有机紫外吸收剂(organic ultraviolet absorbents, OUVs)是一类可吸收紫外线辐射的芳香族化合物,被广泛地添加到塑料、油漆等工业品,织物、家具等生活品和防晒霜、化妆品等个人护理品中。OUVs会通过游泳、冲浪和潜水等海上娱乐活动的直接排放或河流、废水等间接排放进入海洋环境[1-3],之后经历海流迁移、颗粒沉积、生物吸收和微生物降解等复杂的物理、化学和生物过程,最终分布于海水[4-13]、沉积物[4, 9, 14]及海洋生物体中[4, 9]。
珊瑚礁生态系统是地球上最重要的生态系统之一,具有极高的生产力和生物多样性,为人类社会提供了优质的渔业资源、旅游观光资源和海岸生态服务。但是,近年来受全球变暖、海洋酸化等气候变化和过度捕捞[15-16]、废水排放[17-19]等人类活动的影响,珊瑚礁面临着严峻的退化问题[20-21]。其中,OUVs被证实是一类对珊瑚健康产生严重威胁的化学污染物[22]。作为一种“准”持久性有机污染物,OUVs在世界各地珊瑚礁区(中国香港[4]、中国台湾[5-6]、中国南海[7],日本冲绳岛[8],美国夏威夷瓦胡岛[9]、美属维尔京群岛象鼻湾[10]、美属圣约翰岛[11],荷兰博奈尔岛[12],格林纳达[13])水体中的浓度高达214 μg·L-1,在珊瑚组织(美国夏威夷瓦胡岛[9]和中国香港岛屿[4])中的浓度高达56.8 ng·g-1。根据全球OUVs的使用量评估,其对世界海域内高达10%的珊瑚具有致白化风险[22]。以二苯酮-3(BP-3)为例,当以珊瑚虫变形、浮浪幼虫死亡、珊瑚白化或者珊瑚死亡作为毒理效应终点时,中国台湾、日本冲绳岛等多个海域的BP-3对珊瑚的生态风险达到高风险级别[4-13](图1)。目前,有关国家和地区已相继采取措施限制某些OUVs的使用[23]。
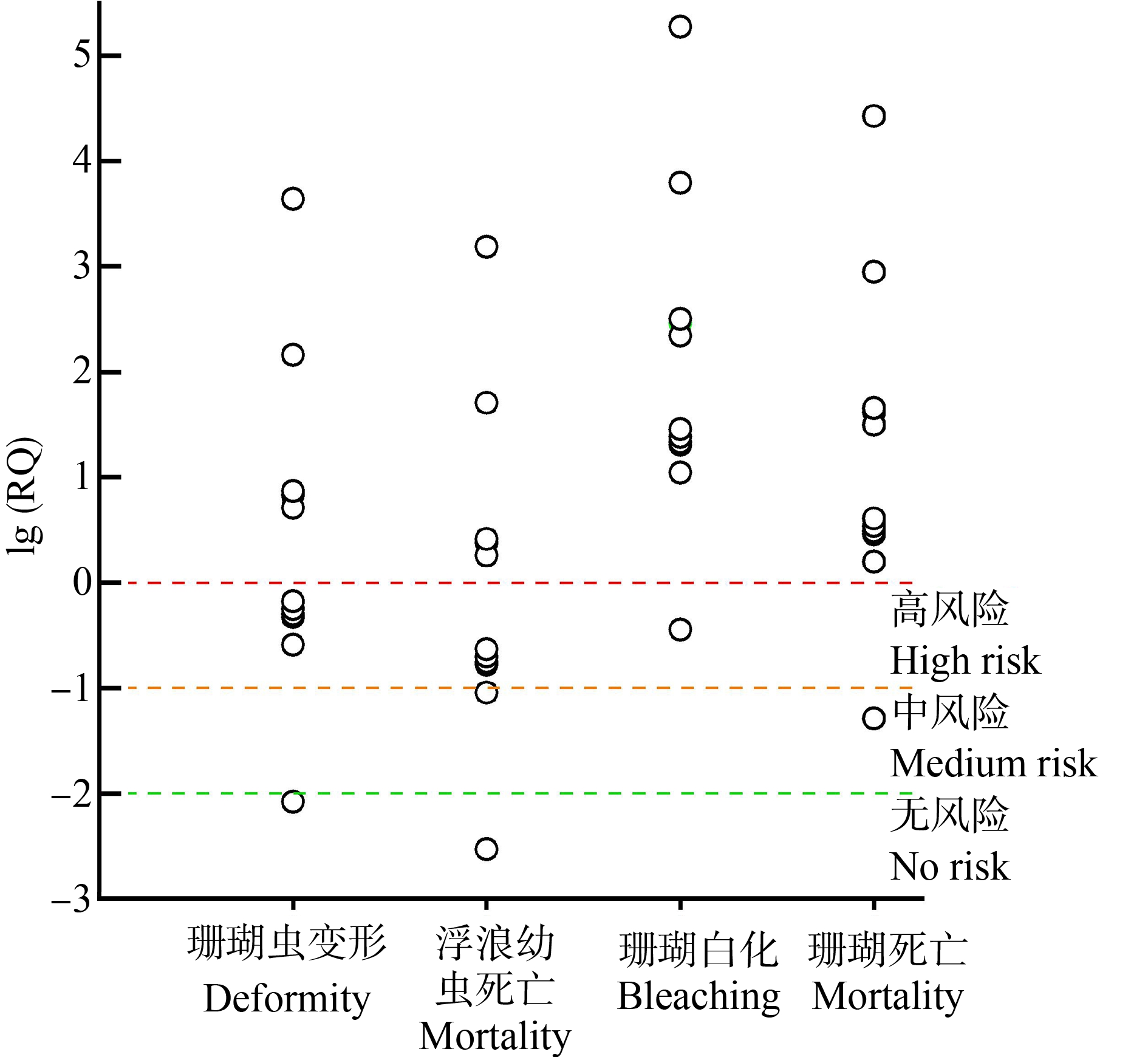
图1 不同珊瑚礁区海水中二苯酮-3(BP-3)浓度对珊瑚风险评价的结果
Fig. 1 Results of coral risk assessment with benzophenone-3(BP-3)concentrations in seawater from different coral reef areas
近年来,关于OUVs对珊瑚的毒理逐渐受到关注,相关论文被陆续发表。为全面地梳理该领域的研究进展,本文从毒理实验设计(OUVs种类选择、暴露浓度、珊瑚受试种类选择)和毒理效应终点(白化、浮浪幼虫或珊瑚虫死亡、珊瑚虫收缩、浮浪幼虫变形、幼虫变态发育受阻、珊瑚组织病变、遗传物质受损、代谢异常等)2个角度进行了综述,并展望了OUVs对珊瑚毒理学的研究方向。
1 毒理实验设计(Design of toxicological experiment)
迄今为止,国内外关于珊瑚暴露OUVs的毒理实验研究大多从个体、组织和分子水平探究OUVs对珊瑚的毒理机制,以及量化珊瑚对OUVs生态响应的时间-效应和剂量-效应关系。珊瑚和OUVs种类繁多,不同种类的珊瑚对不同的OUVs耐受程度具有差异。因此,在珊瑚毒理实验设计中,需针对珊瑚种类和目标OUVs,合理设计暴露条件。
1.1 毒理实验目标OUVs的选择
目前,用于开展珊瑚毒理暴露实验较常见的OUVs包括二苯甲酮-1(BP-1)、二苯酮-3(BP-3)、二苯酮-8(BP-8)、奥克立林(OC)和对甲氧基肉桂酸辛酯(EHMC)等(表1),其中,对BP-3和EHMC的研究最多,这可能与其毒性强或用量大有关。2015—2019年防晒类化妆品中防晒剂的使用情况表明,BP-3和EHMC的使用率分别高达24.0%和82.3%[24]。且多项毒理实验均已表明,BP-3可从分子水平到器官水平上影响人体内分泌及生殖发育[25-26];EHMC可干扰哺乳动物神经系统的发育及人体甲状腺的功能[27]。自2021年起,夏威夷已禁止销售含有BP-3和EHMC的防晒霜[28]。
1.2 毒理实验中OUVs的暴露浓度
在珊瑚的毒理实验设计中,为尽可能还原珊瑚暴露于OUVs的真实海洋环境,实验中OUVs的暴露浓度应尽量覆盖珊瑚礁区实测浓度,且还需考虑不同珊瑚对OUVs耐受力的差异。在对OUVs进行监测的多个珊瑚礁区(中国香港[4]、中国台湾[5-6]、中国南海[7],日本冲绳岛[8],美国夏威夷瓦胡岛[9]、美属维尔京群岛象鼻湾[10]、美属圣约翰岛[11],荷兰博奈尔岛[12],格林纳达[13]),BP-1、BP-3、BP-8、OC和EHMC在海水中的浓度为0.30~213 796.21 ng·L-1;而在沉积物中的浓度为0.26~11.2 ng·g-1;在珊瑚组织中的浓度为0.025~12.71 ng·g-1。不同环境介质储存OUVs的能力不同,故在珊瑚毒理实验中,OUVs暴露浓度应根据暴露介质而设计。目前,OUVs对珊瑚的毒理实验几乎全部基于海水环境介质,且设计浓度范围为ng·L-1到mg·L-1(表1)。另外,不同生长阶段的珊瑚(珊瑚细胞、浮浪幼虫和珊瑚株)对OUVs的敏感程度不同。在光照条件下,BP-3对萼柱珊瑚细胞(Stylophora pistillata)的致死率远高于其对浮浪幼虫的致死率[11];而BP-8对浅杯排孔珊瑚株(Seriatopora caliendrum)最低效应浓度(LOEC)低于其对浮浪幼虫的LOEC[5]。对OUVs毒性越敏感的珊瑚体系,毒理实验中浓度设置越小。以Downs等[11]的研究为例,针对珊瑚细胞、浮浪幼虫和珊瑚共生体,作者将BP-3的暴露浓度分别设置为0.57~246 000μg·L-1、0.1~288 000 μg·L-1和0.1~2 000 μg·L-1。值得注意的是,在珊瑚毒理实验设计过程中,OUVs浓度的设置还需考虑实际浓度和理论浓度是否匹配的问题。这是因为在OUVs加入到珊瑚缸之后,受微生物代谢转化[29]、珊瑚缸吸附[30]和海水金属离子络合等影响,养殖海水中OUVs的游离浓度通常低于表观浓度。因此,在珊瑚毒理实验中,各种因素的复杂性使得确定OUVs对珊瑚毒理的阈值具有较大挑战[31]。
1.3 毒理实验中受试珊瑚种类的选择
不同种类的珊瑚抵抗环境胁迫的能力具有差异。在珊瑚对OUVs的毒理暴露实验中,为了更全面、更保守地评估珊瑚礁受OUVs影响的生态现状,一般选择对环境更敏感的珊瑚种类进行研究。由于鹿角珊瑚科、杯形珊瑚科等枝状珊瑚对环境的敏感度高于块状珊瑚,故常被选做受试对象(表1)。实验结果表明,枝状摩羯鹿角珊瑚(Acropora cervicornis)在4 h光照下暴露于BP-3的LC20和LC50分别为0.063 μg·L-1和9 μg·L-1,而块状萼柱珊瑚(Stylophora pistillata)、大石星珊瑚(Montastrea annularis)、圆菊珊瑚(Montastrea cavernosa)、芥末滨珊瑚(Porites astreoides)和细手指滨珊瑚(Porites divaricata)在该条件下的LC20和LC50分别为0.502~671 μg·L-1和36~340 μg·L-1[11]。显然,枝状珊瑚对BP-3的耐受力更弱。不同种类珊瑚对环境胁迫耐受力的差异和多种因素有关,比如珊瑚组织厚度[32-34]、共生微生物(虫黄藻、细菌、古生菌和病毒等)群落多样性和组成[35-37]等。相关研究表明,组织层越厚的珊瑚(如块状珊瑚>枝状珊瑚),拥有的能量储备越高,应对环境胁迫(高温胁迫[38]、高悬浮颗粒物[32, 39]和沉积物[40]等)的能力越强;含有D系群虫黄藻比例较高的珊瑚,耐高温胁迫的能力较强[41-42]。
表1 珊瑚暴露于有机紫外吸收剂(OUVs)的毒理实验结果
Table 1 Toxicological test results of coral exposed to organic ultraviolet absorbents(OUVs)

OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献ReferencesBP-1浮浪幼虫Larva鹿角杯形珊瑚、浅杯排孔珊瑚Pocillopora dami-cornis, Seriatopo-ra caliendrum0.1~1 000 μg·L-1750 μg·L-1:2.5% P. damicornis浮浪幼虫死亡 (2.5% of P. dam-icornis larvae died)≤ 1 000 μg·L-1:P. dami-cornis浮浪幼虫未白化 (P. damicornis larvae did not bleach),LOEC (14 d,14 h∶10 h光∶暗周期循环14 h∶10 h light∶dark cycle,S.caliendrum)=500 μg·L-1附着失败 (De-settlement):EC50(14 d,14 h∶10 h光∶暗周期循环14 h∶10 h light∶dark cy-cle,S. caliendrum)=184.13 μg·L-1[5]珊瑚共生体Nubbin鹿角杯形珊瑚Pocillopora dami-cornis0.1~1 000 μg·L-11 000 μg·L-1:100%珊瑚虫死亡 (100% of corals died)1 000 μg·L-1:33%珊瑚白化 (33% of corals bleached),NOEC=1 000 μg·L-11 000 μg·L-1:66.7%珊瑚收缩 (The tenta-cles of 66.7% of coral polyps were retracted)[5]BP-2浮浪幼虫Larva萼柱珊瑚Stylophora pistillata2.46~246 000 μg·L-1NOEC=246 μg·L-1 (8 h,光照或黑暗 Light or darkness),LC50=120 mg·L-1 (8 h,光照Light)、165 μg·L-1 (24 h,光照 Light)、144 mg·L-1 (8 h,黑暗 Darkness)、548 μg·L-1 (24 h,黑暗Darkness)>2.28 μg·L-1:珊瑚出现白化迹象(Corals bleached)变形 (Deformity):NOEC (8 h,光照或黑暗Light or darkness)=246 mg·L-1,EC20 (24 h,光照Light)=246 ng·L-1、EC20 (24 h,黑暗Darkness)= 9.6 μg·L-1;组织变异 (Tissue mutation):246 mg·L-1 (光照 Light),珊瑚的表皮和胃真皮中大规模坏死 (Corals experienced catastrophic tissue lysis both within the epi-dermis and gastrodermis),246 mg·L-1 (黑暗 Darkness),珊瑚细胞自噬并死亡(Coral cells ex-perienced autophagy and died)遗传物质受损 (DNA dam-age):EC20 (8 h,光照 Light)=52 ng·L-1、EC20 (8 h,黑暗 Darkness)=4 ng·L-1,EC50 (8 h,光照 Light) =8.6 μg·L-1、EC50 (8 h,黑暗 Darkness)=1.8 μg·L-1,NOEC(8 h,黑暗Darkness)=246 μg·L-1、NOEC(8 h,光照Light)=24.6 μg·L-1[43]续表1OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献References

BP-3浮浪幼虫Larva浮浪幼虫Larva萼柱珊瑚Stylophora pistillata2.28~228 000 μg·L-1LC50 (8 h,光照Light)=3.1 mg·L-1、LC50 (24 h,光照Light)=139 μg·L-1、LC50 (8 h,黑暗Darkness)=16.8 mg·L-1、LC50 (24 h,黑暗Darkness)=779 μg·L-1LOEC (8 h,光照Light)=2.28 μg·L-1、LOEC (8 h,黑暗 Darkness)=22.8 μg·L-1变形 (Deformity):EC50=107 μg·L-1 (8 h,光照Light)、436 μg·L-1 (8 h,黑暗 Darkness)、49 μg·L-1 (24 h,光照Light)、137 μg·L-1 (24 h,黑暗Darkness),EC20=6.5 μg·L-1 (24 h,光照 Light),10 μg·L-1 (24 h,黑暗Darkness);组织变异 (Tissue mutation):22.8 μg·L-1 (光照Light),珊瑚外皮层变白色不透明 (The epider-mis of corals took on a white o-paque hue),288 μg·L-1 (光照Light),珊瑚组织溶解与表皮、胃真皮细胞降解 (Corals experi-enced catastrophic tissue lysis and cellular degradation in both the epidermis and gastrodermis),以及中胶质层部分坍塌 (As well as partial collapse of the mesoglea)[11]鹿角杯形珊瑚、浅杯排孔珊瑚Pocillopora dami-cornis, Seriatopo-ra caliendrum0.1~1 000 μg·L-11 000 μg·L-1:3% P. damicor-nis幼虫死亡 (3% of P. dami-cornis larvae died)1 000 μg·L-1:95% S. cal-iendrum白化 (95% ofS. caliendrum bleached)附着失败(De-settlement):1 000 μg·L-1,S. caliendrum珊瑚幼虫附着率从100%降到 (65.0±21.79)% (Settlement rates of S. caliendrum in the 1 000 μg·L-1 BP-3 were reduced from 100% to (65.0±21.79)%)[5]丛生盔形珊瑚Galaxea fascicu-laris310~10 000μg·L-1LC50 (96 h)=6.5 mg·L-1[45]续表1OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献References

