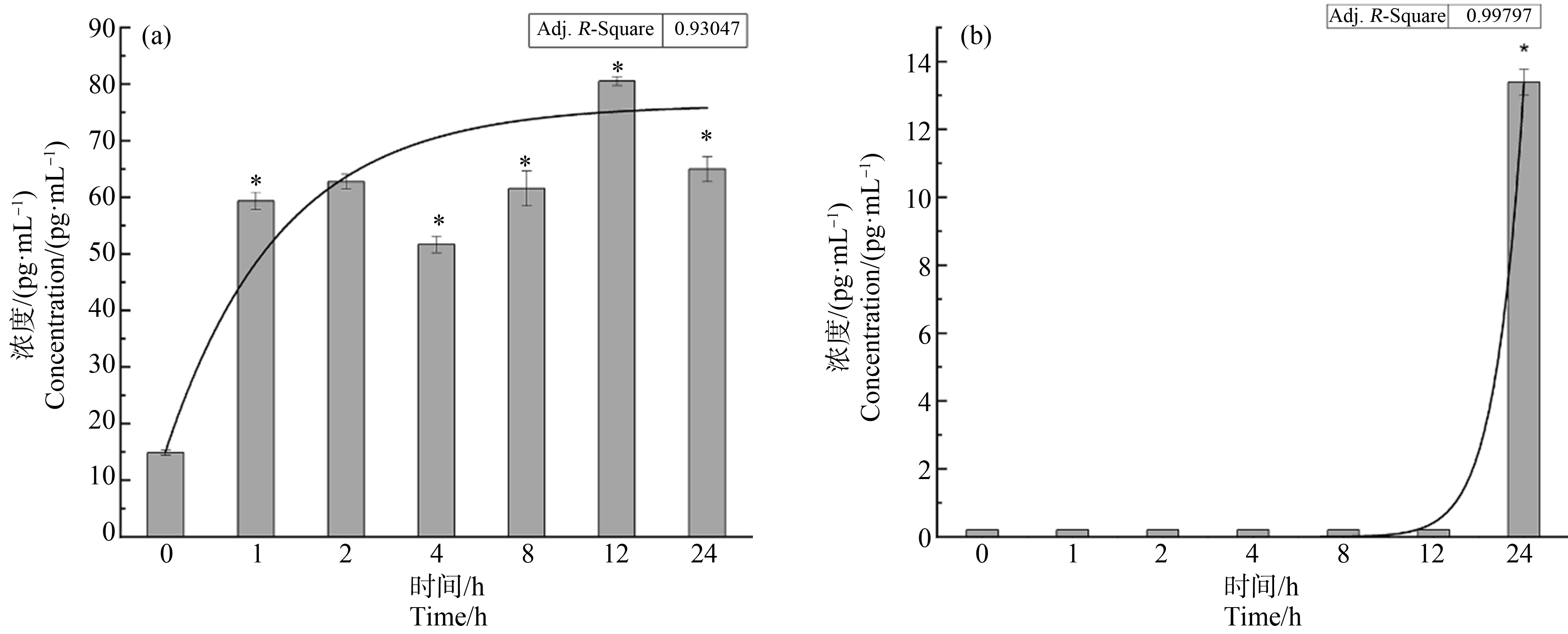
图8 PS-NPs在不同时间点刺激RAW264.7细胞产生的炎症因子水平
注:(a)PS-NPs诱导的细胞TNF-α表达水平;(b)PS-NPs诱导的细胞IL-6表达水平(*P<0.05)。
Fig.8 The levels of inflammatory factors induced by PS-NPs at different time points
Note:(a)The level of TNF-αinduced by PS-NPs;(b)The level of IL-6 induced by PS-NPs(*P<0.05).
