随着人类工农业现代化的发展,采矿、冶金、电镀、电工、染料和纺织等人为因素造成的土壤重金属污染是当下世界不容忽视的环境问题[1]。《全国土壤污染状况调查公报》(2014年)显示,我国耕地土壤点位超标率为19.4%,土壤重金属总超标率为16.1%,受重金属污染的耕地面积约占耕地总面积的1/5,其中Cd超标率为7%[2],As超标率为2.7%[3],分别是耕地土壤位列第一和第三的主要污染物。Cd、As属“五毒元素”,是危险致癌物质,世界范围内土壤Cd背景值为0.01~2.0 mg·kg-1,均值约0.35 mg·kg-1[2],全球土壤As含量均值为6 mg·kg-1,我国土壤As含量均值为11.2 mg·kg-1,是全球土壤As浓度的近2倍[4]。Cd、As的人为来源广泛,包括矿山开采、有色金属冶炼等行业,农药和化肥固体废弃物、农用塑料薄膜,杀虫剂、除草剂和磷酸盐肥料的施放、燃煤、木材保存剂等[5],此外,污水亦是一个主要人为来源,我国耕地污灌污染面积约占总面积的45%[6]。
植物作为初级生产者,是生态系统物质流动的“入口”,而微生物数量众多且分布广泛,参与生物地球化学循环并发挥重要作用。由于重金属污染广泛,为稳定重金属含量并降低其毒性,生物在适应重金属胁迫过程中产生了一系列抗重金属系统,进化出多种抗性特征(趋避、直接排出、减弱生物毒性和降低自身敏感度等)[7]。联合微生物和植物的不同生态结构功能可为重金属污染土壤修复提供可行途径。通过植物在土壤中构成特异性根际系统,直接或间接活化吸收或固化土壤中重金属[8]。植物根际是植物根系与土壤形成的微小生境(根表1~2 mm),在根际微区,与植物发生相互作用的大量微生物被称为根际微生物[9]。利用土壤-微生物-植物的共存关系,充分发挥植物与微生物的作用优势,通过移除或固定化提高重金属污染土壤的修复效率,最终达到削减土壤重金属含量或生物有效性的目的[10]。因此,本文围绕近年来国内外Cd、As污染土壤的微生物-植物联合修复,基于农田土壤及作物Cd、As污染现状分析,重点概述修复机制与效率的研究进展,以期为重金属污染土壤的联合修复提供理论依据和技术参考。
1 农田土壤及作物Cd、As污染现状(Current pollution status of cadmium and arsenic in farmland soils and crops)
据不完全统计,我国农田Cd污染面积达2万hm2,Cd含量超标的农产品年产量达14.6亿kg[11]。我国原农业部2003年的一项调查显示,中国部分地区糙米中Cd含量为0.4~1.0 mg·kg-1,Cd污染糙米量在10%以上[12]。根据《土壤环境质量农用地土壤污染风险管控标准(试行)》(GB 15618—2018),我国农用地土壤Cd浓度标准限值为0.3~0.8 mg·kg-1,土壤As浓度标准限值为20~40 mg·kg-1。依据《食品安全国家标准 食品中污染物限量》(GB 2762—2012),适用新鲜蔬菜的土壤Cd质量标准为≤0.05 mg·kg-1,适用于大米的土壤Cd质量标准为≤0.2 mg·kg-1;适用于谷物、新鲜蔬菜的土壤As质量标准为≤0.5 mg·kg-1。
我国部分地区及其他国家污染农田土壤及农作物Cd含量如表1所示[13]。调查分析我国湖南、云南、贵州和浙江等地区Cd污染较为严重(1.9~30.7 mg·kg-1),青椒、土豆等农作物Cd含量达到国家标准的0.85倍~6.5倍(0.17~3.29 mg·kg-1 vs. 0.2mg·kg-1)。印度、孟加拉和牙买加等国家Cd污染亦较为严重(0.5~13.7 mg·kg-1),黄秋葵、胡萝卜和马铃薯等块茎类、茄果类农作物Cd含量较高(0.4~6.58 mg·kg-1),富集系数(bioconcentration factors, BCF)高达13.4~70(表1),但巴基斯坦、荷兰、牙买加和埃及出现土壤Cd含量与农作物Cd含量呈负相关现象。
表1 我国部分地区及其他部分国家农田土壤及农作物Cd含量
Table 1 Cd concentrations in farmland soils and crops in some regions of China and other countries

中国部分地区Regions of China农作物Crops土壤Cd含量/(mg·kg-1)Soil Cd content/(mg·kg-1)农作物Cd含量/(mg·kg-1)Crop Cd content/(mg·kg-1)Cd富集系数Cd BCF参考文献Reference部分国家Some countries农作物Crops土壤Cd含量/(mg·kg-1)Soil Cd content/(mg·kg-1)农作物Cd含量/(mg·kg-1)Crop Cd content/(mg·kg-1)Cd富集系数Cd BCF参考文献Reference辽宁Liaoning玉米Zea mays0.1040.0870.837[16]甘肃Gansu甘蓝Brassica oleracea0.3640.0100.027[18]印度India菠菜Spinacia oleracea2.35.52.39[17]黄秋葵Abelmoschus moschatus L. Medic.0.074.970[17]上海Shanghai青椒Capsicum annuum0.230.291.24[19]四川Sichuan川芎Ligusticum chuanxiong0.280.762.71[21]孟加拉Bangladesh茄子Solanum melongena11.42.910.25[20]番茄Lycopersicon esculentum Miller.11.42.390.21[20]湖南Hunan黄麻Corchorus capsularis Linn.1.903.291.73[22]浙江Zhejiang黄豆Glycine max Linn. Merr.2.180.350.161[24]巴基斯坦Pakistan胡萝卜Daucus carota Linn. var. sativa Hoffm.0.296.5822.7[23]番茄Lycopersicon esculentum Miller.0.293.8813.4[23]云南Yunnan土豆Solanum tuberosum30.70.260.008[25]广西Guangxi玉米Zea mays0.790.0780.099[27]罗马尼亚Romania胡萝卜Daucus carota Linn. var. sativa Hoffm.9.90.230.02[26]土豆Solanum tuberosum9.90.090.01[26]重庆Chongqing玉米Zea mays5.650.030.005[28]荷兰the Netherlands黄瓜Cucumis sativus Linn.0.50.0030.006[29-30]天津Tianjin小麦Triticum aestivum0.400.0620.155[30]牙买加Jamaica马铃薯Solanum tuberosum13.70.090.007[31]福建Fujian白菜Brassica pekinensis Lour. Rupr.2.110.170.081[32]埃及Egypt玉米Zea mays0.490.010.02[33]贵州贵阳Guiyang, Guizhou茄子Solanum melongena2.611.680.644[34]意大利Italy菠菜Spinacia oleracea Linn.0.150.42.67[35]
注:粗体表示超标含量;BCF表示富集系数。
Note: Bold numbers represent values exceeding the standard limiting values; BCF stands for bioconcentration factors.
我国部分地区及其他国家农田土壤及农作物As污染情况如表2所示[14]。湖南省、云南省个旧、东川As污染严重,土壤中浓度高达63.9~302 mg·kg-1,是我国农用地土壤As标准限值的1.6倍~7.6倍,该地区叶菜类农作物(小苦菜、白菜等)亦表现出较高As含量(1.26~8.86 mg·kg-1),为标准限值(0.5 mg·kg-1)的2.5倍~17.7倍。其他国家As污染差异较大(38~14 800 mg·kg-1),农作物As含量多处于标准值以下,但匈牙利土壤As含量超过标准值,为10~2 000 mg·kg-1,蘑菇As含量高达11.9 mg·kg-1是标准值(0.5 mg·kg-1)的23.8倍。相反,西班牙、澳大利亚和墨西哥土壤As含量高达789~14 800 mg·kg-1,但水稻As含量较低(0.1~0.2 mg·kg-1),这一现象与表1中巴基斯坦、荷兰、牙买加和埃及出现的土壤Cd含量与农作物Cd含量呈负相关现象相似,表明作物中Cd、As含量与土壤Cd、As含量有一定联系,但不完全取决于土壤Cd、As含量,也可能因作物种类、取食部位等而异。总的来说,相较于重金属总量,重金属的生物有效性能更准确地反映可被作物/植物吸收的量[15]。
表2 我国部分地区及其他部分国家农田土壤及农作物As含量
Table 2 As concentration in farmland soils and crops in some regions of China and other countries
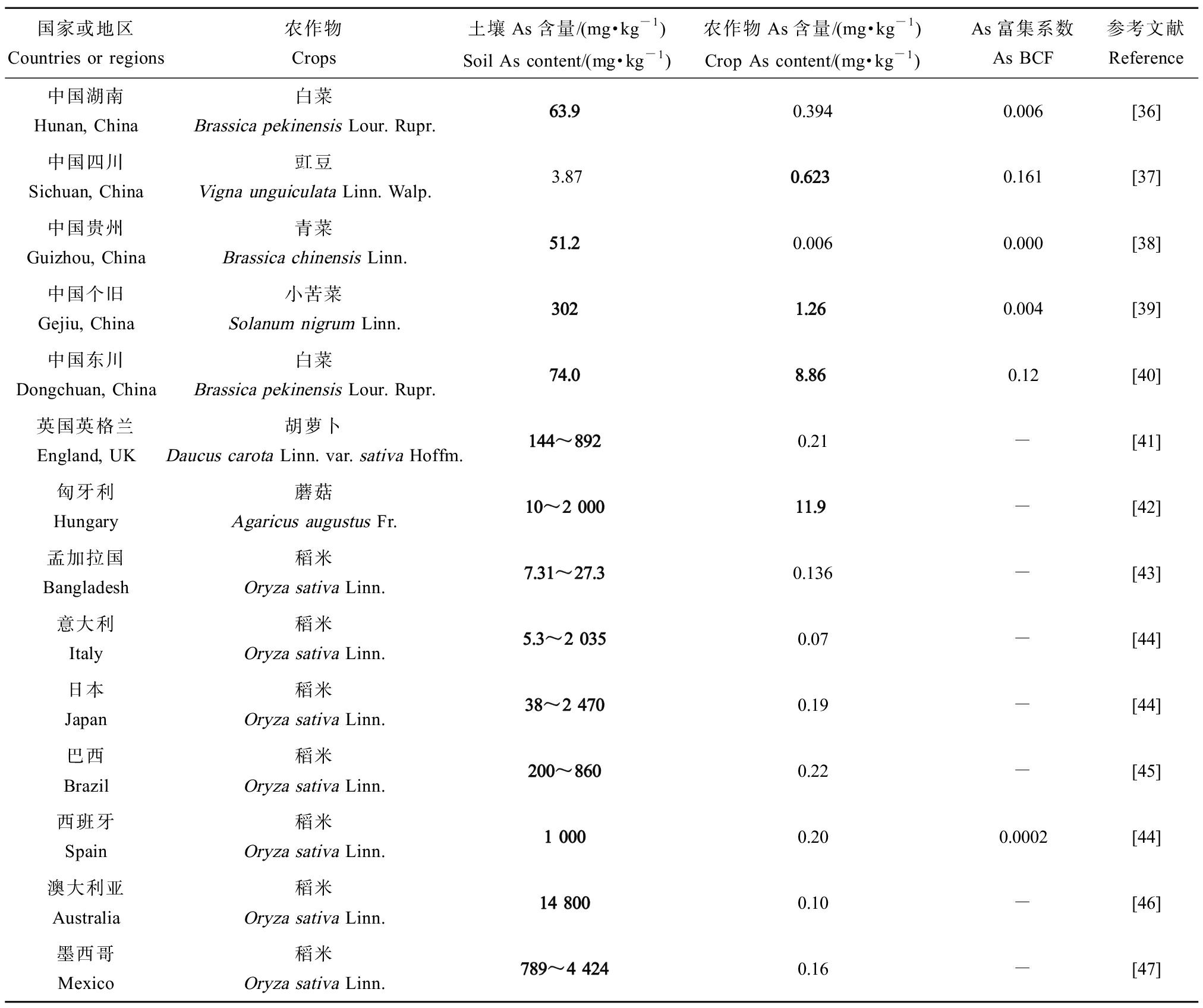
国家或地区Countries or regions农作物Crops土壤As含量/(mg·kg-1)Soil As content/(mg·kg-1)农作物As含量/(mg·kg-1)Crop As content/(mg·kg-1)As富集系数As BCF参考文献Reference中国湖南Hunan, China白菜Brassica pekinensis Lour. Rupr.63.90.3940.006[36]中国四川Sichuan, China豇豆Vigna unguiculata Linn. Walp.3.870.6230.161[37]中国贵州Guizhou, China青菜Brassica chinensis Linn.51.20.0060.000[38]中国个旧Gejiu, China小苦菜Solanum nigrum Linn.3021.260.004[39]中国东川Dongchuan, China白菜Brassica pekinensis Lour. Rupr.74.08.860.12[40]英国英格兰England, UK胡萝卜Daucus carota Linn. var. sativa Hoffm.144~8920.21-[41]匈牙利Hungary蘑菇Agaricus augustus Fr.10~2 00011.9-[42]孟加拉国Bangladesh稻米Oryza sativa Linn.7.31~27.30.136-[43]意大利Italy稻米Oryza sativa Linn.5.3~2 0350.07-[44]日本Japan稻米Oryza sativa Linn.38~2 4700.19-[44]巴西Brazil稻米Oryza sativa Linn.200~8600.22-[45]西班牙Spain稻米Oryza sativa Linn.1 0000.200.0002[44]澳大利亚Australia稻米Oryza sativa Linn.14 8000.10-[46]墨西哥Mexico稻米Oryza sativa Linn.789~4 4240.16-[47]
注:粗体表示超标含量。
Note: Bold numbers represent values exceeding the standard limiting values.