BP-3珊瑚细胞Coral cell珊瑚共生体Nubbin萼柱珊瑚、鹿角杯形珊瑚、摩羯鹿角珊瑚、大石星珊瑚、圆菊珊瑚、芥末滨珊瑚、细手指滨珊瑚Stylophora pistil-lata, Pocillopora damicornis, Acro-pora cervicornis, Montastrea annu-laris,Montastrea cavernosa,Porites astreoides, Porites divaricata0.57~228 000 μg·L-1LC50 (4 h,光照 Light)=42 μg·L-1 (S. pistillata)、8 μg·L-1(P. damicornis)、9 μg·L-1(A. cervicornis)、74 μg·L-1 (M. an-nularis)、52 μg·L-1 (M. caverno-sa)、340 μg·L-1 (P. astreoides)、36 μg·L-1 (P. divaricata),LC50 (4 h,黑暗Darkness)=671 μg·L-1(S. pistillata),LC20 (4 h,光照 Light)=2 μg·L-1(S. pistillata)、0.062 μg·L-1(P. damicornis)、0.063 μg·L-1(A. cervicornis)、0.562 μg·L-1 (M. annularis)、0.502 μg·L-1(M. cavernosa)、8 μg·L-1 (P. astreoides)、0.175 μg·L-1(P. divaricata),LC20 (4 h,黑暗Darkness)=14 μg·L-1(S. pistil-lata)[11]佳丽鹿角珊瑚Acropora pulchra10~100 μL·L-133 μL·L-1:48 h,86% Acropora sp.白化 (86% of Acropora sp. bleached);50 μL·L-1:96 h,93% A. pulchra白化 (93% of A. pulchra bleached)[22]浅杯排孔珊瑚Seriatopora caliendrum0.1~1 000 μg·L-11 000 μg·L-1:6 d后S. calien-drum死亡 (S. caliendrum corals died after 6 d)1 000 μg·L-1:50%珊瑚白化 (50% of corals bleached)10 μg·L-1:16.7%珊瑚收缩 (The tentacles of 16.7% of coral pol-yps were retracted)[5]续表1OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献ReferencesBP-3珊瑚共生体Nubbin鹿角杯形珊瑚Pocillopora dami-cornis2 000 μg·L-1代谢异常(Metabolic abnor-mality):7 d后代谢物中的过氧麦角甾醇增加 (Er-gosterol peroxide in metab-olites increased after 7 d)[51]萼柱珊瑚、鹿角珊瑚Stylophora pistil-lata, Acropora tenuis26 ℃:(0.05±0.03) μg·L-1,33 ℃:(0.06±0.04) μg·L-10.06 μg·L-1:A. tenuis死亡率达100% (The mortality of A. tenuis was 100%),对S. pistil-lata存活无影响 (The survival of S. pistillata was unaffected)0.06 μg·L-1:S. pistillata白化 (S. pistillata bleached),光合产量降低22%~33% (The photosyn-thetic yield decreased by 22%~33%);A. tenuis 光合产量降低5% (Photo-synthetic yield of A. tenuis decreased by 5%),虫黄藻密度无变化 (The zooxan-thellae density did not change)[44]
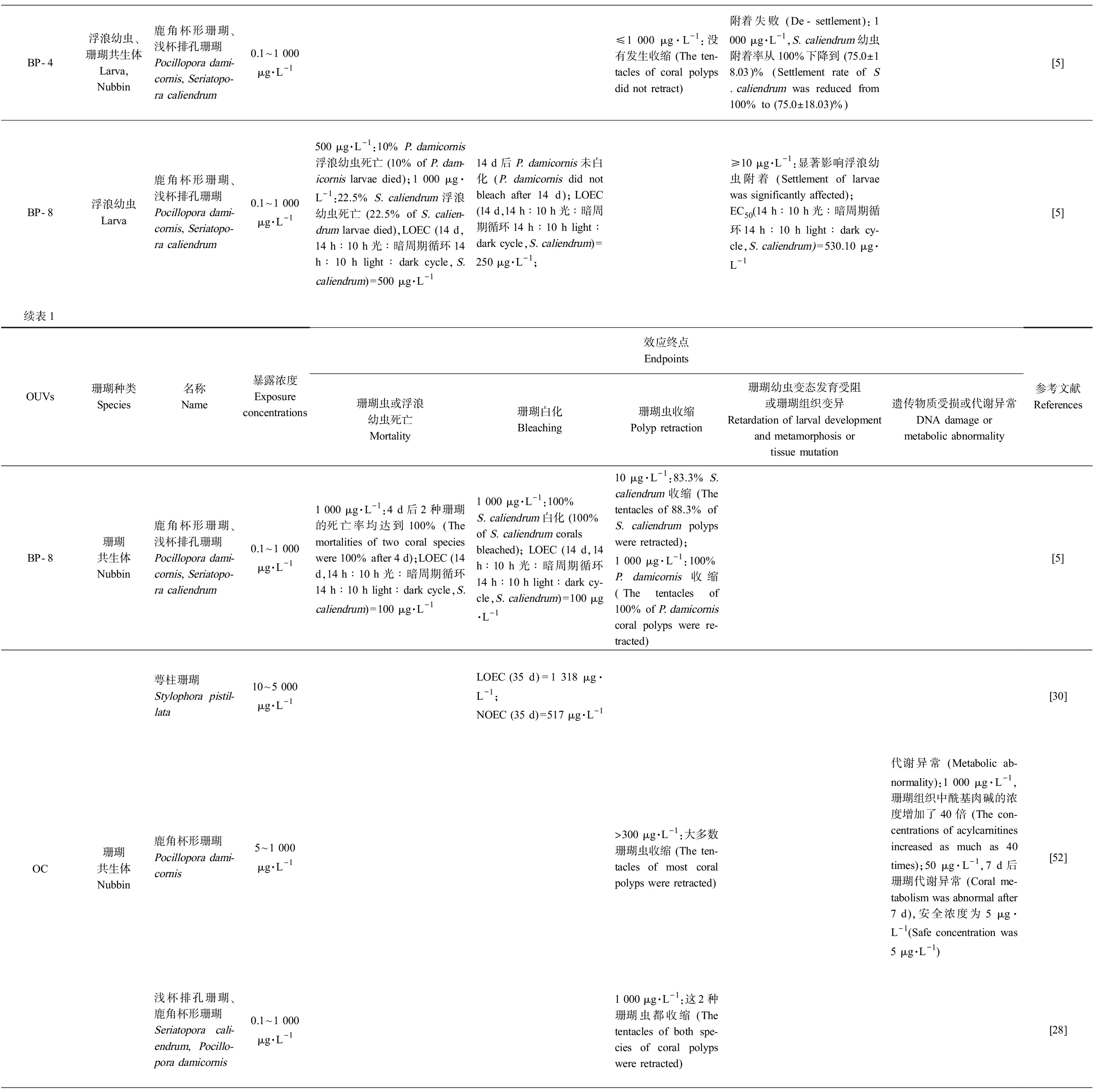
BP-4浮浪幼虫、珊瑚共生体Larva,Nubbin鹿角杯形珊瑚、浅杯排孔珊瑚Pocillopora dami-cornis, Seriatopo-ra caliendrum0.1~1 000 μg·L-1≤1 000 μg·L-1:没有发生收缩 (The ten-tacles of coral polyps did not retract)附着失败 (De-settlement):1 000 μg·L-1,S. caliendrum幼虫附着率从100%下降到 (75.0±18.03)% (Settlement rate of S. caliendrum was reduced from 100% to (75.0±18.03)%)[5]BP-8浮浪幼虫Larva鹿角杯形珊瑚、浅杯排孔珊瑚Pocillopora dami-cornis, Seriatopo-ra caliendrum0.1~1 000 μg·L-1500 μg·L-1:10% P. damicornis浮浪幼虫死亡 (10% of P. dam-icornis larvae died);1 000 μg·L-1:22.5% S. caliendrum浮浪幼虫死亡 (22.5% of S. calien-drum larvae died),LOEC (14 d,14 h∶10 h光∶暗周期循环14 h∶10 h light∶dark cycle,S. caliendrum)=500 μg·L-114 d后P. damicornis未白化 (P. damicornis did not bleach after 14 d);LOEC (14 d,14 h∶10 h光∶暗周期循环14 h∶10 h light∶dark cycle,S. caliendrum)=250 μg·L-1;≥10 μg·L-1:显著影响浮浪幼虫附着 (Settlement of larvae was significantly affected);EC50(14 h∶10 h光∶暗周期循环14 h∶10 h light∶dark cy-cle,S. caliendrum)=530.10 μg·L-1[5]续表1OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献ReferencesBP-8珊瑚共生体Nubbin鹿角杯形珊瑚、浅杯排孔珊瑚Pocillopora dami-cornis, Seriatopo-ra caliendrum0.1~1 000 μg·L-11 000 μg·L-1:4 d后2种珊瑚的死亡率均达到100% (The mortalities of two coral species were 100% after 4 d);LOEC (14 d,14 h∶10 h光∶暗周期循环14 h∶10 h light∶dark cycle,S. caliendrum)=100 μg·L-11 000 μg·L-1:100% S. caliendrum白化 (100% of S. caliendrum coralsbleached); LOEC (14 d,14 h∶10 h光∶暗周期循环14 h∶10 h light∶dark cy-cle,S. caliendrum)=100 μg·L-110 μg·L-1:83.3% S. caliendrum收缩 (The tentacles of 88.3% of S. caliendrum polyps were retracted);1 000 μg·L-1:100% P. damicornis收缩 (The tentacles of 100% of P. damicornis coral polyps were re-tracted)[5]OC珊瑚共生体Nubbin萼柱珊瑚Stylophora pistil-lata10~5 000 μg·L-1LOEC (35 d)=1 318 μg·L-1;NOEC (35 d)=517 μg·L-1[30]鹿角杯形珊瑚Pocillopora dami-cornis5~1 000 μg·L-1>300 μg·L-1:大多数珊瑚虫收缩 (The ten-tacles of most coral polyps were retracted)代谢异常 (Metabolic ab-normality):1 000 μg·L-1,珊瑚组织中酰基肉碱的浓度增加了40倍 (The con-centrations of acylcarnitines increased as much as 40 times);50 μg·L-1,7 d后珊瑚代谢异常 (Coral me-tabolism was abnormal after 7 d),安全浓度为5 μg·L-1(Safe concentration was 5 μg·L-1)[52]浅杯排孔珊瑚、鹿角杯形珊瑚Seriatopora cali-endrum,Pocillo-pora damicornis0.1~1 000 μg·L-11 000 μg·L-1:这2种珊瑚虫都收缩 (The tentacles of both spe-cies of coral polyps were retracted)[28]