3 讨论(Discussion)
近年在市售的瓶装矿泉水、海鲜、海盐和饮用茶包中均发现塑料颗粒,引发对塑料颗粒通过饮食途径摄入造成人体暴露风险的高度关注[9-10]。动物模型已证实通过饮食途径摄入的微/纳米塑料颗粒不仅会在肠道大量沉积,影响肠道结构功能,还会在肝脏、肾脏和肺积累,造成脏器的损伤,尤其纳米粒径颗粒能透过生殖屏障和血脑屏障(表2)。但不同研究暴露时间不同,不能阐明其迁移规律。本研究以小鼠为模型,模拟了纳米塑料摄入后对胃肠道的动态积累影响,结果表明通过饮食途径摄入的纳米塑料在1 h内快速由胃向肠道转移,但是在24 h内荧光信号已衰减至难以检测,可能是一部分纳米塑料随代谢物排出体外,如Peng等[22]用串联液相色谱质谱检测到,大鼠通过粪便排泄纳米聚酰胺的半衰期为36.9 h;另一部分纳米塑料可能进入血液循环系统迁移至其他器官富集,后续我们将进一步研究不同器官的PS-NPs积累情况。本研究表明PS-NPs急性暴露并不对会胃肠道有明显的影响,但长期暴露下,存在剂量依赖的炎症效应,表明即使是低剂量的摄入也会因为累积效应造成严重的组织结构损伤。
表2 微/纳米塑料暴露的体内分布和毒性结果统计
Table 2 A statistical table of toxicity and distribution of microplastics and nanoplastics

塑料种类Kind of plastic暴露方式Way of exposure暴露时间Time of exposure主要累积部位Main accumulation site主要效应Main effect文献ReferencesPS、PS-COOH、PS-NH2(80 nm)吸入Inhalation7 d大脑、肝Brain, liver神经毒性 Neurotoxicity[23]PS (100 nm、3 μm、10 μm)灌胃Gavage4 h肠、肝、脂肪Intestine, liver, fat未检测Undetected[24]PS (4 μm、10 μm)灌胃Gavage28 d睾丸Testis破坏生殖屏障Disruption of reproductive barrier[25]PS (20 nm、220 nm、1 μm、6 μm)灌胃Gavage48 h胃、肠Stomach, intestine未检测Undetected[26]PS (50 nm、500 nm、5 μm)灌胃Gavage28 d胃、肠Stomach, intestine破坏肠道屏障Disruption of intestinal barrier[27]PS、PS-COOH、PS-NH2(100 nm)灌胃Gavage28 d胃、肠、睾丸、肾Stomach, intestine, testis, kidney局部炎症Local inflammation[12]PS (5 μm、20 μm)灌胃Gavage28 d肝、肾、肠Liver, kidney, intestine脂质代谢异常Abnormal lipid metabolism[28]PE (10~150 μm)混入食物Added to food5周 5 weeks肠Intestine肠道菌群失调、炎症Alteration of intestinal flora, inflammation[29]PS (5 μm)混入饮水Dissolved in drinking water28 d未统计Uncounted加重DSS诱导的结肠炎Exacerbation of DSS-induced colitis[30]PS (157 nm)尾静脉注射Tail vein injection21 d肝、肺、脂肪Liver, lung, fat血糖升高、脂质代谢异常Elevated blood glucose, abnormal lipid metabolism[31]PS (50 nm)灌胃Gavage7 d肠、脑Intestine, brain破坏血脑屏障、炎症Disruption of blood-brain barrier, inflammation[32]
注:PS-COOH表示表面用羧基修饰的PS-NPs,PS-NH2表示表面用氨基修饰的PS-NPs;PE表示聚乙烯;DSS表示葡聚糖硫酸钠。
Note: PS-COOH means carboxyl functionalized PS-NPs, PS-NH2means amino functionalized PS-NPs; PE means polyethylene; DSS means dextransulfatesodium.