2 微生物-植物联合修复机制(Mechanism of microbe-plant combined remediation)
2.1 修复材料
微生物-植物联合修复技术中微生物主要包括丛枝菌根真菌(arbuscular mycorrhizal fungi, AMF)、内生菌(endophytes)和根际促生菌(plant growth promoting rhizobacteria, PGPR)等。
(1)根际真菌包括腐生和菌根真菌,重金属污染土壤修复中应用较多的为菌根真菌。AMF是菌根真菌中分布最广、与农林生产关系最为密切的一类菌根真菌,它能与植物根系形成共生体,可有效改善植物生长状况,提升植物对重金属的抗逆性、吸收、转运和富集能力,强化重金属的植物提取、根系稳定化过程,进而提高植物修复效率[48]。
(2)内生菌是指生活于健康植物各种组织和器官的细胞间隙或细胞内的细菌或真菌,一般指内生细菌和内生真菌[49],接种内生真菌N. coenophialum不仅可以增强宿主植物(例如,苇状羊茅(Lolium arundinaceum))对重金属的耐受性,促进植物根部生长和吸收、转运Cd,亦可显著增加其生物量,提高植物提取重金属能力及重金属提取修复效率[50]。
(3)PGPR指能够在根际或根表面稳定存活且在根际附近生产和分泌各种物质,直接或间接促生的有益微生物的总称[51],大部分PGPR能够产生生长素、细胞分裂素、赤霉素和乙烯等影响植物生长发育的植物激素,促进侧根和根毛的生长和伸长,增加根系表面积,促进植物对营养和水分的吸收,从而产生促生效果[52]。
用于修复的植物主要为超积累植物,判定标准为地上部分和根部之间的元素浓度比,其中比率>1.0表明污染物主要积聚在地上部分[53]。超积累植物一般具备以下特点:根系具有活化(植物提取)或固定化(植物稳定)重金属的作用;极强的重金属转运能力,可将吸收的重金属转运至地上部,表现为较高的地上部/根浓度比;体内重金属浓度大于一定的临界值,通常为普通植物在同一生长条件下的100倍[54];地上部对积累的重金属具有解毒作用,可在重金属污染土壤生长,且无重金属毒害现象[55]。今已发现的重金属超积累植物已超过500多种[56],目前已发现的Cd超富集植物约80余种,如东南景天(Sedum alfredii)、龙葵(Solanum nigrum)等[57],As超富集植物约10余种,如蜈蚣草(Pteris vittata)、大叶井口边草(Pteris cretica)等[58]。
2.2 修复机制
植物修复利用一种或几种植物来修复受污染的土壤,灵活且具有成本效益,并被归类为环境友好型方法,因降低污染物扩散的风险,并通过避免挖掘受污染的场地来保护原始生态型,可考虑在全球范围大力实施[59-60]。去除重金属的3种主要植物修复机制是植物稳定、植物提取和植物挥发。植物稳定限制了重金属进入食物链的流动性;植物提取是从土壤中吸附重金属并积聚在植物组织中;植物挥发是利用植物的吸收、积累和挥发而减少土壤中一些挥发性污染物,即植物将污染物吸收到体内后将其转化为气态物质,并以毒性较小或无害的形式释放到大气中。同时,这些机制可能会受到多种因素的影响,例如植物物种、介质的性质、金属的生物利用度以及螯合剂的添加等[61-62]。
微生物自身的解毒机理分生物吸附和生物积累2步进行,微生物累积过程又可分为胞外富集或沉淀,胞内富集、细胞表面吸附、络合。当重金属被吸收转运至细胞内,微生物通过区隔化作用将其转运到代谢不活跃区域(如液泡)封闭起来,或将金属离子和热稳定蛋白结合,使其转变为低毒形态。
微生物-植物联合修复中,微生物能够在重金属胁迫下诱导植物抗氧化防御系统、金属电阻/封存系统启动,以抵御有氧胁迫对植物的危害,而抗氧化防御系统能力增强可提高植物对重金属的耐受性,加强植物重金属抗性来降低植物毒性效应。植物修复效率在很大程度上依赖于重金属的生物有效性,因此可利用根际细菌、内生菌及菌根真菌作用改变重金属形态与价态,活化土壤重金属增强植物的吸收积累[63],不仅如此,其分泌的螯合物还可与植物体内重金属结合,改变重金属在植物体内的存在形态,促进重金属向地上部的转运[64-65],由此提高植物的修复效率(图1)。
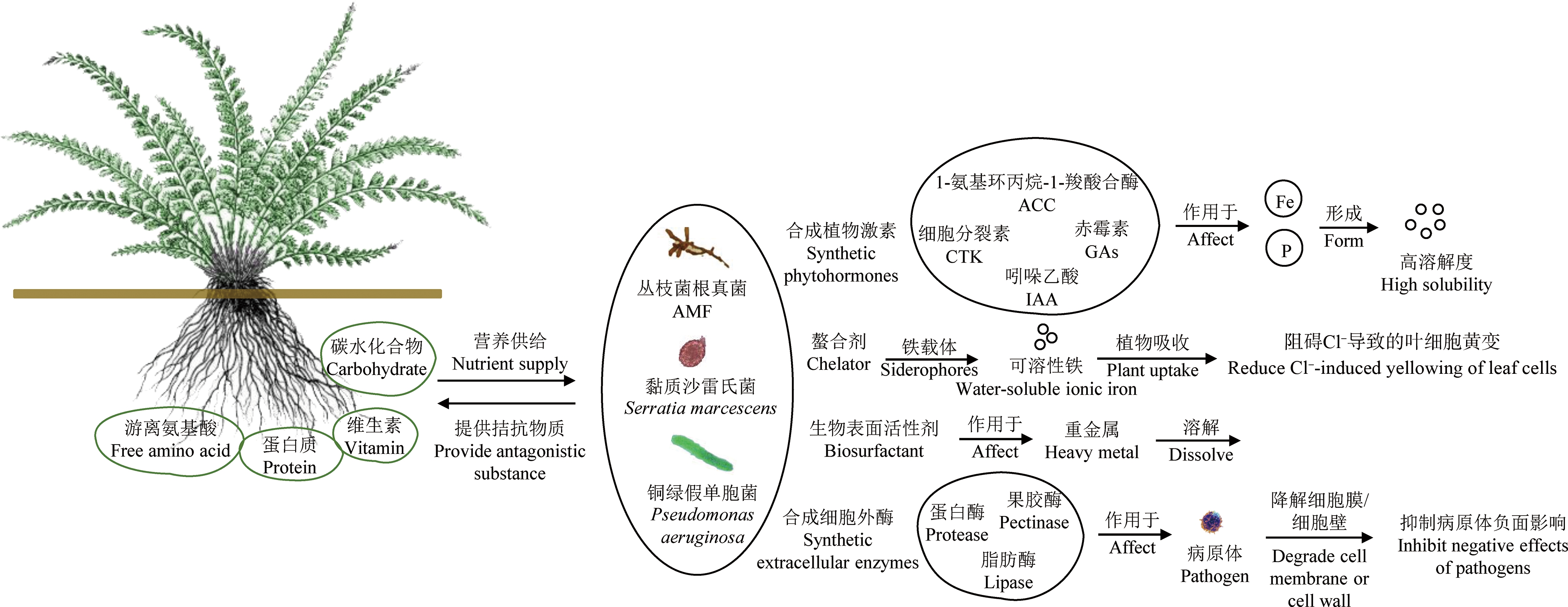
图1 植物-微生物联合修复作用机制
注:AMF为丛枝菌根真菌;ACC为1-氨基环丙烷-1-羧酸合酶;CTK为细胞分裂素;GAs为赤霉素;IAA为吲哚乙酸。
Fig. 1 The mechanism of microbe-plant combined remediation
Note: AMF means arbuscular mycorrhizal fungi; ACC means 1-aminocyclopropane-1-carboxylate synthase;
CTK means cytokinin; GAs means gibberellins; IAA means indoleacetic acid.