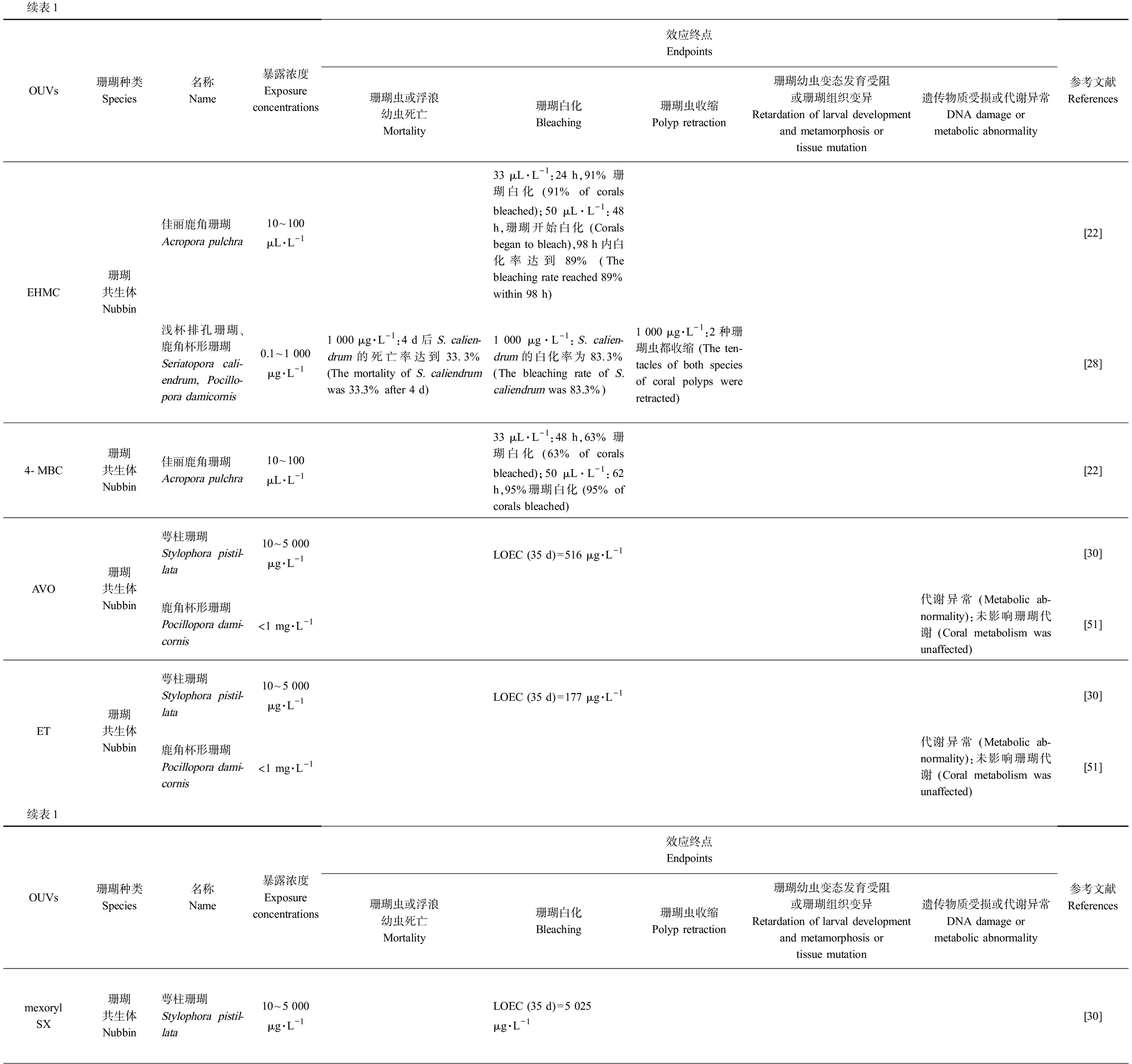
续表1OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献ReferencesEHMC珊瑚共生体Nubbin佳丽鹿角珊瑚Acropora pulchra10~100 μL·L-133 μL·L-1:24 h,91% 珊瑚白化 (91% of corals bleached);50 μL·L-1:48 h,珊瑚开始白化 (Corals began to bleach),98 h内白化率达到89% (The bleaching rate reached 89% within 98 h)[22]浅杯排孔珊瑚、鹿角杯形珊瑚Seriatopora cali-endrum, Pocillo-pora damicornis0.1~1 000 μg·L-11 000 μg·L-1:4 d后S. calien-drum的死亡率达到33.3% (The mortality of S. caliendrum was 33.3% after 4 d)1 000 μg·L-1:S. calien-drum的白化率为83.3% (The bleaching rate of S. caliendrum was 83.3%)1 000 μg·L-1:2种珊瑚虫都收缩 (The ten-tacles of both species of coral polyps were retracted)[28]4-MBC珊瑚共生体Nubbin佳丽鹿角珊瑚Acropora pulchra10~100 μL·L-133 μL·L-1:48 h,63% 珊瑚白化 (63% of corals bleached);50 μL·L-1:62 h,95%珊瑚白化 (95% of corals bleached)[22]AVO珊瑚共生体Nubbin萼柱珊瑚 Stylophora pistil-lata10~5 000 μg·L-1LOEC (35 d)=516 μg·L-1[30]鹿角杯形珊瑚 Pocillopora dami-cornis<1 mg·L-1代谢异常 (Metabolic ab-normality):未影响珊瑚代谢 (Coral metabolism was unaffected)[51]ET珊瑚共生体Nubbin萼柱珊瑚Stylophora pistil-lata10~5 000 μg·L-1LOEC (35 d)=177 μg·L-1[30]鹿角杯形珊瑚Pocillopora dami-cornis<1 mg·L-1代谢异常 (Metabolic ab-normality):未影响珊瑚代谢 (Coral metabolism was unaffected)[51]续表1OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献Referencesmexoryl SX珊瑚共生体Nubbin萼柱珊瑚Stylophora pistil-lata10~5 000 μg·L-1LOEC (35 d)=5 025 μg·L-1[30]
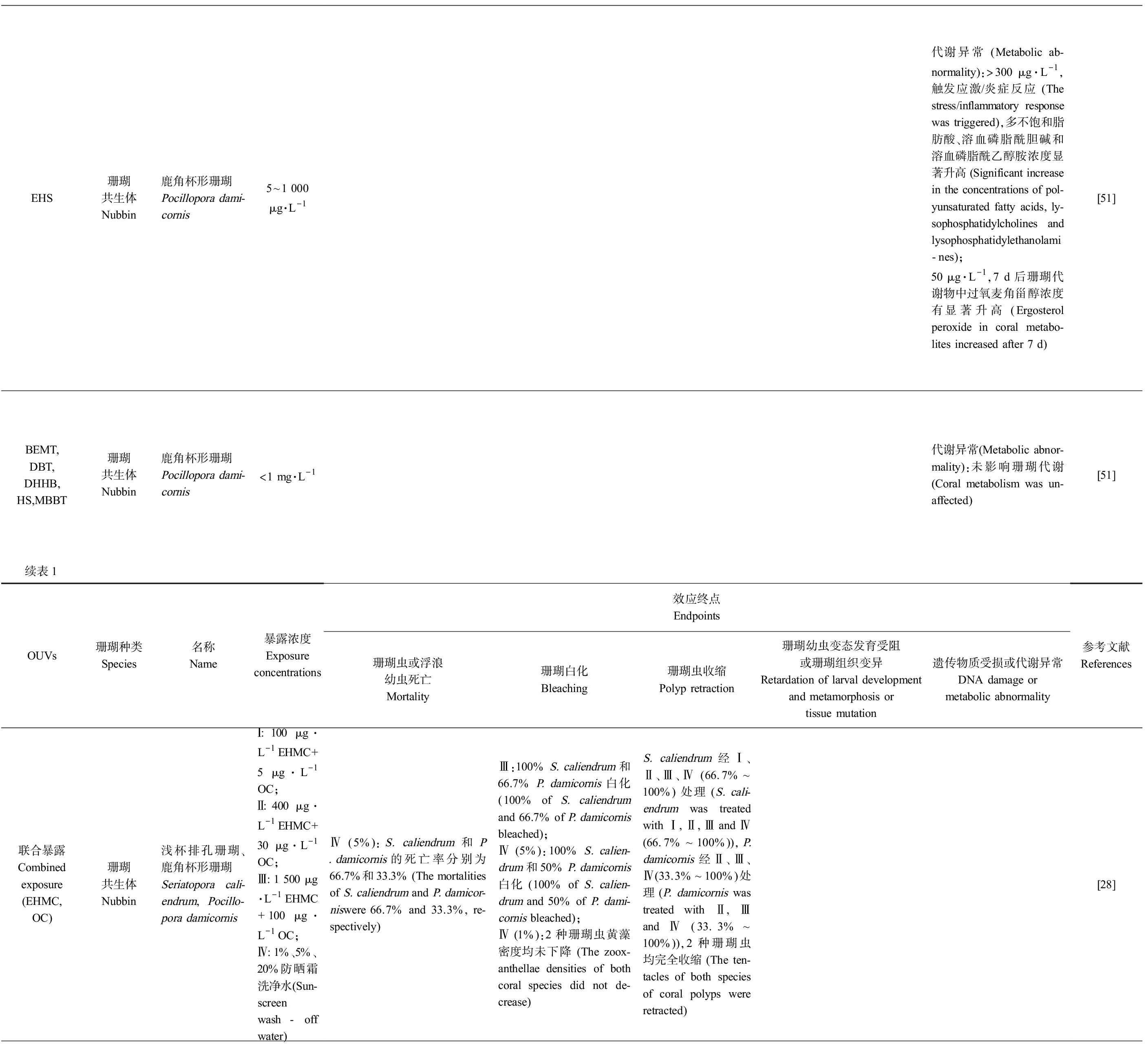
EHS珊瑚共生体Nubbin鹿角杯形珊瑚Pocillopora dami-cornis5~1 000 μg·L-1代谢异常 (Metabolic ab-normality):>300 μg·L-1,触发应激/炎症反应 (The stress/inflammatory response was triggered),多不饱和脂肪酸、溶血磷脂酰胆碱和溶血磷脂酰乙醇胺浓度显著升高 (Significant increase in the concentrations of pol-yunsaturated fatty acids, ly-sophosphatidylcholines and lysophosphatidylethanolami-nes);50 μg·L-1,7 d后珊瑚代谢物中过氧麦角甾醇浓度有显著升高 (Ergosterol peroxide in coral metabo-lites increased after 7 d)[51]BEMT,DBT,DHHB,HS,MBBT珊瑚共生体Nubbin鹿角杯形珊瑚Pocillopora dami-cornis<1 mg·L-1代谢异常(Metabolic abnor-mality):未影响珊瑚代谢 (Coral metabolism was un-affected)[51]续表1OUVs珊瑚种类Species名称Name暴露浓度Exposureconcentrations效应终点Endpoints珊瑚虫或浮浪幼虫死亡Mortality珊瑚白化Bleaching珊瑚虫收缩Polyp retraction珊瑚幼虫变态发育受阻或珊瑚组织变异Retardation of larval development and metamorphosis or tissue mutation遗传物质受损或代谢异常DNA damage ormetabolic abnormality参考文献References联合暴露Combined exposure(EHMC,OC)珊瑚共生体Nubbin浅杯排孔珊瑚、鹿角杯形珊瑚Seriatopora cali-endrum,Pocillo-pora damicornisⅠ: 100 μg·L-1 EHMC+5 μg·L-1 OC;Ⅱ: 400 μg·L-1 EHMC+30 μg·L-1 OC;Ⅲ: 1 500 μg·L-1 EHMC+100 μg·L-1 OC;Ⅳ: 1%、5%、 20%防晒霜洗净水(Sun-screen wash-off water)Ⅳ (5%):S. caliendrum和P. damicornis的死亡率分别为66.7%和33.3% (The mortalities of S. caliendrum and P. damicor-niswere 66.7% and 33.3%, re-spectively)Ⅲ:100% S. caliendrum和66.7% P. damicornis白化 (100% of S. caliendrum and 66.7% of P. damicornis bleached);Ⅳ (5%):100% S. calien-drum和50% P. damicornis白化 (100% of S. calien-drum and 50% of P. dami-cornis bleached);Ⅳ (1%):2种珊瑚虫黄藻密度均未下降 (The zoox-anthellae densities of both coral species did not de-crease)S. caliendrum经Ⅰ、Ⅱ、Ⅲ、Ⅳ (66.7%~100%) 处理 (S. cali-endrum was treated with Ⅰ, Ⅱ, Ⅲ and Ⅳ (66.7%~100%)),P. damicornis经Ⅱ、Ⅲ、Ⅳ(33.3%~100%)处理 (P. damicornis was treated with Ⅱ, Ⅲ and Ⅳ (33.3%~100%)),2种珊瑚虫均完全收缩 (The ten-tacles of both species of coral polyps were retracted)[28]