小鼠肠道的病理分析表明PS-NPs暴露导致了大量炎症细胞的浸润,其中肠道巨噬细胞是其免疫功能的重要执行者,进入肠道的塑料颗粒主要被巨噬细胞吞噬,从而维持其肠道稳态[33]。本研究在细胞水平上探索了PS-NPs被巨噬细胞吞噬的动力学规律及其对细胞的急性毒性。研究结果表明,纳米塑料被细胞吞噬同时存在吐出的代谢过程,如Liu等[20]在大鼠嗜碱性粒细胞中研究50、500和5 000 nm粒径的PS塑料颗粒转运规律,发现胞内的PS浓度也在8 h时达到峰值,随后开始下降;且撤除暴露源后,再培养基中观察到塑料颗粒,证实细胞存在外排机制。本实验中,在8 h内塑料颗粒进入细胞的量与时间呈显著正相关,而12 h后,胞内的塑料颗粒趋向于饱和,表明开始细胞对塑料的摄入量大于排出量,最后二者达成动态平衡,使得胞内的塑料颗粒数量维持在稳定状态,但是巨噬细胞对纳米塑料的胞吞、胞吐调控机制有待进一步明晰。
细胞对纳米塑料的应激响应主要为氧化应激损伤及炎症反应。我们的研究也证明PS-NPs在进入细胞后会刺激细胞产生氧化应激损伤,并在12 h内与PS-NPs浓度成正相关,随后ROS水平下降,这可能是由于细胞内超氧化物歧化酶、过氧化氢酶和谷胱甘肽过氧化物酶等抗氧化物酶被活化,激活胞内抗氧化机制[34]。在细胞炎症响应上,PS-NPs对TNF-α表现出较明显的激活作用,但只诱导极少量的IL-6产生,这可能与TNF-α和IL-6的生理功能有关。TNF-α和IL-6都是参与炎症调控的重要细胞因子,是机体对抗外来病原体入侵时的重要信号分子,但是在效应细胞上有所区别,TNF-α分泌会进一步激活CD4+、CD8+T细胞以及Treg细胞,调控细胞免疫过程[35];而IL-6调控B细胞,在体液免疫中具有重要作用[36]。因此PS-NPs主要诱导肠道细胞免疫反应。Xu等[37]在A549细胞中同样发现70 nm PS-NPs刺激后TNF-α基因表达远高于IL-6,表明纳米塑料在不同组织间的致炎效应存在一致性。
目前对纳米塑料在动物体内甚至细胞内的动力学分布规律研究较少,且不少研究的结果之间也存在差异。一方面可能是由于选用模型生物不同,个体差异性较大,另一方面是由于纳米塑料难以检测,目前常用的检测方式是对纳米塑料标记荧光信号,但是荧光技术在应用于动物时容易受到生物自发荧光的干扰,对结果影响较大[38]。近年来有研究团队通过对纳米塑料标记金属锆的同位素,解决了荧光信号干扰大的问题,为后续检测纳米塑料的体内分布和转运规律提供新的研究方法[26]。
[1] Eriksen M, Lebreton L C, Carson H S, et al.Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250, 000 tons afloat at sea[J].PLoS One, 2014, 9(12): e111913
[2] Zhang G S, Liu Y F.The distribution of microplastics in soil aggregate fractions in southwestern China[J].The Science of the Total Environment, 2018, 642: 12-20
[3] Zhang K, Shi H H, Peng J P, et al.Microplastic pollution in China’s inland water systems: A review of findings, methods, characteristics, effects, and management[J].The Science of the Total Environment, 2018, 630: 1641-1653
[4] Zhang K, Xiong X, Hu H J, et al.Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir, China[J].Environmental Science &Technology, 2017, 51(7): 3794-3801
[5] Rowley K H, Cucknell A C, Smith B D, et al.London’s river of plastic: High levels of microplastics in the Thames water column[J].The Science of the Total Environment, 2020, 740: 140018
[6] Lenz R, Enders K, Nielsen T G.Microplastic exposure studies should be environmentally realistic[J].Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(29): E4121-E4122
[7] Ho B T, Roberts T K, Lucas S.An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach[J].Critical Reviews in Biotechnology, 2018, 38(2): 308-320
[8] Pironti C, Ricciardi M, Motta O, et al.Microplastics in the environment: Intake through the food web, human exposure and toxicological effects[J].Toxics, 2021, 9(9): 224
[9] Ibrahim Y S, Tuan Anuar S, Azmi A A, et al.Detection of microplastics in human colectomy specimens[J].JGH Open: An Open Access Journal of Gastroenterology and Hepatology, 2020, 5(1): 116-121
[10] Schwabl P, Köppel S, Königshofer P, et al.Detection of various microplastics in human stool: A prospective case series[J].Annals of Internal Medicine, 2019, 171(7): 453-457
[11] Leslie H A, van Velzen M J M, Brandsma S H, et al.Discovery and quantification of plastic particle pollution in human blood[J].Environment International, 2022, 163: 107199
[12] Xu D H, Ma Y H, Han X D, et al.Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells[J].Journal of Hazardous Materials, 2021, 417: 126092
[13] Yong C Q Y, Valiyaveettil S, Tang B L.Toxicity of microplastics and nanoplastics in mammalian systems[J].International Journal of Environmental Research and Public Health, 2020, 17(5): 1509
[14] Meng X M, Zhang J W, Wang W J, et al.Effects of nano-and microplastics on kidney: Physicochemical properties, bioaccumulation, oxidative stress and immunoreaction[J].Chemosphere, 2022, 288(Pt 3): 132631
[15] Nikolic S, Gazdic-Jankovic M, Rosic G, et al.Orally administered fluorescent nanosized polystyrene particles affect cell viability, hormonal and inflammatory profile, and behavior in treated mice[J].Environmental Pollution, 2022, 305: 119206