2.2.1 微生物及植物重金属抗性机制
微生物-植物联合修复中,关于功能性微生物菌种、微生物解毒机理和植物抗性的过程机理等已有较多报道[66-67]。菌根真菌菌丝外表面的松结合态黏液、真菌分泌物(摩西球囊霉等)、真菌细胞壁和原生质膜组分(壳聚糖、黑色素、纤维素及其衍生物等)均能吸附或螯合重金属,其中壳聚糖对外源重金属的吸附/螯合率高达90%,重金属大量固定吸收积累在植物根及真菌中,由此降低重金属对植物的毒害作用,提高植物金属抗性[68-70]。
超富集植物具有极强的重金属富集能力归因于其特有的重金属耐受、吸收、转运和解毒机制,Cd可通过与三叶鬼针草(Bidens pilosa)体内小分子有机酸和植物螯合肽等物质结合及细胞壁吸附和液泡隔离等方式降低细胞质中游离金属离子浓度,降低金属离子毒性,通过高效解毒提高植物的重金属抗性[71],同时激活抗氧化酶抵抗重金属毒性,包括过氧化氢酶(catalase, CAT)、过氧化物酶(peroxidase, POD)、超氧化物歧化酶(superoxide dismutase, SOD)、抗坏血酸过氧化物酶(aseorbate peroxidase, APX)和谷胱甘肽还原酶(glutathione reductase, GR)等[72]。抗氧化酶可清除重金属毒性所产生的活性氧(reactive oxygen species, ROS),增强植物对重金属诱导的氧化应激的耐受性,抗氧化酶大多是电子供体,可与自由基反应,ROS在与其特定位点结合后将转化为无毒和无活性产物(H2O等)[73]。另外,Cd处理诱导龙葵(Solanum nigrum)大量产生ROS和积累硫代巴比妥酸反应物质(thiobarbituric acid-reacting substances, TBARS),同时根系抗氧化酶CAT和SOD活性分别增加57%和38%,植物非蛋白巯基(NPT)总量提高2.4倍,半胱氨酸(Cys)和谷胱甘肽(GSH)分别被消耗34.8 mol·g-1和13.32 mol·g-1[74]。豨莶(Siegesbeckia orientalis L.)在Cd胁迫下通过上调热激蛋白70基因、重金属耐受蛋白基因和捕光叶绿素蛋白前体基因等,提高Cd的耐受性,有效抵御氧化损失,此外,通过天然抗性巨噬细胞蛋白家族(NRAMP)调控Cd跨膜运输,ABC转运蛋白家族、P型-ATPase调控Cd在液泡中的区隔化,提高对Cd的解毒能力[75]。芥菜(Brassica juncea L.)在160 mmol·L-1 Cd水培处理下,GR活性提高3倍,非蛋白巯基化合物(non-protein thiols, NP-SH)、GSH含量的增加和GR的高活性使Brassica juncea L.对Cd的抗性增强[76]。
As通过产生各种ROS在植物中诱导氧化应激,引起运输、代谢过程变化和抑制生长等一系列反应[77],因此激发抗氧化系统被认为是植物响应As等重(类)金属胁迫的重要机制[78-79],而SOD在As超富集植物蜈蚣草(P. vittata)和井栏边草(P. multifida)中As积累和解毒中发挥重要作用[80]。P. vittata等As超富集植物可高效消除As暴露产生的氧化胁迫,As对细胞产生氧化胁迫,形成ROS而毒害细胞。用As处理P. vittata时,其体内非酶类抗氧化物和抗氧化酶等均随As浓度的升高而增加[81],表明As暴露可对P. vittata产生氧化胁迫。对比超富集植物P. vittata与非超富集植物剑叶凤尾蕨(P. ensiformis)对As的胁迫响应,发现非超富集植物P. ensiformis体内抗氧化酶活性显著低于超富集植物P. vittata,且膜脂过氧化产物丙二醛(malondialdehyde, MDA)和TBARS浓度显著高于P. vittata,表明超富集植物具有较强的抗氧化胁迫能力[82]。水培体系中,P. multifida体内As(Ⅲ)占比高达33%,可能是将As(Ⅲ)通过水/甘油转运蛋白运输、储存和隔离在液泡中以降低其对植物的毒性[83]。土培实验中,随土壤As含量增加,P. vittata和P. multifida根/叶片砷酸盐还原酶(arsenate reductase, AR)活性增加,P. vittata根/叶片SOD活性显著增加,表明AR和SOD在As积累和解毒方面具有重要作用[80]。另外,欧洲凤尾蕨(Pteris cretica)中PcACR2和PcACR3基因编码的功能蛋白可在As胁迫下执行As(Ⅴ)还原和As(Ⅲ)跨膜转运,且转录水平与非超富集植物相比分别增加了6.5倍和45倍,表明PcACR2和PcACR3的转录明显具有As响应性[84]。
2.2.2 微生物-植物共生机制
植物在重金属污染土壤生长过程中伴生数量庞大、种类繁多的可耐受高浓度重金属的微生物,其进化过程中产生了重金属耐性,并与植物产生协同效应[64],这一现象可归因于菌根共生体形成的物理性防御体系和产生的生化拮抗物质,以及通过改善宿主植物的营养状况、改变植物根系形态和改变根际环境的理化情况等途径调控植物的生理代谢过程[85]。在协同与共生作用下,菌根、内生菌与根系形成共生体,通过改变土壤pH、释放螯合剂及诱导氧化还原反应改变重金属的形态和生物有效性,强化植物修复过程[85],促进根系发育生长,提高抗毒能力、存活率和生长率,加强植物根系对重金属的吸收和向植物地上部的转运。
从Cd超积累植物东南景天(S. alfredii)体内分离的鞘氨醇单胞菌(Sphingomonas sp.)SaMR12能够分泌大量吲哚乙酸(IAA)[86],增加植物根长及根表面积,分泌有机酸络合Cd,提高对Cd的耐受能力,上调天然抵抗相关巨噬细胞蛋白(Nramp)、重金属ATP酶(HMA)等家族基因的表达和对Cd的吸收[87]。同时,As超富集植物蜈蚣草(P. vittata)内生菌芽孢杆菌(Bacillus sp.)、类芽孢杆菌(Paenibacillus sp.)等亦能分泌IAA来提高植物对As耐性[88],其地上部寄生的抗As细菌(AsRB1)可将As(Ⅴ)还原成As(Ⅲ)[89]。丛枝菌根真菌(Glomus mosseae)对P. vittata根系的侵染率约为50%,显著提高P. vittata地上部生物量[90]。
目前,关于微生物对植物重金属抗性与解毒机理的研究主要集中在运用植物激素、有机酸等提高重金属抗性的作用,在分子水平上对其抗性机制的研究已有报道,研究表明一些细菌质粒中含有Hg2+、Cd2+、Cu2+、Ag+、Co2+和Pb2+等抗性基因,如在汞抗性系统中含有汞还原酶基因,可将有毒的汞离子转换为毒性较小的挥发性金属汞[91]。
2.2.3 土壤重金属活化机制
一般植物在吸收非必需金属元素时均会表现出一定的中毒症状,但超富集植物可以吸收数倍甚至数百倍于普通植物所能吸收的量,解释这种现象的可能机制是超积累植物能够活化根际土壤中重金属[92]。微生物可分泌氨基酸、有机酸等代谢产物,溶解重金属化合物和矿物,提高金属元素的生物有效性,促进植物根系对重金属的吸收[65]。生物表面活性剂可通过与土壤液相中的游离金属离子络合、降低界面张力使土壤中重金属离子与表面活性剂直接接触2种方式促进对土壤中重金属的吸收[93]。根系分泌物中低分子量有机酸(如羧酸、酚酸等),可通过酸化作用降低根际土壤pH、改变土壤氧化还原电位及重金属络合作用,促进土壤重金属溶解、活化,进而促进植物吸收[94]。根系分泌物的分泌机制主要包括:根内分泌物合成与代谢、细胞质膜阴离子通道启动和促进胞内有机阴离子物质外排,该过程受环境胁迫影响,研究表明,重金属胁迫可诱导植物有机酸分泌增强,进而促进土壤重金属活化[95]。
研究发现,东南景天(S. alfredii)可能通过诱导根系分泌癸酸、苯甲酸、月桂酸、壬酸、果糖、赤藓糖醇、羟基乙酸、甘露醇、海藻糖、核糖醇和磷酸等活化Cd促进植物吸收,亦可通过降低有机酸分泌减少Cd活化以提高植物耐受力,表明S. alfredii通过改变根系分泌物的组成或含量来耐受或吸收Cd[96]。
蜈蚣草(P. vittata)具有独特稳定的植酸酶,使其能够在低磷土壤环境中获取磷,除了对缺磷土壤的自然适应性外,包括植酸酶在内的磷酸酶可有效地从植酸盐和其他有机磷中水解无机磷,与As进行竞争吸收,提高对As的抗性[97]。As胁迫下,As超富集植物P. vittata、Pteris multifida等蕨类植物主要根系分泌物植酸和草酸的占比达93%以上,而在其他植物根系分泌物未检测出植酸,表明植酸是蕨类植物特异性根系分泌物。As胁迫使蕨类植物植酸分泌量增加,P. vittata植酸分泌量比非超富集植物剑叶凤尾蕨(P. ensiformis)高262%,植酸含有6个磷酸根基团和12个可解离质子,与Fe有较强的配位能力,从而促进根际FeAsO4溶解,有效增强P. vittata吸收Fe和As[98]。
2.2.4 叶细胞重金属解毒机制
液泡隔离是植物维持体内重金属稳态、降低毒性的重要方式。液泡中的硫肽和有机酸可与重金属离子螯合,降低游离金属离子活性,降低其对细胞器的毒性[99]。例如,As超积累植物P. vittata根系可将As(Ⅴ)还原为As(Ⅲ),经木质部被高效转运至地上叶片(木质部As(Ⅲ)转运速率是As(Ⅴ)的近9倍)[100],而后通过谷氧还蛋白基因GRX5直接或间接与液泡膜跨膜蛋白作用,将As(Ⅲ)转运至液泡[101],通过液泡隔离降低As毒性。另外,金属硫蛋白(metallothionein, MTS)是一类普遍存在具有结合金属能力和高诱导性蛋白质,当拟南芥金属硫蛋白基因At MT2a和At MT3在蚕豆中表达时,可降低Cd对保卫细胞的毒性作用,避免叶绿体降解[102],植物感应Cd胁迫后,可产生植物螯合肽合成酶(phytochelatin synthase, PCS)促进植物螯合肽(phytochelatin, PC)的形成,螯合肽捕获Cd并形成低毒螯合物,将其隔离在液泡中[103]。此外,细胞壁果胶被认为是将Cd阻滞在细胞壁的关键物质[104],西红柿(Solanum lycopersicum)细胞壁果胶含量及果胶甲酯酶(pectin methylesterase, PME)活性与细胞壁Cd累积量呈正相关[105]。
3 联合修复技术应用效率与限制因素(Efficiency and influencing factors of combined remediation)
3.1 应用效率
植物/微生物在重金属胁迫条件下可诱导抗氧化防御系统降低重金属的毒性作用,提高植物/微生物对重金属的耐受性,以此提高微生物对重金属的活化/钝化和植物对重金属的吸收富集[90]。
一些特定的微生物可以明显提高植物对于土壤中重金属污染物的去除效率[106]。绿脓杆菌(Pseudomonas aeruginosa)的添加可能使污染土壤中的Ca2+、Mg2+等离子浸出,并对植物吸收Cd产生竞争,降低水稻籽粒Cd含量,增加干质量和籽粒中矿质养分的积累[107],同时具有溶解磷酸盐产生生长素的作用[108]。印度芥菜(Brassica juncea)根系分泌的特殊物质,可专一性地溶解根系土壤难溶态Cd,接种25 mL菌液后,Brassica juncea地上、地下部Cd含量达到21.1 mg·kg-1和8.76 mg·kg-1,同时土壤Cd降低4.43%[109]。接种巨大芽孢杆菌(Bacillus megateriumde Bary)使得伴矿景天(Sedum plumbizincicola)地上部和地下部生物量分别提高8.7%~66.7%和13.6%~81.8%,且地上部Cd含量提高29.2%~60.4%,土壤Cd去除率26.7%~42.9%[110]。张云霞等[111]发现鬼针草(B. pilosa)对Cd表现出稳定积累特性和较强富集能力,鬼针草修复Cd污染土壤的Cd去除率为12.9%~18.6%。从污染土中筛选出的丝核菌(Rhizoctonia sp.)可产生类似赤霉素的活性物质,在一定程度上可促进超积累植物P. vittata生长,减轻P. vittata根系质膜损伤,提高其生物量和对As的吸收能力,且促进As从地下部向地上部转运,接种后P. vittata地上部、地下部As含量高达4 916 mg·kg-1和1 207 mg·kg-1[112]。与未接种的对照组相比,接种内生菌短小芽孢杆菌(B. pumilus)E2S2显著增加了东南景天(S. plumbizincicola)中Cd的积累,增加了43%[113]。接种卷边网褶菌(Paxillus involutus)可使毛枝柳(Salix dasyclados)茎中Cd总含量显著增加,从每株植物0.89 g增至1.08 g,增量高达22%,与非生物增强土壤相比,Paxillus involutus的可萃取Zn、Cd、Pb和Cu的浓度分别提高了1.33倍、1.22倍、1.33倍和1.11倍[114]。
对近几年研究运用微生物-植物联合修复Cd、As污染农田土壤的部分应用案例进行了系统归纳总结,如表3所示。
表3 微生物-植物联合修复Cd、As污染土壤应用
Table 3 Application of microbial-plant combined remediation of Cd and/or As contaminated soils
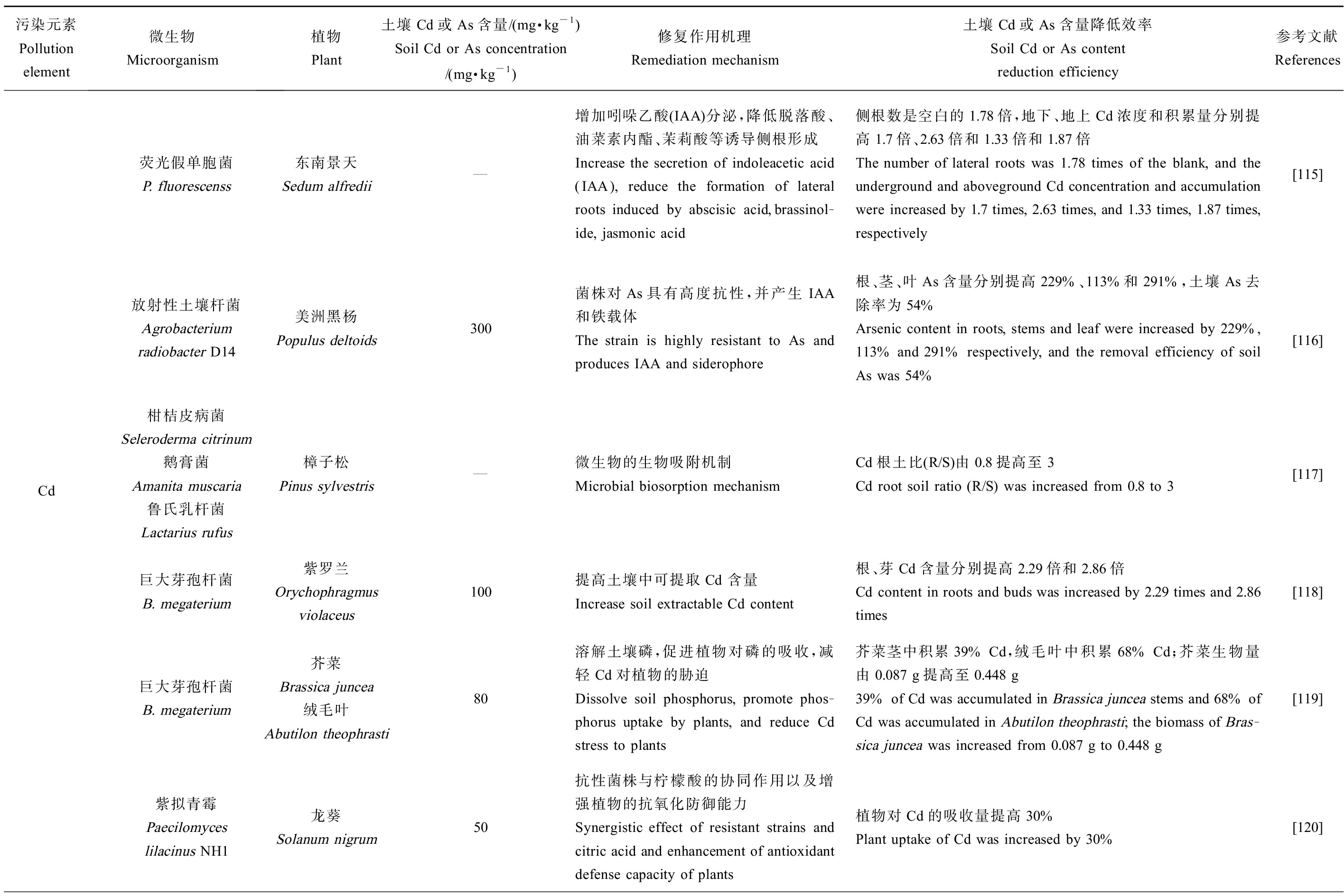