联合暴露Combined exposure(EHMC,OC,BP-3,EHS,BP,BMDBM,4-MBC)珊瑚共生体Nubbin佳丽鹿角珊瑚Acropora pulchra防晒霜洗净水 (Sun-screen wash-off water):10~100 μL·L-1100 μL·L-1:24 h内95%珊瑚白化 (95% of corals bleached within 24 h);33 μL·L-1:48 h内89%珊瑚白化 (89% of corals bleached within 48 h)代谢异常 (Metabolic abnor-mality):珊瑚周围海水中病毒的丰度增长15倍 (Viral abundance in seawater sur-rounding corals increased as much as 15 times),在虫黄藻周围和内部出现病毒状颗粒(VLPs) (The virus-like particles (VLPs) appeared a-round and inside the zoox-anthellae)[22]
注:OUVs 表示有机紫外吸收剂;BP-1、BP-2、BP-3、BP-4、BP-8、OC、EHMC、4-MBC、AVO、ET、mexoryl SX、EHS、BEMT、DBT、DHHB、HS、MBBT分别为二苯甲酮-1、二苯甲酮-2、二苯甲酮-3、二苯甲酮-4、二苯甲酮-8、奥克立林、对甲氧基肉桂酸辛酯、3-(4-甲基苄烯)-樟脑、阿伏苯宗、乙基己基三嗪酮、依莰舒、水杨酸异辛酯、双-乙基己氧苯酚甲氧苯基三嗪、二乙基己基丁酰胺基三嗪酮、二乙氨基羟苯甲酰基苯甲酸己酯、胡莫柳酯、并奥克三唑;LC50为半数致死浓度;EC50为半数效应浓度;LC20为20%致死浓度;EC20为20%效应浓度;NOEC为无效应浓度;LOEC为最低效应浓度。
Note: OUVs means organic ultraviolet absorbents; BP-1, BP-2, BP-3, BP-4, BP-8, OC, EHMC, 4-MBC, AVO, ET, mexoryl SX, EHS, BEMT, DBT, DHHB, HS, MBBT are benzophenone-1, benzophenone-2, benzophenone-3, benzophenone-4, benzophenone-8, octocrylene, ethylhexyl methoxycinnamate, 4-methylbenzylidene camphor, avobenzone, ethylhexyl triazone, terephthalylidene dicamphor sulfonic acid, 2-ethylhexyl salicylate, bis-ethylhexyloxyphenol ethoxyphenyl triazine, diethylhexyl butamido triazone, diethylamino hydroxybenzoyl hexyl enzoate, homosalate, methylene bis-benzotriazolyl etramethylbutylphenol, respectively; LC50means median lethal concentration; EC50means median effect concentration; LC20means 20% lethal concentration; EC20means 20% effect concentration; NOEC means no observed effect concentration; LOEC means lowest observed effect concentration.
2 OUVs对珊瑚的毒理效应(Toxicological effects of OUVs on corals)
目前,OUVs对珊瑚毒理研究的效应终点包括白化、死亡、触角收缩、幼虫变形、幼虫变态发育受阻、遗传物质受损和代谢异常等(图2)。珊瑚共生体、浮浪幼虫和珊瑚细胞响应OUVs污染的生物学机制不同,故应根据受试对象合理选择效应终点。
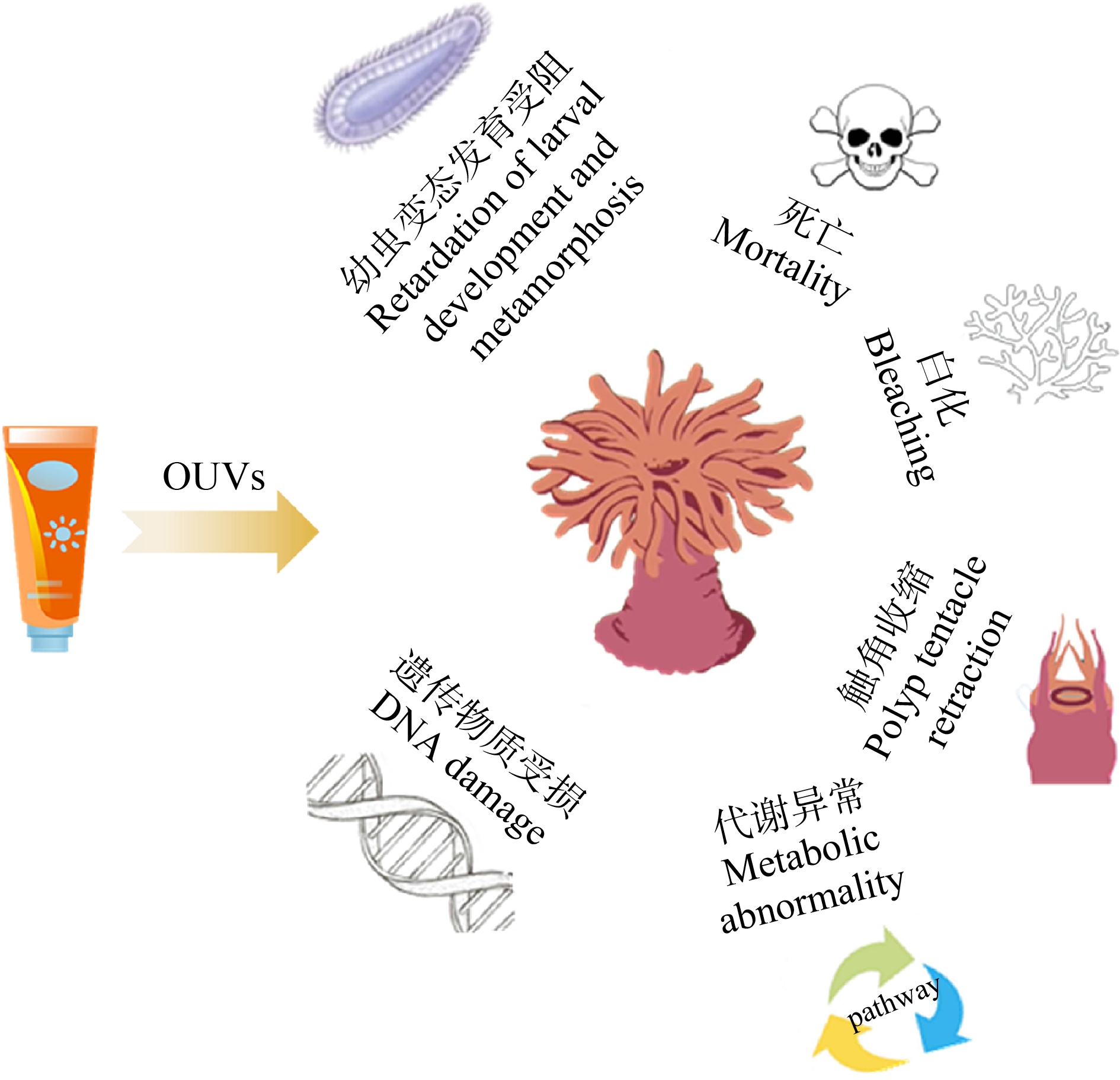
图2 OUVs对珊瑚的毒理效应
Fig. 2 Toxicological effects of OUVs on coral
2.1 珊瑚白化
OUVs可诱导珊瑚发生白化(图3(a))。白化是指珊瑚由于失去体内共生的虫黄藻和(或)共生的虫黄藻失去体内色素而导致五彩缤纷的珊瑚变白的一种生态现象[43]。它是珊瑚毒理实验中最常用的毒性终点。珊瑚白化的定量手段包括根据珊瑚颜色[5, 11, 43]、叶绿素荧光参数[44]和虫黄藻密度变化[5, 22]等进行计算。珊瑚对OUVs的耐白化能力与其种类有关,而且多种OUVs联合暴露会加剧其白化程度。

图3 OUVs对珊瑚的毒理效应
注:(a)白化、触角收缩;珊瑚暴露于5%防晒霜洗净水后出现触手闭合和白化现象[28](从左至右分别为控制组、暴露5 d和7 d的实验组);(b)附着失败;珊瑚浮浪幼虫暴露于1 000 mg·L-1BP-8后出现附着失败现象[5](从左至右分别为控制组、暴露7 d和8 d的实验组);(c)幼虫变形;珊瑚浮浪幼虫暴露于24.6 mg·L-1BP-2下出现变形现象(从上至下分别为控制组、暴露8 h的实验组)。
Fig.3 Toxicological effects of OUVs on corals
Note:(a)bleaching, tentacle retraction; occurrence of tentacle retraction and bleaching when corals were exposed to 5% sunscreen wash-off water [28](from left to right: the control group, test groups with exposure time of 5 d and 7 d, respectively);(b)failure of larval settlement; occurrence of settlement failure when coral larvae were exposed to 1 000 μg·L-1BP-8[5](from left to right: the control group, test groups with exposure time of 7 d and 8 d, respectively);(c)deformation of larvae; occurrence of deformation when coral larvae were exposed to 24.6 mg·L-1BP-2(from top to bottom: the control group, test groups with exposure time of 8 h, respectively).