[16] Birben E, Sahiner U M, Sackesen C, et al.Oxidative stress and antioxidant defense[J].The World Allergy Organization Journal, 2012, 5(1): 9-19
[17] Kuhn D A, Vanhecke D, Michen B, et al.Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages[J].Beilstein Journal of Nanotechnology, 2014, 5: 1625-1636
[18] Florance I, Ramasubbu S, Mukherjee A, et al.Polystyrene nanoplastics dysregulate lipid metabolism in murine macrophages in vitro[J].Toxicology, 2021, 458: 152850
[19] Liang B X, Zhong Y Z, Huang Y J, et al.Underestimated health risks: Polystyrene micro-and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis[J].Particle and Fibre Toxicology, 2021, 18(1): 20
[20] Liu L, Xu K X, Zhang B W, et al.Cellular internalization and release of polystyrene microplastics and nanoplastics[J].The Science of the Total Environment, 2021, 779: 146523
[21] Kik K, Bukowska B, Krokosz A, et al.Oxidative properties of polystyrene nanoparticles with different diameters in human peripheral blood mononuclear cells(in vitrostudy)[J].International Journal of Molecular Sciences, 2021, 22(9): 4406
[22] Peng C, He N, Wu Y H, et al.Excretion characteristics of nylon microplastics and absorption risk of nanoplastics in rats[J].Ecotoxicology and Environmental Safety, 2022, 238: 113586
[23] Liu X Y, Zhao Y C, Dou J B, et al.Bioeffects of inhaled nanoplastics on neurons and alteration of animal behaviors through deposition in the brain[J].Nano Letters, 2022, 22(3): 1091-1099
[24] 谢琼, 于慧慧, 周长赫, 等.微纳米塑料在小鼠消化器官吸收分布及与脂肪亲和性[J].中国公共卫生, 2021, 37(3): 446-450
Xie Q, Yu H H, Zhou C H, et al.Absorption, distribution and affinity with adipose tissue of nanomicroplastics in mouse digestive organs[J].Chinese Journal of Public Health, 2021, 37(3): 446-450(in Chinese)
[25] Wei Y X, Zhou Y, Long C L, et al.Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-mediated imbalance of mTORC1 and mTORC2[J].Environmental Pollution, 2021, 289: 117904
[26] Keinänen O, Dayts E J, Rodriguez C, et al.Harnessing PET to track micro-and nanoplastics in vivo[J].Scientific Reports, 2021, 11(1): 11463
[27] Liang B X, Zhong Y Z, Huang Y J, et al.Underestimated health risks: Polystyrene micro-and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis[J].Particle and Fibre Toxicology, 2021, 18(1): 20
[28] Deng Y F, Zhang Y, Lemos B, et al.Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure[J].Scientific Reports, 2017, 7: 46687
[29] Li B Q, Ding Y F, Cheng X, et al.Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice[J].Chemosphere, 2020, 244: 125492
[30] Zheng H B, Wang J, Wei X Y, et al.Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis[J].The Science of the Total Environment, 2021, 750: 143085
[31] Fan X P, Wei X J, Hu H L, et al.Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice[J].Chemosphere, 2022, 288(Pt 3): 132607
[32] Shan S, Zhang Y F, Zhao H W, et al.Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice[J].Chemosphere, 2022, 298: 134261
[33] Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al.Macrophage plasticity, polarization, and function in health and disease[J].Journal of Cellular Physiology, 2018, 233(9): 6425-6440
[34] Li G L, Li Y Y, Xiao B P, et al.Antioxidant activity of docosahexaenoic acid(DHA)and its regulatory roles in mitochondria[J].Journal of Agricultural and Food Chemistry, 2021, 69(5): 1647-1655
[35] Mehta A K, Gracias D T, Croft M.TNF activity and T cells[J].Cytokine, 2018, 101: 14-18
[36] Mudter J, Neurath M F.IL-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance[J].Inflammatory Bowel Diseases, 2007, 13(8): 1016-1023
[37] Xu M K, Halimu G, Zhang Q R, et al.Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell[J].The Science of the Total Environment, 2019, 694: 133794
[38] Catarino A I, Frutos A, Henry T B.Use of fluorescent-labelled nanoplastics(NPs)to demonstrate NP absorption is inconclusive without adequate controls[J].The Science of the Total Environment, 2019, 670: 915-920