污染元素Pollution element微生物Microorganism植物Plant土壤Cd或As含量/(mg·kg-1)Soil Cd or As concentration/(mg·kg-1)修复作用机理Remediation mechanism土壤Cd或As含量降低效率Soil Cd or As content reduction efficiency参考文献ReferencesCd荧光假单胞菌P. fluorescenss放射性土壤杆菌Agrobacterium radiobacter D14柑桔皮病菌Seleroderma citrinum鹅膏菌Amanita muscaria鲁氏乳杆菌Lactarius rufus巨大芽孢杆菌B. megaterium巨大芽孢杆菌B. megaterium紫拟青霉Paecilomyces lilacinus NH1东南景天Sedum alfredii—增加吲哚乙酸(IAA)分泌,降低脱落酸、油菜素内酯、茉莉酸等诱导侧根形成Increase the secretion of indoleacetic acid (IAA), reduce the formation of lateral roots induced by abscisic acid,brassinol-ide, jasmonic acid侧根数是空白的1.78倍,地下、地上Cd浓度和积累量分别提高1.7倍、2.63倍和1.33倍和1.87倍The number of lateral roots was 1.78 times of the blank, and the underground and aboveground Cd concentration and accumulation were increased by 1.7 times, 2.63 times, and 1.33 times, 1.87 times, respectively[115]美洲黑杨Populus deltoids300菌株对As具有高度抗性,并产生IAA和铁载体The strain is highly resistant to As and produces IAA and siderophore根、茎、叶As含量分别提高229%、113%和291%,土壤As去除率为54%Arsenic content in roots, stems and leaf were increased by 229%, 113% and 291% respectively, and the removal efficiency of soil As was 54%[116]樟子松Pinus sylvestris—微生物的生物吸附机制Microbial biosorption mechanism Cd根土比(R/S)由0.8提高至3Cd root soil ratio (R/S) was increased from 0.8 to 3[117]紫罗兰Orychophragmus violaceus100提高土壤中可提取Cd 含量Increase soil extractable Cd content 根、芽Cd含量分别提高2.29倍和2.86倍Cd content in roots and buds was increased by 2.29 times and 2.86 times[118]芥菜Brassica juncea绒毛叶Abutilon theophrasti80溶解土壤磷,促进植物对磷的吸收,减轻Cd对植物的胁迫Dissolve soil phosphorus, promote phos-phorus uptake by plants, and reduce Cd stress to plants芥菜茎中积累39% Cd,绒毛叶中积累68% Cd;芥菜生物量由0.087 g提高至0.448 g39% of Cd was accumulated in Brassica juncea stems and 68% of Cd was accumulated in Abutilon theophrasti; the biomass of Bras-sica juncea was increased from 0.087 g to 0.448 g[119]龙葵Solanum nigrum50抗性菌株与柠檬酸的协同作用以及增强植物的抗氧化防御能力Synergistic effect of resistant strains and citric acid and enhancement of antioxidant defense capacity of plants植物对Cd的吸收量提高30%Plant uptake of Cd was increased by 30%[120]
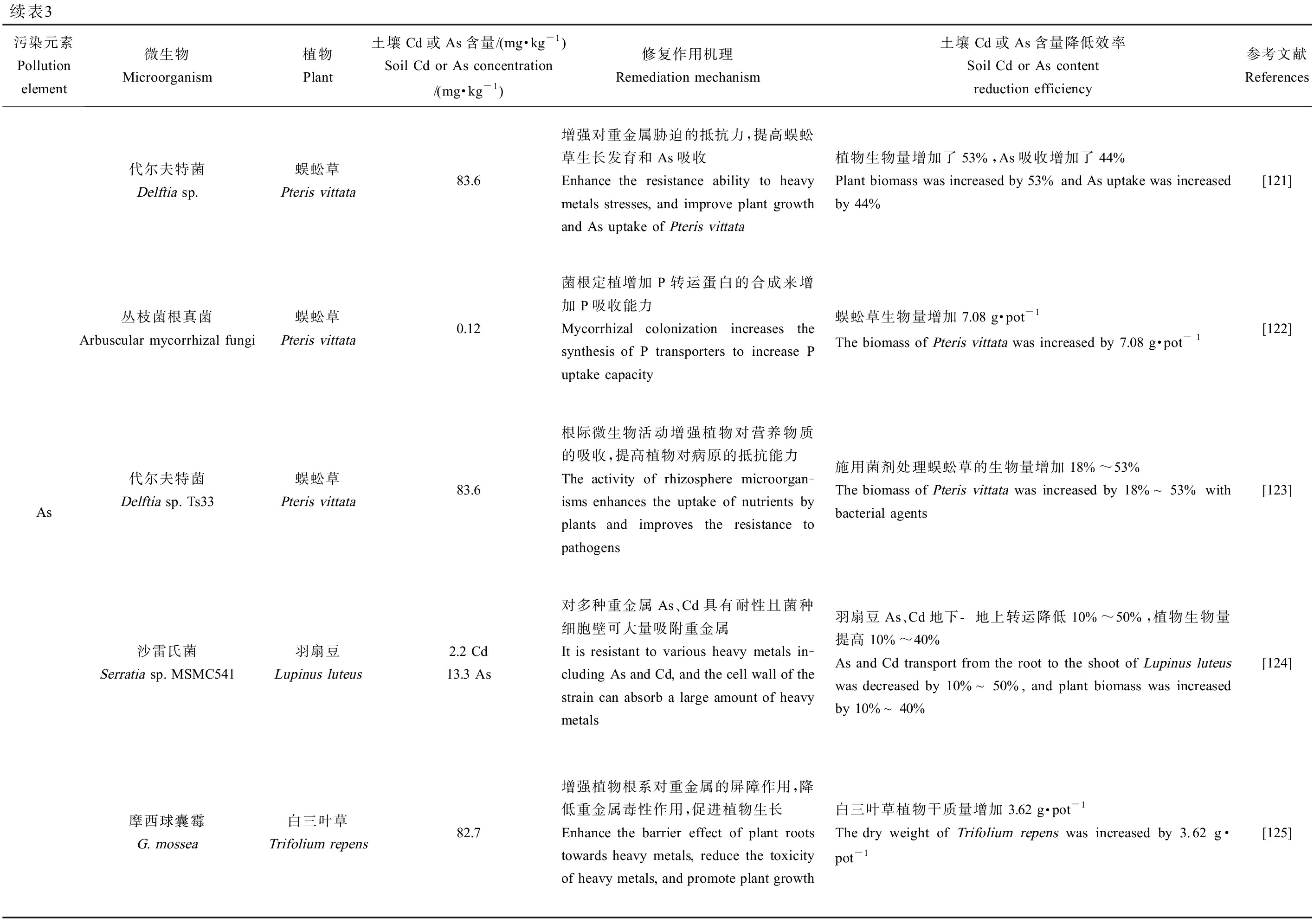
续表3污染元素Pollution element微生物Microorganism植物Plant土壤Cd或As含量/(mg·kg-1)Soil Cd or As concentration/(mg·kg-1)修复作用机理Remediation mechanism土壤Cd或As含量降低效率Soil Cd or As content reduction efficiency参考文献ReferencesAs代尔夫特菌Delftia sp.蜈蚣草Pteris vittata83.6增强对重金属胁迫的抵抗力,提高蜈蚣草生长发育和As吸收Enhance the resistance ability to heavy metals stresses, and improve plant growth and As uptake of Pteris vittata植物生物量增加了53%,As吸收增加了44%Plant biomass was increased by 53% and As uptake was increased by 44%[121]丛枝菌根真菌Arbuscular mycorrhizal fungi蜈蚣草Pteris vittata0.12菌根定植增加P转运蛋白的合成来增加P吸收能力Mycorrhizal colonization increases the synthesis of P transporters to increase P uptake capacity蜈蚣草生物量增加7.08 g·pot-1The biomass of Pteris vittata was increased by 7.08 g·pot - 1[122]代尔夫特菌Delftia sp. Ts33蜈蚣草Pteris vittata83.6根际微生物活动增强植物对营养物质的吸收,提高植物对病原的抵抗能力The activity of rhizosphere microorgan-isms enhances the uptake of nutrients by plants and improves the resistance to pathogens施用菌剂处理蜈蚣草的生物量增加18%~53%The biomass of Pteris vittata was increased by 18%~53% with bacterial agents[123]沙雷氏菌Serratia sp. MSMC541羽扇豆Lupinus luteus2.2 Cd13.3 As对多种重金属As、Cd具有耐性且菌种细胞壁可大量吸附重金属It is resistant to various heavy metals in-cluding As and Cd, and the cell wall of the strain can absorb a large amount of heavy metals羽扇豆As、Cd地下-地上转运降低10%~50%,植物生物量提高10%~40%As and Cd transport from the root to the shoot of Lupinus luteus was decreased by 10%~50%, and plant biomass was increased by 10%~40%[124]摩西球囊霉G. mossea白三叶草Trifolium repens82.7增强植物根系对重金属的屏障作用,降低重金属毒性作用,促进植物生长Enhance the barrier effect of plant roots towards heavy metals, reduce the toxicity of heavy metals, and promote plant growth白三叶草植物干质量增加3.62 g·pot-1The dry weight of Trifolium repens was increased by 3.62 g·pot-1[125]
3.2 限制因素
3.2.1 土壤理化性质
植物、微生物生存均需特定的适宜环境条件范围,超出或低于均会影响生存,从而导致土壤pH值、水分和温度等对联合修复效率具有一定程度的影响。当pH<5时,植物和微生物活性均受抑制[10],在土壤pH=3极酸条件下,多年生黑麦草(Lolium perenne)和蒲公英(Taraxacum mongolicum)种子无法萌发,影响植株生长和Cd吸收富集能力,当土壤pH由5.5降至4.5时,Lolium perenne地上部、地下部Cd含量分别降低12 mg·kg-1和106 mg·kg-1,Cd富集系数显著降低[126]。一些常见微生物如摩西球囊霉(Glomus mosseae)适宜中性或略碱性(pH 5.8~6.8)土壤环境,珍珠巨孢囊霉(Gigaspora margarita)适宜微酸性(pH 4.3~5.1)土壤条件。此外,不同AMF对pH的适应范围也不尽相同,如球囊霉属(Glomus)多出现在pH=5~9土壤中,而无梗囊霉(Acaulospora)、巨孢囊霉属(Gigaspora)和盾巨孢囊霉属(Scutellospora)则主要出现在pH<7土壤中[127]。
3.2.2 土壤重金属形态与生物有效性
重金属的吸附解吸、溶解沉淀和氧化还原平衡等决定着土壤溶液中重金属浓度。在一定条件下,吸附态和沉淀态可相互转换,通过降低pH使吸附态重金属解吸进入土壤溶液,从而增加植物对重金属的吸收积累。植物对Cd的敏感性可能与Cd在土壤中主要以可交换态和有机质结合态形式存在有关,其结合力较弱,Cd易释放至土壤溶液,增加土壤生物有效态Cd含量[15]。As则与其相反,在土壤中以阴离子形式存在,提高pH使土壤颗粒表面负电荷增加,可减弱As的吸附作用,提高土壤溶液中有效态As含量,促进植物As吸收[128]。
3.2.3 根际微生物与修复植物种类
植物类型影响根际微生物群落结构,根系脱落物、分泌物等物质是维持根际微生物数量和活性的重要因素[129]。根际微生物通过定殖、竞争、共生和调节土壤营养动态等活动影响植物生长,不同微生物在植物根际的功能不同[130]。主要体现在根际细菌通过改变重金属价态及赋存形态(络合态、脱烷基)来降低重金属毒性;根际真菌通过辅助宿主植物获取矿质营养[131],提高寄主植物生物量。同时,外部因素对根际微生物产生影响,通过根际微生物影响植物[132]。例如,棉花枯萎孢子萌发实验中,在抗病健株根分泌物作用下枯萎孢子萌发率为22.4%~26.3%,而在感病健株根分泌物中孢子萌发率为34.9%~57.7%,说明感病健株根分泌物可刺激孢子萌发[133],而根分泌物中的糖类、氨基酸可能作为营养源,通过影响根际微生物的种类、数量及分布,提高微生物种群的数量,间接制约病原微生物的生长[134]。另外,植物种类不同,根际微生物的多样性和优势种有显著差异[135],重金属土壤中根际细菌的群落结构会因为植物的种植而发生改变,同时根际细菌群落的相互作用也会影响植物的多样性与修复效果。不同As浓度下,考察荆(Cirsium arvense)和簇毛草(Deschampsia caespitosa)的根系异养细菌数量,由EUB338探针混合物估计的细菌数量D. caespitosa显著高于C. arvense样品中,分别为7.27 logCFU·g-1和6.29 logCFU·g-1 [135]。
4 结论与展望(Conclusion and prospect)
近年来,微生物-植物联合修复Cd、As污染土壤的研究与应用因其成本低、对土壤和环境的破坏较小以及适用于多金属污染场地等优点,前期关于植物根际细菌已有较多文献报道,植物内生菌可更高效提高污染土壤的植物修复效率,但其在重金属污染农田土壤修复的实际应用较少。目前,一些低累积农作物品种-微生物的协同作用和低累积农作物品种替代种植等“边产出边修复”的模式不仅可维持农田土壤的种植价值,亦可控制Cd、As等重金属的食品安全风险,但同时针对不同重金属污染物种类、污染程度和栽培方式等情况筛选高抗、低累积作物品种亦是亟待解决的问题。
现有关于微生物-植物联合修复Cd、As污染土壤的研究报道多为实验室研究成果,农田应用相对较少。这是由于田间条件通常极端且恶劣,细菌接种物一般只能存活数小时到数天;各超富集植物对重金属耐受性不同,导致大规模任务需要农业设备的经验和专业知识,且完全修复场地所需的时间相对较长等,这一系列问题阻碍了农田修复的有效实施。
农田土壤重金属污染复杂多元,污染来源多样且具有协同效应,修复过程困难、对象单一、效率较低。现有修复技术在Cd、As污染土壤治理中已取得一定发展,但面临农田土壤Cd、As污染的严峻形势,可从多手段联合修复角度开展深入研究,具体包括:
(1)进一步筛选根际抗性促生微生物和高效植物,建立微生物、植物资源库,优化微生物-植物联合修复配置组合,挖掘微生物和植物互作机制及协同机制,结合重金属污染结构、污染特征、污染程度,准确、高效、针对性修复重金属污染。
(2)以现有微生物-植物联合修复技术为基础发展微生物-植物-改良剂、复合微生物-植物等技术,联合多种修复技术实现重金属复合污染修复并进行实际应用。
(3)根际微生物多样且大量不可培养微生物有待挖掘,可充分利用现代分子生物学,通过基因筛选、分离、克隆和编辑等手段,加强微生物遗传改良,为获得具有多种重金属抗性的微生物增加可能性。
(4)目前大量农田因农药、化肥的大量使用使土壤肥力下降,理化性质破坏严重,在修复方法实际应用中,结合植物物种和微生物对生态环境的适应性,研究不同组合方式联合修复在不同生态环境的实际应用效果,因地制宜达到修复目的。
[1] 王帆宇. 新时期中国社会转型进程中的生态文明建设研究[D]. 苏州: 苏州大学, 2016: 1-298
Wang F Y. Study on the construction of ecological civilization in the process of transformation of China society in the new period [D]. Suzhou: Soochow University, 2016: 1-298 (in Chinese)
[2] 李婧, 周艳文, 陈森, 等. 我国土壤镉污染现状、危害及其治理方法综述[J]. 安徽农学通报, 2015, 21(24): 104-107
Li J, Zhou Y W, Chen S, et al. Actualities, damage and management of soil cadmium pollution in China [J]. Anhui Agricultural Science Bulletin, 2015, 21(24): 104-107 (in Chinese)
[3] 黄毅, 邓志英. 我国重金属污染区耕地轮作休耕存在的问题及对策——以湖南省为例[J]. 环境保护, 2019, 47(13): 22-26
Huang Y, Deng Z Y. Problems and countermeasures on farmland rotation and fallow system in the heavy metal polluted region of China [J]. Environmental Protection, 2019, 47(13): 22-26 (in Chinese)
[4] 纪冬丽, 孟凡生, 薛浩, 等. 国内外土壤砷污染及其修复技术现状与展望[J]. 环境工程技术学报, 2016, 6(1): 90-99
Ji D L, Meng F S, Xue H, et al. Situation and prospect of soil arsenic pollution and its remediation techniques at home and abroad [J]. Journal of Environmental Engineering Technology, 2016, 6(1): 90-99 (in Chinese)
[5] 陈文艳, 耿庆芬, 王燕, 等. 重金属污染土壤的植物修复及植物联合修复研究进展[J]. 广东化工, 2020, 47(2): 87-88, 95
Chen W Y, Geng Q F, Wang Y, et al. Review in phytoremediation and phytoremediation of heavy metal contaminated soils [J]. Guangdong Chemical Industry, 2020, 47(2): 87-88, 95 (in Chinese)
[6] 王维薇, 林清. 国内外土壤镉污染及其修复技术的现状与展望[J]. 绿色科技, 2017(4): 90-93, 102