如上文所述,珊瑚耐受性与其形态紧密相关。块状珊瑚的耐受性通常高于片状或枝状珊瑚。同样,珊瑚对OUVs的耐白化能力亦是如此。如,块状丛生盔形珊瑚(Galaxea fascicularis)在10 mg·L-1的BP-3溶液中4 d内未发生白化[45];而枝状浅杯排孔珊瑚(S. caliendrum)在1 mg·L-1的BP-3溶液中7 d内的白化率高达95%[5]。另外,同种珊瑚对不同OUVs的耐白化能力亦具有差异,如对浅杯排孔珊瑚(S. caliendrum)而言,BP-8造成其白化的LOEC值为250 μg·L-1,而BP-3则高达1 000 μg·L-1。显然,浅杯排孔珊瑚对BP-3的耐受性高于BP-8[5]。
为了同时吸收多种不同波段的紫外光,防晒霜生产厂家通常会在防晒霜中加入多种OUVs,如对UVB(290~320 nm)有吸收的EHMC、对UVA(320~340 nm)和UVB(290~320 nm)同时吸收的OC[46]等。然而,多种OUVs联合暴露可能会加剧珊瑚白化。He等[28]将浅杯排孔珊瑚(S. caliendrum)暴露于1 000 mg·L-1的EHMC溶液7 d(光照∶黑暗时间为14 h∶10 h,循环处理),发现83.3%的珊瑚白化;而同等光照条件下,5%的防晒霜洗净水(防晒霜洗净水由志愿者把涂上防晒霜的双手放入海水中浸泡一段时间后,将海水进行不同程度的稀释所得,对应EHMC和OC浓度分别为(422.34±37.34)μg·L-1和33.50 μg·L-1),会造成100%的珊瑚发生白化。当前,有关于珊瑚白化的细胞假说包括活性氧物质(reactive oxygen species, ROS)、光系统II(photosystem II, PSII)、DNA损伤和细胞凋亡等[47],其中ROS产生和积累所导致的细胞氧化应激反应被认为是珊瑚白化现象的关键机制[48]。故推测在多种OUVs联合暴露时,珊瑚的氧化应激系统可能受到了更为严重的损伤。
2.2 珊瑚虫或浮浪幼虫死亡
OUVs对珊瑚的致死率与珊瑚种类及暴露条件有关。浮浪幼虫或珊瑚虫死亡的效应终点通常选择LC50或NOEC值;鉴别珊瑚死亡的方法有台盼蓝拒染法和肉眼观察法,其中肉眼观察法以珊瑚幼虫静止且组织腐烂作为死亡判定标准。Downs等[11, 43]通过台盼蓝拒染法测得萼柱珊瑚(S. pistillata)浮浪幼虫在光照和黑暗条件下暴露于BP-3溶液24 h的LC50值分别为139 μg·L-1和779 μg·L-1;而He等[5]通过肉眼观察法发现浅杯排孔珊瑚(S. caliendrum)浮浪幼虫暴露于1 000 μg·L-1的BP-3溶液7 d(光照∶黑暗时间为14 h∶10 h,循环处理),其死亡率仅为3%。在这2个研究工作中,BP-3对珊瑚的致死LC50值具有较大差异,这可能是因为如下原因。(1)珊瑚种类不同。相对于浅杯排孔珊瑚(S. caliendrum)和鹿角杯形珊瑚(P. damicornis),萼柱珊瑚(S. pistillata)对环境变化较为敏感。(2)2组毒理实验设计的暴露条件存在差异,如光照条件、海水来源(人工海水vs天然海水)、有机溶剂添加等。
同样,多种OUVs联合暴露会加剧珊瑚虫的死亡[28]及进一步降低珊瑚的繁殖能力[49]。He等[28]将浅杯排孔珊瑚(S. caliendrum)、鹿角杯形珊瑚(P. damicornis)分别暴露于1 000 μg·L-1EHMC溶液、1 000 μg·L-1OC溶液和5%防晒霜洗净水4 d。结果显示,1 000 μg·L-1的EHMC溶液造成33.3%的浅杯排孔珊瑚(S. caliendrum)及0%的鹿角杯形珊瑚(P. damicornis)死亡;而1 000 μg·L-1的OC溶液未造成任何一种珊瑚死亡;相比较而言,5%的防晒霜洗净水即可造成66.7%~83.3%的浅杯排孔珊瑚(S. caliendrum)和33.3%~50%的鹿角杯形珊瑚(P. damicornis)死亡。由此可见,防晒霜洗净水对2种珊瑚的死亡危害远远高于EHMC或OC的单独溶液。当多种毒物同时作用在同一个生物体时,可表现为独立作用、加和作用、协同作用或拮抗作用。尽管每一种OUV在珊瑚礁区的浓度可能较低,但当多种OUVs联合作用时,其对珊瑚死亡率的影响将趋于复杂化。故为更加准确评估多种OUVs联合暴露或OUV与其他污染物联合暴露对珊瑚的毒理效应,应适当发挥等效线图法、混合物毒性指数法、析因分析法等多种评价方法[50]在毒理实验中的作用。
2.3 珊瑚遗传物质或代谢异常
OUVs对珊瑚的毒性除了表现在个体(死亡)或者组织水平(白化)上,还表现在分子水平上,如诱导珊瑚基因变异[11, 43]、代谢紊乱[51-52]等。当萼柱珊瑚(S. pistillata)浮浪幼虫暴露于BP-3溶液时,其遗传物质受到损伤[11],这可能和BP-3的基因毒性有关。BP-3通过诱导umu操纵子产生阳性诱变反应,进而增加DNA损伤率[53],且在阳光照射下,其基因毒性增强[54]。Stien等[52]利用高分辨率质谱对暴露于OC的珊瑚进行代谢组学分析,发现OC在珊瑚体内通过与脂肪酸形成络合物干扰脂质的代谢,并导致线粒体功能紊乱。ES同样会诱导珊瑚应激/炎症反应,引起多不饱和脂肪酸、溶血磷脂酰胆碱和溶血磷脂酰乙醇胺等代谢物的上调[51]。OUVs对珊瑚代谢的干扰可能是通过激发珊瑚共生体的氧化应激系统,进而破坏珊瑚细胞的结构;或通过病毒感染破坏珊瑚细胞[22],造成珊瑚内分泌紊乱[11, 55]。
2.4 其他影响
OUVs除了造成珊瑚白化、死亡、遗传物质受损及代谢异常外,还影响珊瑚触角闭合、幼虫变态发育等。健康的珊瑚虫对触摸敏感,被触摸时触角闭合,停止触摸后触角很快再次张开[28],但暴露于5%防晒霜洗净水下的珊瑚,其闭合/张开行为受到干扰[28](图3(a)),表现在触角完全收缩并伴随白化,对触摸行为无响应等。触角收缩可能是珊瑚适应OUVs污染物胁迫的一种生理适应机制[56-57]。除此之外,幼虫附着是珊瑚幼虫变态发育的重要过程之一,但OUVs胁迫会干扰该生理过程。例如,BP-8暴露会引起浅杯排孔珊瑚(S. caliendrum)的浮浪幼虫附着失败(图3(b));BP-2暴露会引起该珊瑚幼虫形态由正常的棒状变成球状(图3(c))[11, 43]。
OUVs不仅危害珊瑚健康,还会通过连锁反应对该生态系统中的其他生物产生危害。McCoshum等[49]将一个完整的珊瑚群落(包括扁形虫(Convolutriloba macropyga)、光合硅藻(Nitzschiasp.)、海葵(Aiptasia)、珊瑚(Xenia sp.))暴露于防晒霜稀释液中21 d,发现珊瑚的繁殖能力下降,海葵的健康指数降低(表现为脚盘附着力减弱,反应迟缓,身体颜色发生变化等),扁虫部分死亡、且无性繁殖能力下降,硅藻的叶绿素a含量下降,即OUVs引起了珊瑚礁整个生态系统的健康指数下降。珊瑚礁作为全球物种多样性最高的生态系统之一,食物链涉及多个生物营养级,一旦其中某个营养级被中断,整个生态系统将可能面临土崩瓦解的风险。
2.5 OUVs和其他自然因素的协同效应
珊瑚的生存不仅面临人类活动带来的各种污染物的威胁,还面临着全球变暖、海洋酸化等诸多自然条件恶化的挑战。当OUVs与高温胁迫、光照辐射等其他自然条件联合作用时,珊瑚受到的危害进一步增强[44]。如,高温胁迫会通过产生活性氧物质(ROS)破坏珊瑚共生虫黄藻的PSⅡ系统[58-60],进而降低其光合效率[30],而当OUVs和高温胁迫联合作用时,其光合效率进一步降低[44]。OUVs和高温联合作用同样会改变珊瑚共生微生物的群落结构[44],而微生物群落改变会引发珊瑚共生体内诸如固氮能力下降等众多生理生化反应的改变[61]。
光照是珊瑚礁生态系统的另外一个重要限制因子。一方面,造礁珊瑚及附礁生物中的共生虫黄藻需要足够的辐照度进行光合作用;另一方面,珊瑚繁殖需要一定的黑暗条件。因此,合适的光照条件对珊瑚的生长发育至关重要。当萼柱珊瑚(S. pistillata)浮浪幼虫分别在光照和黑暗条件下暴露于BP-3溶液8 h时,其LC50值分别为3.1 mg·L-1和16.8 mg·L-1,即光照增加了BP-3对珊瑚的毒性[11, 43]。值得注意的是,珊瑚礁生态系统主要分布在热带海域,在清澈的海水中,光合有效辐射的强度高达2 000 μmol·m-2·s-1,在如此强烈的光照条件下,OUVs对珊瑚的毒理效应可能会进一步增强[11]。OUVs作为一类对紫外光有吸收作用的化合物,它和光照的联合作用可能导致对珊瑚的致毒机制有别于其他毒物。
3 结语与展望(Summary and perspective)
OUVs作为一种新型有机污染物,广泛存在于珊瑚礁区的各种环境介质中,对珊瑚具有不可忽视的毒害作用。在个体层面,OUVs可致珊瑚白化、死亡、触角收缩、幼虫变形和附着失败;在组织层面,OUVs可致珊瑚组织发生病变;在分子层面,OUVs可使珊瑚遗传物质受损、代谢异常等。当OUVs与其他环境因子(如高温、紫外线)联合胁迫,其毒性可进一步增强。
虽然国内外已陆续开展OUVs对珊瑚的毒理研究,但多数停留于对毒害现象的描述,受害过程及致毒机理的研究仍很薄弱。为更全面探究OUVs对珊瑚的致毒机理,未来需要在以下几个方面继续开展工作。(1)开展新型OUVs对珊瑚的毒理研究。随着某些传统OUVs的禁用,一些新型OUVs(如天然防晒剂[62]、光控智能防晒剂[63])应运而生,其对珊瑚的毒性需要受到关注。(2)开展多种OUVs的联合毒性研究。目前大多研究只采用1种或2种OUVs暴露,而环境中通常多种OUVs共存。因此,多种OUVs的联合毒性亟需重点关注。(3)从共生体的不同角度探索珊瑚对OUVs的毒理响应。珊瑚是由珊瑚虫、共生虫黄藻、微生物和病毒等组成的共生体,它们在维护珊瑚共生体稳定方面均发挥着重要作用,单独对每个对象进行研究可加深对OUVs致毒机理的认知。(4)多组学技术联合使用,深挖OUVs对珊瑚的致毒机理。近年来迅速发展的高通量和高灵敏度的组学(Omics)技术(如基因组学、转录组学、蛋白质组学和代谢组学等)有助于揭示污染物的致毒过程、机制及致毒后的典型特征,有望成为解决OUVs毒理学中诸多问题的强有力工具。(5)珊瑚毒性效应终点量化方法有待改进。精准量化效应终点是评价OUVs对珊瑚毒性的基础,然而,目前定量珊瑚白化、触角收缩和致死等的方法具有一定的局限性,开发新型、高效的效应终点量化方法是未来需要努力的方向。
[1] 朱小山, 黄静颖, 吕小慧, 等.防晒剂的海洋环境行为与生物毒性[J].环境科学, 2018, 39(6): 2991-3002
Zhu X S, Huang J Y, Lv X H, et al.Fate and toxicity of UV filters in marine environments[J].Environmental Science, 2018, 39(6): 2991-3002(in Chinese)
[2] Sánchez-Quiles D, Tovar-Sánchez A.Are sunscreens a new environmental risk associated with coastal tourism?[J].Environment International, 2015, 83: 158-170
[3] Lyu Y, Zhong F Y, Tang Z W, et al.Bioaccumulation and trophic transfer of organic ultraviolet absorbents in the food web of a freshwater lake: Implications for risk estimation[J].Environmental Pollution, 2022, 294: 118612
[4] Tsui M M P, Lam J C W, Ng T Y, et al.Occurrence, distribution, and fate of organic UV filters in coral communities[J].Environmental Science &Technology, 2017, 51(8): 4182-4190
[5] He T T, Tsui M M P, Tan C J, et al.Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species[J].The Science of the Total Environment, 2019, 651(Pt 2): 2391-2399
[6] Kung T A, Lee S H, Yang T C, et al.Survey of selected personal care products in surface water of coral reefs in Kenting National Park, Taiwan[J].The Science of the Total Environment, 2018, 635: 1302-1307
[7] Tsui M M P, Chen L G, He T T, et al.Organic ultraviolet(UV)filters in the South China Sea coastal region: Environmental occurrence, toxicological effects and risk assessment[J].Ecotoxicology and Environmental Safety, 2019, 181: 26-33