Wang W W, Lin Q. Present situation and prospect of soil cadmium pollution and remediation technology at home and abroad [J]. Journal of Green Science and Technology, 2017(4): 90-93, 102 (in Chinese)
[7] 钱前, 瞿礼嘉, 袁明, 等. 2012年中国植物科学若干领域重要研究进展[J]. 植物学报, 2013, 48(3): 231-287
Qian Q, Qu L J, Yuan M, et al. Research advances on plant science in China in 2012 [J]. Chinese Bulletin of Botany, 2013, 48(3): 231-287 (in Chinese)
[8] 倪妮, 宋洋, 王芳, 等. 多环芳烃污染土壤生物联合强化修复研究进展[J]. 土壤学报, 2016, 53(3): 561-571
Ni N, Song Y, Wang F, et al. A review of researches on intensified bio-remediation of polycyclic aromatic hydrocarbons contaminated soils [J]. Acta Pedologica Sinica, 2016, 53(3): 561-571 (in Chinese)
[9] 刘京伟, 李香真, 姚敏杰. 植物根际微生物群落构建的研究进展[J]. 微生物学报, 2021, 61(2): 231-248
Liu J W, Li X Z, Yao M J. Research progress on assembly of plant rhizosphere microbial community [J]. Acta Microbiologica Sinica, 2021, 61(2): 231-248 (in Chinese)
[10] 王梦姣, 杨国鹏, 乔帅, 等. 植物-根际微生物协同修复有机物污染土壤的研究进展[J]. 江苏农业科学, 2017, 45(1): 5-8
Wang M J, Yang G P, Qiao S, et al. Research progress of plant-rhizosphere microbe synergistic remediation of organic contaminated soil [J]. Jiangsu Agricultural Sciences, 2017, 45(1): 5-8 (in Chinese)
[11] 刘洋, 张玉烛, 方宝华, 等. 栽培模式对水稻镉积累差异及其与光合生理关系的研究[J]. 农业资源与环境学报, 2014, 31(5): 450-455
Liu Y, Zhang Y Z, Fang B H, et al. Relationships between cadmium uptake characteristics and photosynthetic physiology under different cultivation modes of rice [J]. Journal of Agricultural Resources and Environment, 2014, 31(5): 450-455 (in Chinese)
[12] Li J R, Xu Y M. Immobilization of Cd in paddy soil using moisture management and amendment [J]. Environmental Science and Pollution Research International, 2015, 22(7): 5580-5586
[13] 董萌, 赵运林, 周小梅, 等. 土壤镉污染现状与重金属修复方法研究[J]. 绿色科技, 2012(4): 212-215
Dong M, Zhao Y L, Zhou X M, et al.Current situation of soil Cd pollution and research progress of heavy metal repairing [J]. Journal of Green Science and Technology, 2012(4): 212-215 (in Chinese)
[14] Pendias H, Kabata-Pendias A. Trace elements in soils and plants [J]. Experimental Agriculture, 2011, 47(4): 739
[15] 胡文. 土壤—植物系统中重金属的生物有效性及其影响因素的研究[D]. 北京: 北京林业大学, 2008: 1-224
Hu W. Heavy metal bio-availability and its affecting factors in soil-plant system [D]. Beijing: Beijing Forestry University, 2008: 1-224 (in Chinese)
[16] 王姗姗, 王颜红, 王世成, 等. 辽北地区农田土壤-作物系统中Cd、Pb的分布及富集特征[J]. 土壤通报, 2010, 41(5): 1175-1179
Wang S S, Wang Y H, Wang S C, et al. Distribution and accumulation of heavy metals in agricultural soil-crop systems of Tieling Area, Liaoning Province [J]. Chinese Journal of Soil Science, 2010, 41(5): 1175-1179 (in Chinese)
[17] Singh S, Kumar M. Heavy metal load of soil, water and vegetables in peri-urban Delhi [J]. Environmental Monitoring and Assessment, 2006, 120(1): 79-91
[18] 王彦斌, 杨一鸣, 曾亮, 等. 甘肃省榆中县菜地土壤与蔬菜中重金属含量及健康风险评估[J]. 干旱地区农业研究, 2015, 33(6): 234-241
Wang Y B, Yang Y M, Zeng L, et al. A survey of heavy metals concentrations in vegetables and soils in Yuzhong County of Gansu Province and their health risk [J]. Agricultural Research in the Arid Areas, 2015, 33(6): 234-241 (in Chinese)
[19] 陈小华, 沈根祥, 白玉杰, 等. 不同作物对土壤中Cd的富集特征及低累积品种筛选[J]. 环境科学, 2019, 40(10): 4647-4653
Chen X H, Shen G X, Bai Y J, et al. Accumulation of Cd in different crops and screening of low-Cd accumulation cultivars [J]. Environmental Science, 2019, 40(10): 4647-4653 (in Chinese)
[20] Ahmad J U, Goni M A. Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh [J]. Environmental Monitoring and Assessment, 2010, 166(1): 347-357
[21] 陈林, 银玲, 陈鸿平, 等. 不同种植区土壤对川芎药材无机元素富集影响研究[J]. 时珍国医国药, 2014, 25(8): 2004-2006
Chen L, Yin L, Chen H P, et al. Study on the origin soil impacts on the enrichment of inorganic elements in Ligusticum chuanxiong form different planting regions [J]. Lishizhen Medicine and Materia Medica Research, 2014, 25(8): 2004-2006 (in Chinese)
[22] 尹明, 杨大为, 唐慧娟, 等. 黄麻修复重度镉污染农田的品种筛选[J]. 中国麻业科学, 2020, 42(4): 150-156
Yin M, Yang D W, Tang H J, et al. Comparison of the capacity of different varieties of jute (Corchorus capsularis L.) to remediate heavily cadmium-contaminated farmland [J]. Plant Fiber Sciences in China, 2020, 42(4): 150-156 (in Chinese)
[23] Khan A, Javid S, Muhmood A, et al. Heavy metal status of soil and vegetables grown on peri-urban area of Lahore District [J]. Plant, Soil and Environment, 2013, 32(1): 49-54
[24] 黄涂海. 镉污染农田土壤的分类管控实践[D]. 杭州: 浙江大学, 2019: 1-81
Huang T H. Classified management and control practice of cadmium contaminated farmland soil [D]. Hangzhou: Zhejiang University, 2019: 1-81 (in Chinese)
[25] 李小琦. 云南典型红壤农田Pb、Cd污染特征及其风险评价[D]. 昆明: 云南大学, 2018: 1-84
Li X Q. The characteristics and risk assessment of Pb and Cd in the red soil areas of farmland in Yunnan Province [D]. Kunming: Yunnan University, 2018: 1-84 (in Chinese)
[26] Laˇcˇtu u R, Rău
u R, Rău ă C, C
ă C, C rstea S, et al. Soil-plant-man relationships in heavy metal polluted areas in Romania [J]. Applied Geochemistry, 1996, 11(1-2): 105-107
rstea S, et al. Soil-plant-man relationships in heavy metal polluted areas in Romania [J]. Applied Geochemistry, 1996, 11(1-2): 105-107
[27] 宋波, 杨子杰, 张云霞, 等. 广西西江流域土壤镉含量特征及风险评估[J]. 环境科学, 2018, 39(4): 1888-1900
Song B, Yang Z J, Zhang Y X, et al. Accumulation of Cd and its risks in the soils of the Xijiang River drainage basin in Guangxi [J]. Environmental Science, 2018, 39(4): 1888-1900 (in Chinese)
[28] 刘意章, 肖唐付, 熊燕, 等. 西南高镉地质背景区农田土壤与农作物的重金属富集特征[J]. 环境科学, 2019, 40(6): 2877-2884
Liu Y Z, Xiao T F, Xiong Y, et al. Accumulation of heavy metals in agricultural soils and crops from an area with a high geochemical background of cadmium, southwestern China [J]. Environmental Science, 2019, 40(6): 2877-2884 (in Chinese)
[29] Wiersma D, van Goor B J, van der Veen N G. Cadmium, lead, mercury and arsenic concentrations in crops and corresponding soils in the Netherlands [J]. Journal of Agricultural and Food Chemistry, 1986, 34(6): 1067-1074
[30] 孙亚芳, 王祖伟, 孟伟庆, 等. 天津污灌区小麦和水稻重金属的含量及健康风险评价[J]. 农业环境科学学报, 2015, 34(4): 679-685
Sun Y F, Wang Z W, Meng W Q, et al. Contents and health risk assessment of heavy metals in wheat and rice grown in Tianjin sewage irrigation area, China [J]. Journal of Agro-Environment Science, 2015, 34(4): 679-685 (in Chinese)
[31] Sanderson D V, Voutchkov M, Benkeblia N. Bioaccumulation of cadmium in potato tuber grown on naturally high levels cadmium soils in Jamaica [J]. Science of the Total Environment, 2019, 649: 909-915
[32] 谢团辉, 郭京霞, 陈炎辉, 等. 福建省某矿区周边土壤-农作物重金属空间变异特征与健康风险评价[J]. 农业环境科学学报, 2019, 38(3): 544-554
Xie T H, Guo J X, Chen Y H, et al. Spatial variability and health risk assessment of heavy metals in soils and crops around the mining area in Fujian Province, China [J]. Journal of Agro-Environment Science, 2019, 38(3): 544-554 (in Chinese)
[33] El-Hassanin A S, Samak M R, Abdel-Rahman G N, et al. Risk assessment of human exposure to lead and cadmium in maize grains cultivated in soils irrigated either with low-quality water or freshwater [J]. Toxicology Reports, 2020, 7: 10-15
[34] 张桂玲, 罗绪强, 廖艳梅, 等. 贵阳市南明河中下游水东段沿岸菜地农作物重金属污染评价[J]. 山地农业生物学报, 2019, 38(3): 56-62
Zhang G L, Luo X Q, Liao Y M, et al. Evaluation of heavy metal contamination of vegetables in the fields along the Shuidong section of the middle and lower reaches of Nanming River in Guiyang City [J]. Journal of Mountain Agriculture and Biology, 2019, 38(3): 56-62 (in Chinese)
[35] Coppola S, Dumontet S, Pontonio M, et al. Effect of cadmium-bearing sewage sludge on crop plants and microorganisms in two different soils [J]. Agriculture, Ecosystems & Environment, 1988, 20(3): 181-194
[36] 蔡保松, 陈同斌, 廖晓勇, 等. 土壤砷污染对蔬菜砷含量及食用安全性的影响[J]. 生态学报, 2004, 24(4): 711-717
Cai B S, Chen T B, Liao X Y, et al. Arsenic concentrations in soils and vegetables and their risk assessments in highly contaminated area in Hu’nan Province [J]. Acta Ecologica Sinica, 2004, 24(4): 711-717 (in Chinese)
[37] 李伟, 刘晖. 成都地区典型土壤与农作物中砷含量研究[J]. 四川环境, 2008, 27(5): 27-30, 43
Li W, Liu H. Study on the arsenical content of the typical soils and crops in Chengdu region [J]. Sichuan Environment, 2008, 27(5): 27-30, 43 (in Chinese)
[38] 马先杰. 贵州水城典型铅锌矿区蔬菜重金属污染特征及效应研究[D]. 贵阳: 贵州大学, 2020: 1-80
Ma X J. Characteristics and effects of heavy metals pollution in vegetables in the typical lead-zinc mines in Shuicheng, Guizhou [D]. Guiyang: Guizhou University, 2020: 1-80 (in Chinese)
[39] 冉继伟, 宁平, 孙鑫, 等. 云南个旧土壤农作物重金属污染特征及潜在风险[J]. 中国环境监测, 2019, 35(5): 62-68
Ran J W, Ning P, Sun X, et al. Heavy metal pollution characteristics and potential risks of soil and crops in Gejiu, Yunnan [J]. Environmental Monitoring in China, 2019, 35(5): 62-68 (in Chinese)
[40] 夏立江, 华珞, 韦东普. 部分地区蔬菜中的含砷量[J]. 土壤, 1996, 28(2): 105-109
Xia L J, Hua L, Wei D P. Arsenic content in vegetables in some areas [J]. Soils, 1996, 28(2):105-109 (in Chinese)
[41] Xu J L, Thornton I. Arsenic in garden soils and vegetable crops in Cornwall, England: Implications for human health [J]. Environmental Geochemistry and Health, 1985, 7(4): 131-133
[42] Vetter J. Arsenic content of some edible mushroom species [J]. European Food Research and Technology, 2004, 219(1): 71-74
[43] Das H K, Mitra A K, Sengupta P K, et al. Arsenic concentrations in rice, vegetables, and fish in Bangladesh: A preliminary study [J]. Environment International, 2004, 30(3): 383-387
[44] Meharg A A, Williams P N, Adomako E, et al. Geographical variation in total and inorganic arsenic content of polished (white) rice [J]. Environmental Science & Technology, 2009, 43(5): 1612-1617
[45] Batista B L Jr, Souza J M, De Souza S S, et al. Speciation of arsenic in rice and estimation of daily intake of different arsenic species by Brazilians through rice consumption [J]. Journal of Hazardous Materials, 2011, 191(1-3): 342-348
[46] Tinggi U, Schoendorfer N, Scheelings P, et al. Arsenic in rice and diets of children [J]. Food Additives & Contaminants Part B, 2015, 8(2): 149-156
[47] García-Rico L, Valenzuela-Rodríguez M P, Meza-Montenegro M M, et al. Arsenic in rice and rice products in Northwestern Mexico and health risk assessment [J]. Food Additives & Contaminants Part B, 2020, 13(1): 25-33
[48] 李婷, 吴明辉, 杨馨婷, 等. 植物与微生物对重金属的抗性机制及联合修复研究进展[J]. 应用与环境生物学报, 2021, 27(5): 1405-1414
Li T, Wu M H, Yang X T, et al. Advances in the mechanism of heavy metal resistance and combined remediation of plants and microorganisms [J]. Chinese Journal of Applied and Environmental Biology, 2021, 27(5): 1405-1414 (in Chinese)
[49] Stone J, Bacon C, White J. An Overview of Endophytic Microbes: Endophytism Defined [M]// Bacon C W. Microbial Endophytes. New York: Marcel Dekker, 2000: 17-44
[50] Ren A Z, Li C, Gao Y B. Endophytic fungus improves growth and metal uptake of Lolium arundinaceum Darbyshire ex. Schreb [J]. International Journal of Phytoremediation, 2011, 13(3): 233-243
[51] Chang H X, Haudenshield J S, Bowen C R, et al. Metagenome-wide association study and machine learning prediction of bulk soil microbiome and crop productivity [J]. Frontiers in Microbiology, 2017, 8: 519
[52] Glick B R, Cheng Z Y, Czarny J, et al. Promotion of plant growth by ACC deaminase-producing soil bacteria [J]. European Journal of Plant Pathology, 2007, 119(3): 329-339
[53] Baker A J M, Reeves R D, Hajar A S M. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae) [J]. The New Phytologist, 1994, 127(1): 61-68
[54] 孙瑞莲. 镉超积累植物的生态特征及污染耐性机理分析[D]. 北京: 中国科学院研究生院, 2006: 1-169
Sun R L. Ecological characteristics of cadmium-hyperaccumulators and their mechanism analysis of pollution endurance [D]. Beijing: Graduate School of Chinese Academy of Sciences, 2006: 1-169 (in Chinese)
[55] 张新成. 东南景天内生菌分离鉴定及其强化重金属超积累效应与机制[D]. 杭州: 浙江大学, 2012: 1-189
Zhang X C. Isolation and identification of endophytes from Sedum alfredii and the mechanisms of their enhancement on heavy metal hyperaccumulation [D]. Hangzhou: Zhejiang University, 2012: 1-189 (in Chinese)
[56] Sarma H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology [J]. Journal of Environmental Science and Technology, 2011, 4(2): 118-138
[57] 李熠, 陈熹, 肖丕显, 等. 中国镉超富集植物种类组成及分布特征研究[J]. 中国野生植物资源, 2020, 39(6): 11-16
Li Y, Chen X, Xiao P X, et al. Study on the species composition, geographical distribution and flora characteristics of Cd hyperaccumulators in China [J]. Chinese Wild Plant Resources, 2020, 39(6): 11-16 (in Chinese)
[58] 段桂兰, 王利红, 陈玉, 等. 植物超富集砷机制研究的最新进展[J]. 环境科学学报, 2007, 27(5): 714-720
Duan G L, Wang L H, Chen Y, et al. Recent developments in understanding the mechanisms of arsenic hyperaccumulation in plants [J]. Acta Scientiae Circumstantiae, 2007, 27(5): 714-720 (in Chinese)
[59] Trippe R C, Pilon-Smits E. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications [J]. Journal of Hazardous Materials, 2021, 404: 124178
[60] Nejad Z D, Jung M C, Kim K H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology [J]. Environmental Geochemistry and Health, 2018, 40(3): 927-953
[61] Desai M, Haigh M, Walkington H. Phytoremediation: Metal decontamination of soils after the sequential forestation of former opencast coal land [J]. The Science of the Total Environment, 2019, 656: 670-680
![]() ńska A, Wiszniewska A, Hanus-Fajerska E, et al. Recent strategies of increasing metal tolerance and phytoremediation potential using genetic transformation of plants [J]. Plant Biotechnology Reports, 2018, 12(1): 1-14
ńska A, Wiszniewska A, Hanus-Fajerska E, et al. Recent strategies of increasing metal tolerance and phytoremediation potential using genetic transformation of plants [J]. Plant Biotechnology Reports, 2018, 12(1): 1-14