[8] Tashiro Y, Kameda Y.Concentration of organic sun-blocking agents in seawater of beaches and coral reefs of Okinawa Island, Japan[J].Marine Pollution Bulletin, 2013, 77(1-2): 333-340
[9] Mitchelmore C L, He K, Gonsior M, et al.Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii[J].The Science of the Total Environment, 2019, 670: 398-410
[10] Bargar T A, Alvarez D A, Garrison V H.Synthetic ultraviolet light filtering chemical contamination of coastal waters of Virgin Islands National Park, St.John, US Virgin Islands[J].Marine Pollution Bulletin, 2015, 101(1): 193-199
[11] Downs C A, Kramarsky-Winter E, Segal R, et al.Toxicopathological effects of the sunscreen UV filter, oxybenzone(benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US virgin Islands[J].Archives of Environmental Contamination and Toxicology, 2016, 70(2): 265-288
[12] Schaap I, Slijkerman D M E.An environmental risk assessment of three organic UV-filters at Lac Bay, Bonaire, Southern Caribbean[J].Marine Pollution Bulletin, 2018, 135: 490-495
[13] Horricks R A, Tabin S K, Edwards J J, et al.Organic ultraviolet filters in nearshore waters and in the invasive lionfish(Pterois volitans)in Grenada, West Indies[J].PLoS One, 2019, 14(7): e0220280
[14] Tsui M M, Leung H W, Kwan B K, et al.Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in marine sediments in Hong Kong and Japan[J].Journal of Hazardous Materials, 2015, 292: 180-187
[15] Ellis J I, Jamil T, Anlauf H, et al.Multiple stressor effects on coral reef ecosystems[J].Global Change Biology, 2019, 25(12): 4131-4146
[16] Teh L S L, Teh L C L, Sumaila U R.A global estimate of the number of coral reef fishers[J].PLoS One, 2013, 8(6): e65397
[17] Zhong M Y, Tang J H, Guo X Y, et al.Occurrence and spatial distribution of organophosphorus flame retardants and plasticizers in the Bohai, Yellow and East China Seas[J].Science of the Total Environment, 2020, 741: 140434
[18] 陆昊, 刘红岩, 黄秀铭, 等.洗涤剂主成分LAS和AEO对软珊瑚氧化应激水平的影响[J].海洋环境科学, 2021, 40(1): 133-138
Lu H, Liu H Y, Huang X M, et al.Effect of main components of detergent on oxidative stress in soft corals[J].Marine Environmental Science, 2021, 40(1): 133-138(in Chinese)
[19] Zhou Z, Wan L, Cai W Q, et al.Species-specific microplastic enrichment characteristics of scleractinian corals from reef environment: Insights from an in situstudy at the Xisha Islands[J].The Science of the Total Environment, 2022, 815: 152845
[20] Putnam H M, Barott K L, Ainsworth T D, et al.The vulnerability and resilience of reef-building corals[J].Current Biology, 2017, 27(11): R528-R540
[21] 江志坚, 黄小平.富营养化对珊瑚礁生态系统影响的研究进展[J].海洋环境科学, 2010, 29(2): 280-285
Jiang Z J, Huang X P.Recent progress on effect of eutrophication on coral reef ecosystem[J].Marine Environmental Science, 2010, 29(2): 280-285(in Chinese)
[22] Danovaro R, Bongiorni L, Corinaldesi C, et al.Sunscreens cause coral bleaching by promoting viral infections[J].Environmental Health Perspectives, 2008, 116(4): 441-447
[23] 刘玮, 李航, 赵欣研, 等.防晒剂对海洋生态环境的污染及潜在影响[J].中华皮肤科杂志, 2021, 54(5): 456-458
Liu W, Li H, Zhao X Y, et al.Sunscreen pollution of marine ecosystems and its potential impact[J].Chinese Journal of Dermatology, 2021, 54(5): 456-458(in Chinese)
[24] 刘小娟, 张弦, 周桓, 等.2015—2019年防晒类化妆品中防晒剂的使用情况分析[J].广州化工, 2020, 48(24): 78-80
Liu X J, Zhang X, Zhou H, et al.Analysis of sunscreen use in sunscreen cosmetics from 2015 to 2019[J].Guangzhou Chemical Industry, 2020, 48(24): 78-80(in Chinese)
[25] 卢婍, 周义军, 田英.有机紫外线吸收剂二苯甲酮-3的环境污染及其内分泌干扰作用研究进展[J].上海交通大学学报: 医学版, 2019, 39(11): 1320-1324
Lu Q, Zhou Y J, Tian Y.Research progress in environmental pollution and endocrine disruption of the UV filter benzophenone-3[J].Journal of Shanghai Jiao Tong University: Medical Science, 2019, 39(11): 1320-1324(in Chinese)
[26] Ghazipura M, McGowan R, Arslan A, et al.Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies[J].Reproductive Toxicology, 2017, 73: 175-183
[27] Axelstad M, Boberg J, Hougaard K S, et al.Effects of pre-and postnatal exposure to the UV-filter octyl methoxycinnamate(OMC)on the reproductive, auditory and neurological development of rat offspring[J].Toxicology and Applied Pharmacology, 2011, 250(3): 278-290
[28] He T T, Tsui M M P, Tan C J, et al.Toxicological effects of two organic ultraviolet filters and a related commercial sunscreen product in adult corals[J].Environmental Pollution, 2019, 245: 462-471
[29] 刘世光.纳米二氧化钛在水中的聚集沉积特性研究[D].哈尔滨: 哈尔滨工业大学, 2013: 25-59
Liu S G.Study on aggregation and deposition characteristics of titanium dioxide nanoparticles in aqueous systems[D].Harbin: Harbin Institute of Technology, 2013: 25-59(in Chinese)
[30] Fel J P, Lacherez C, Bensetra A, et al.Photochemical response of the scleractinian coral Stylophora pistillatato some sunscreen ingredients[J].Coral Reefs, 2019, 38(1): 109-122
[31] Watkins Y S D, Sallach J B.Investigating the exposure and impact of chemical UV filters on coral reef ecosystems: Review and research gap prioritization[J].Integrated Environmental Assessment and Management, 2021, 17(5): 967-981
[32] Rotmann S, Thomas S.Coral tissue thickness as a bio-indicator of mine-related turbidity stress on coral reefs at Lihir Island, Papua New Guinea[J].Oceanography, 2012, 25(4): 52-63
[33] Wiedenmann J, D’Angelo C, Smith E G, et al.Nutrient enrichment can increase the susceptibility of reef corals to bleaching[J].Nature Climate Change, 2013, 3(2): 160-164
[34] Qin Z J, Yu K F, Liang Y T, et al.Latitudinal variation in reef coral tissue thickness in the South China Sea: Potential linkage with coral tolerance to environmental stress[J].The Science of the Total Environment, 2020, 711: 134610