[63] Sun R, Sheng X, Li Y, et al. Phyto-accumulation of heavy metals and characteristics of rhizosphere microbes in heavy metal contaminated soils, Qixia, Nanjing[J]. Acta Pedologica Sinica, 2011, 48(5): 1013-1020
[64] 李韵诗, 冯冲凌, 吴晓芙, 等. 重金属污染土壤植物修复中的微生物功能研究进展[J]. 生态学报, 2015, 35(20): 6881-6890
Li Y S, Feng C L, Wu X F, et al. A review on the functions of microorganisms in the phytoremediation of heavy metal-contaminated soils [J]. Acta Ecologica Sinica, 2015, 35(20): 6881-6890 (in Chinese)
[65] Sheng X F, Xia J J, Jiang C Y, et al. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape [J]. Environmental Pollution, 2008, 156(3): 1164-1170
[66] Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization [J]. Soil Biology and Biochemistry, 2010, 42(5): 669-678
[67] Abou-Shanab R A, Ghanem K, Ghanem N, et al. The role of bacteria on heavy-metal extraction and uptake by plants growing on multi-metal-contaminated soils [J]. World Journal of Microbiology and Biotechnology, 2008, 24(2): 253-262
[68] Kuffner M, de Maria S, Puschenreiter M, et al. Culturable bacteria from Zn- and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability [J]. Journal of Applied Microbiology, 2010, 108(4): 1471-1484
[69] 廖继佩, 林先贵, 曹志洪. 内外生菌根真菌对重金属的耐受性及机理[J]. 土壤, 2003, 35(5): 370-377
Liao J P, Lin X G, Cao Z H. Tolerance of mycorrhizal fungi to heavy metals and mechanisms [J]. Soils, 2003, 35(5): 370-377 (in Chinese)
[70] 罗巧玉, 王晓娟, 林双双, 等. AM真菌对重金属污染土壤生物修复的应用与机理[J]. 生态学报, 2013, 33(13): 3898-3906
Luo Q Y, Wang X J, Lin S S, et al. Mechanism and application of bioremediation to heavy metal polluted soil using arbuscular mycorrhizal fungi [J]. Acta Ecologica Sinica, 2013, 33(13): 3898-3906 (in Chinese)
[71] 谌金吾. 三叶鬼针草(Bidens pilosa L.)对重金属Cd、Pb胁迫的响应与修复潜能研究[D]. 重庆: 西南大学, 2013: 1-155
Chen J W. Study on response and potential phytoremediation of Bidens pilosa L. in cadmium and lead stress [D]. Chongqing: Southwest University, 2013: 1-155 (in Chinese)
[72] Hakeem K R. Crop Production and Global Environmental Issues [M]. Cham: Springer International Publishing, 2015: 103-122
[73] 何玉君, 孙梦荷, 沈亚婷, 等. 超富集植物与重金属相互作用机制及应用研究进展[J]. 岩矿测试, 2020, 39(5): 639-657
He Y J, Sun M H, Shen Y T, et al. Research progress on the interaction mechanism between hyperaccumulator and heavy metals and its application [J]. Rock and Mineral Analysis, 2020, 39(5): 639-657 (in Chinese)
[74] 邓小鹏. 超量积累植物龙葵(Solanum nigrum L.)对镉的吸收、积累及耐性机理研究[D]. 南京: 南京农业大学, 2010: 1-147
Deng X P. Study on absorption, accumulation and tolerance mechanisms of cadmium in hyperaccumulator Solanum nigrum L. [D]. Nanjing: Nanjing Agricultural University, 2010: 1-147 (in Chinese)
[75] 徐小逊. 超富集植物豨莶(Siegesbeckia orientalis L.)对镉的吸收和耐性机理研究[D]. 雅安: 四川农业大学, 2018: 1-147
Xu X X. Mechanism of cadmium absorption and tolerance of hyperaccumulator Siegesbeckia orientalis L. [D]. Yaan: Sichuan Agricultural University, 2018: 1-147 (in Chinese)
[76] Seth C S, Kumar Chaturvedi P, Misra V. The role of phytochelatins and antioxidants in tolerance to Cd accumulation in Brassica juncea L. [J]. Ecotoxicology and Environmental Safety, 2008, 71(1): 76-85
[77] Tu C, Ma L Q. Effects of arsenate and phosphate on their accumulation by an arsenic-hyperaccumulator Pteris vittata L. [J]. Plant and Soil, 2003, 249(2): 373-382
[78] Abercrombie J M, Halfhill M D, Ranjan P, et al. Transcriptional responses of Arabidopsis thaliana plants to As (Ⅴ) stress [J]. BMC Plant Biology, 2008, 8: 87
[79] Chen J, Shiyab S, Han F X, et al. Bioaccumulation and physiological effects of mercury in Pteris vittata and Nephrolepis exaltata [J]. Ecotoxicology, 2009, 18(1): 110-121
[80] Liu Y, Wang H B, Wong M H, et al. The role of arsenate reductase and superoxide dismutase in As accumulation in four Pteris species [J]. Environment International, 2009, 35(3): 491-495
[81] Cao X D, Ma L Q, Tu C. Antioxidative responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.) [J]. Environmental Pollution, 2004, 128(3): 317-325
[82] Srivastava M, Ma L Q, Singh N, et al. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic [J]. Journal of Experimental Botany, 2005, 56(415): 1335-1342
[83] Rahman F, Sugawara K, Huang Y, et al. Arsenic, lead and cadmium removal potential of Pteris multifida from contaminated water and soil [J]. International Journal of Phytoremediation, 2018, 20(12): 1187-1193
[84] Popov M, Zemanová V, Sá![]() J, et al. Arsenic accumulation and speciation in two cultivars of Pteris cretica L. and characterization of arsenate reductase PcACR2 and arsenite transporter PcACR3 genes in the hyperaccumulating cv. Albo-lineata [J]. Ecotoxicology and Environmental Safety, 2021, 216: 112196
J, et al. Arsenic accumulation and speciation in two cultivars of Pteris cretica L. and characterization of arsenate reductase PcACR2 and arsenite transporter PcACR3 genes in the hyperaccumulating cv. Albo-lineata [J]. Ecotoxicology and Environmental Safety, 2021, 216: 112196
[85] Khan M S, Zaidi A, Wani P A, et al. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils [J]. Environmental Chemistry Letters, 2009, 7(1): 1-19
[86] Chen B, Zhang Y B, Rafiq M T, et al. Improvement of cadmium uptake and accumulation in Sedum alfredii by endophytic bacteria Sphingomonas SaMR12: Effects on plant growth and root exudates [J]. Chemosphere, 2014, 117: 367-373
[87] Pan F S, Luo S, Shen J, et al. The effects of endophytic bacterium SaMR12 on Sedum alfredii Hance metal ion uptake and the expression of three transporter family genes after cadmium exposure [J]. Environmental Science and Pollution Research International, 2017, 24(10): 9350-9360
[88] Zhu L J, Guan D X, Luo J, et al. Characterization of arsenic-resistant endophytic bacteria from hyperaccumulators Pteris vittata and Pteris multifida [J]. Chemosphere, 2014, 113: 9-16
[89] Rathinasabapathi B, Raman S B, Kertulis G, et al. Arsenic-resistant proteobacterium from the phyllosphere of arsenic-hyperaccumulating fern (Pteris vittata L.) reduces arsenate to arsenite [J]. Canadian Journal of Microbiology, 2006, 52(7): 695-700
[90] Liu Y, Zhu Y G, Chen B D, et al. Influence of the arbuscular mycorrhizal fungus Glomus mosseae on uptake of arsenate by the As hyperaccumulator fern Pteris vittata L. [J]. Mycorrhiza, 2005, 15(3): 187-192
[91] Chien C C, Huang C H, Lin Y W. Characterization of a heavy metal translocating P-type ATPase gene from an environmental heavy metal resistance Enterobacter sp. [J]. Applied Biochemistry and Biotechnology, 2013, 169(6): 1837-1846
[92] 廉梅花. 根际土壤中重金属的活化因素及作用机理研究[D]. 沈阳: 东北大学, 2016: 1-180