[35] Qin Z J, Yu K F, Liang J Y, et al.Significant changes in microbial communities associated with reef corals in the southern South China Sea during the 2015/2016 global-scale coral bleaching event[J].Journal of Geophysical Research: Oceans, 2020, 125(7): e2019JC015579
[36] Hume B C, Voolstra C R, Arif C, et al.Ancestral genetic diversity associated with the rapid spread of stress-tolerant coral symbionts in response to Holocene climate change[J].Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(16): 4416-4421
[37] Liang J Y, Yu K F, Wang Y H, et al.Distinct bacterial communities associated with massive and branching scleractinian corals and potential linkages to coral susceptibility to thermal or cold stress[J].Frontiers in Microbiology, 2017, 8: 979
[38] Loya Y, Sakai K, Yamazato K, et al.Coral bleaching: The winners and the losers[J].Ecology Letters, 2001, 4(2): 122-131
[39] Rotmann S.Tissue thickness as a tool to monitor the stress response of massive Poritescorals to turbidity impact on Lihir Island, Papua New Guinea[D].Townsville: James Cook University, 2004: 12-44
[40] Weber M, de Beer D, Lott C, et al.Mechanisms of damage to corals exposed to sedimentation[J].Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(24): E1558-E1567
[41] Qin Z J, Yu K F, Chen B, et al.Diversity of Symbiodiniaceaein 15 coral species from the southern South China Sea: Potential relationship with coral thermal adaptability[J].Frontiers in Microbiology, 2019, 10: 2343
[42] Ziegler M, Eguíluz V M, Duarte C M, et al.Rare symbionts may contribute to the resilience of coral-algal assemblages[J].The ISME Journal, 2018, 12(1): 161-172
[43] Downs C A, Kramarsky-Winter E, Fauth J E, et al.Toxicological effects of the sunscreen UV filter, benzophenone-2, on planulae and in vitrocells of the coral, Stylophora pistillata[J].Ecotoxicology, 2014, 23(2): 175-191
[44] Wijgerde T, van Ballegooijen M, Nijland R, et al.Adding insult to injury: Effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals[J].The Science of the Total Environment, 2020, 733: 139030
[45] Conway A J, Gonsior M, Clark C, et al.Acute toxicity of the UV filter oxybenzone to the coral Galaxea fascicularis[J].The Science of the Total Environment, 2021, 796: 148666
[46] Santos A J M, Miranda M S, Esteves da Silva J C G.The degradation products of UV filters in aqueous and chlorinated aqueous solutions[J].Water Research, 2012, 46(10): 3167-3176
[47] 林镇跃.珊瑚共生的生物相互作用机制及其响应海洋变化的组学研究[D].厦门: 厦门大学, 2018: 40-56
Lin Z Y.Mechanisms of biotic interaction for coral symbiosis and the related omics responses to the ocean changes[D].Xiamen: Xiamen University, 2018: 40-56(in Chinese)
[48] Lesser M P.Oxidative stress causes coral bleaching during exposure to elevated temperatures[J].Coral Reefs, 1997, 16(3): 187-192
[49] McCoshum S M, Schlarb A M, Baum K A.Direct and indirect effects of sunscreen exposure for reef biota[J].Hydrobiologia, 2016, 776(1): 139-146
[50] 杨蓉, 李娜, 饶凯锋, 等.环境混合物的联合毒性研究方法[J].生态毒理学报, 2016, 11(1): 1-13
Yang R, Li N, Rao K F, et al.Review on methodology for environmental mixture toxicity study[J].Asian Journal of Ecotoxicology, 2016, 11(1): 1-13(in Chinese)
[51] Stien D, Suzuki M, Rodrigues A M S, et al.A unique approach to monitor stress in coral exposed to emerging pollutants[J].Scientific Reports, 2020, 10(1): 9601
[52] Stien D, Clergeaud F, Rodrigues A M S, et al.Metabolomics reveal that octocrylene accumulates in Pocillopora damicornistissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction[J].Analytical Chemistry, 2019, 91(1): 990-995
[53] Nakajima D, Asada S, Kageyama S, et al.Activity related to the carcinogenicity of plastic additives in the benzophenone group[J].Journal of UOEH, 2006, 28(2): 143-156
[54] Knowland J, McKenzie E A, McHugh P J, et al.Sunlight-induced mutagenicity of a common sunscreen ingredient[J].FEBS Letters, 1993, 324(3): 309-313
[55] Tang C H, Lin C Y, Lee S H, et al.Membrane lipid profiles of coral responded to zinc oxide nanoparticle-induced perturbations on the cellular membrane[J].Aquatic Toxicology, 2017, 187: 72-81
[56] 李秀保, 黄晖, 练健生, 等.珊瑚及共生藻在白化过程中的适应机制研究进展[J].生态学报, 2007, 27(3): 1217-1225
Li X B, Huang H, Lian J S, et al.Progress of adaptive mechanism of coral and symbiotic algae during bleaching[J].Acta Ecologica Sinica, 2007, 27(3): 1217-1225(in Chinese)
[57] Malul D, Holzman R, Shavit U.Coral tentacle elasticity promotes an out-of-phase motion that improves mass transfer[J].Proceedings Biological Sciences, 2020, 287(1929): 20200180
[58] Higuchi T, Yuyama I, Nakamura T.The combined effects of nitrate with high temperature and high light intensity on coral bleaching and antioxidant enzyme activities[J].Regional Studies in Marine Science, 2015, 2: 27-31
[59] 项楠, 杨婷寒, 程华民, 等.化学污染物对珊瑚礁生态系统的影响研究进展[J].生态毒理学报, 2017, 12(6): 19-26
Xiang N, Yang T H, Cheng H M, et al.Research progress on effects of chemical pollutants on the coral reef ecosystem[J].Asian Journal of Ecotoxicology, 2017, 12(6): 19-26(in Chinese)
[60] Dove S, Ortiz J C, Enríquez S, et al.Response of holosymbiont pigments from the scleractinian coral Montipora monasteriatato short-term heat stress[J].Limnology and Oceanography, 2006, 51(2): 1149-1158
[61] van Oppen M J H, Blackall L L.Coral microbiome dynamics, functions and design in a changing world[J].Nature Reviews Microbiology, 2019, 17(9): 557-567
[62] 陈玲林, 代彦, 赵华.生物防晒剂活酵母细胞衍生物制备中酶解条件的优化[J].日用化学工业, 2014, 44(1): 31-34
Chen L L, Dai Y, Zhao H.Optimization of enzymolysis conditions in preparation of biological sunscreen live yeast cell derivative[J].China Surfactant Detergent &Cosmetics, 2014, 44(1): 31-34(in Chinese)
[63] 杨永鹏, 董萍, 左夏林, 等.皮肤防晒化妆品的技术革命之五篇: 光控智能防晒化妆品研制的设想和设计[J].中国化妆品(行业), 2011(1): 72-77
Yang Y P, Dong P, Zuo X L, et al.Revolution of skin sunscreen cosmetics: Assumption and design of light-controlled intelligent sunscreen cosmetics[J].China Cosmetics Review, 2011(1): 72-77(in Chinese)