Lian M H. Study on the activating factors and mechanisms of heavy metals in rhizosphere soil [D]. Shenyang: Northeastern University, 2016: 1-180 (in Chinese)
[93] Miller R M. Biosurfactant-facilitated remediation of metal-contaminated soils [J]. Environmental Health Perspectives, 1995, 103(Suppl.1): 59-62
[94] Ding Y Z, Song Z G, Feng R W, et al. Interaction of organic acids and pH on multi-heavy metal extraction from alkaline and acid mine soils [J]. International Journal of Environmental Science and Technology, 2014, 11(1): 33-42
[95] Quartacci M F, Irtelli B, Gonnelli C, et al. Naturally-assisted metal phytoextraction by Brassica carinata: Role of root exudates [J]. Environmental Pollution, 2009, 157(10): 2697-2703
[96] 罗庆, 孙丽娜, 胡筱敏. 镉超富集植物东南景天根系分泌物的代谢组学研究[J]. 分析化学, 2015, 43(1): 7-12
Luo Q, Sun L N, Hu X M. Metabonomics study on root exudates of Cd hyperaccumulator Sedum alfredii [J]. Chinese Journal of Analytical Chemistry, 2015, 43(1): 7-12 (in Chinese)
[97] Lessl J T, Ma L Q, Rathinasabapathi B, et al. Novel phytase from Pteris vittata resistant to arsenate, high temperature, and soil deactivation [J]. Environmental Science & Technology, 2013, 47(5): 2204-2211
[98] Liu X, Fu J W, Guan D X, et al. Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata [J]. Environmental Science & Technology, 2016, 50(17): 9070-9077
[99] Bhargava A, Carmona F F, Bhargava M, et al. Approaches for enhanced phytoextraction of heavy metals [J]. Journal of Environmental Management, 2012, 105: 103-120
[100] Wang X, Ma L Q, Rathinasabapathi B, et al. Mechanisms of efficient arsenite uptake by arsenic hyperaccumulator Pteris vittata [J]. Environmental Science & Technology, 2011, 45(22): 9719-9725
[101] Sundaram S, Rathinasabapathi B, Ma L Q, et al. An arsenate-activated glutaredoxin from the arsenic hyperaccumulator fern Pteris vittata L. regulates intracellular arsenite [J]. The Journal of Biological Chemistry, 2008, 283(10): 6095-6101
[102] Lee J, Shim D, Song W Y, et al. Arabidopsis metallothioneins 2a and 3 enhance resistance to cadmium when expressed in Vicia faba guard cells [J]. Plant Molecular Biology, 2004, 54(6): 805-815
[103] 张星雨, 叶志彪, 张余洋. 植物响应镉胁迫的生理与分子机制研究进展[J]. 植物生理学报, 2021, 57(7): 1437-1450
Zhang X Y, Ye Z B, Zhang Y Y. Advances in physiological and molecular mechanism of plant response to cadmium stress [J]. Plant Physiology Journal, 2021, 57(7): 1437-1450 (in Chinese)
[104] 王学华, 戴力. 作物根系镉滞留作用及其生理生化机制[J]. 中国农业科学, 2016, 49(22): 4323-4341
Wang X H, Dai L. Immobilization effect and its physiology and biochemical mechanism of the cadmium in crop roots [J]. Scientia Agricultura Sinica, 2016, 49(22): 4323-4341 (in Chinese)
[105] 郭军康, 周冉, 任心豪, 等. 不同年限设施菜地番茄细胞壁果胶Cd累积的研究[J]. 农业环境科学学报, 2018, 37(1): 45-51
Guo J K, Zhou R, Ren X H, et al. Accumulation of Cd in cell wall pectin of tomato plants grown in greenhouse soil of different planting years [J]. Journal of Agro-Environment Science, 2018, 37(1): 45-51 (in Chinese)
[106] 丁禺乔, 柳晓光. 土壤重金属污染修复技术及展望[J]. 资源节约与环保, 2021(6): 77-78
Ding Y Q, Liu X G. Remediation technology and prospect of heavy metal pollution in soil [J]. Resources Economization & Environmental Protection, 2021(6): 77-78 (in Chinese)
[107] Suksabye P, Pimthong A, Dhurakit P, et al. Effect of biochars and microorganisms on cadmium accumulation in rice grains grown in Cd-contaminated soil [J]. Environmental Science and Pollution Research International, 2016, 23(2): 962-973
[108] Treesubsuntorn C, Dhurakit P, Khaksar G, et al. Effect of microorganisms on reducing cadmium uptake and toxicity in rice (Oryza sativa L.) [J]. Environmental Science and Pollution Research International, 2018, 25(26): 25690-25701
[109] 杨榕, 李博文, 刘微, 等. 胶质芽孢杆菌对印度芥菜富集土壤Cd的效果[J]. 水土保持学报, 2012, 26(5): 164-168
Yang R, Li B W, Liu W, et al. Effects of Bacillus mucilaginosus on sorption and accumulation for Brassica juncea with Cd in the soil [J]. Journal of Soil and Water Conservation, 2012, 26(5): 164-168 (in Chinese)
[110] 邓月强, 曹雪莹, 谭长银, 等. 巨大芽孢杆菌对伴矿景天修复镉污染农田土壤的强化作用[J]. 应用生态学报, 2020, 31(9): 3111-3118
Deng Y Q, Cao X Y, Tan C Y, et al. Strengthening the effect of Bacillus megaterium on remediation of Cd-contaminated farmland soil by Sedum plumbizincicola [J]. Chinese Journal of Applied Ecology, 2020, 31(9): 3111-3118 (in Chinese)
[111] 张云霞, 周浪, 肖乃川, 等. 鬼针草(Bidens pilosa L.)对镉污染农田的修复潜力[J]. 生态学报, 2020, 40(16): 5805-5813
Zhang Y X, Zhou L, Xiao N C, et al. Remediation potential of B. pilosa L. in cadmium-contaminated farmland [J]. Acta Ecologica Sinica, 2020, 40(16): 5805-5813 (in Chinese)
[112] 曾东, 许振成. 抗砷菌对蜈蚣草生长及其砷吸收能力的影响[J]. 环境污染与防治, 2010, 32(5): 43-46
Zeng D, Xu Z C. Effect of arsenite-resistent bacteria on growth and arsenite adsorption capacity of Pteris vittata L. [J]. Environmental Pollution & Control, 2010, 32(5): 43-46 (in Chinese)
[113] Ma Y, Oliveira R S, Nai F J, et al. The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil [J]. Journal of Environmental Management, 2015, 156: 62-69
[114] Baum C, Hrynkiewicz K, Leinweber P, et al. Heavy‐metal mobilization and uptake by mycorrhizal and nonmycorrhizal willows (Salix × dasyclados) [J]. Journal of Plant Nutrition and Soil Science, 2006, 169(4): 516-522
[115] Wu Y J, Ma L Y, Liu Q Z, et al. The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii [J]. Journal of Hazardous Materials, 2020, 395: 122661
[116] Wang Q, Xiong D, Zhao P, et al. Effect of applying an arsenic-resistant and plant growth-promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoides LH05-17 [J]. Journal of Applied Microbiology, 2011, 111(5): 1065-1074
[117] Krupa P, Kozdrój J. Ectomycorrhizal fungi and associated bacteria provide protection against heavy metals in inoculated pine (Pinus sylvestris L.) seedlings [J]. Water, Air, and Soil Pollution, 2007, 182(1): 83-90
[118] Liang X, Chi-Quan H, Gang N, et al. Growth and Cd accumulation of Orychophragmus violaceus as affected by inoculation of Cd-tolerant bacterial strains [J]. Pedosphere, 2014, 24(3): 322-329
[119] Jeong S, Moon H S, Nam K, et al. Application of phosphate-solubilizing bacteria for enhancing bioavailability and phytoextraction of cadmium (Cd) from polluted soil [J]. Chemosphere, 2012, 88(2): 204-210
[120] Gao Y, Miao C, Mao L, et al. Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid [J]. Journal of Hazardous Materials, 2010, 181(1-3): 771-777
[121] Yang Q, Tu S, Wang G, et al. Effectiveness of applying arsenate reducing bacteria to enhance arsenic removal from polluted soils by Pteris vittata L. [J]. International Journal of Phytoremediation, 2012, 14(1): 89-99
[122] Leung H M, Ye Z, Wong M H. Interactions of mycorrhizal fungi with Pteris vittata (As hyperaccumulator) in As-contaminated soils [J]. Environmental Pollution, 2006, 139(1): 1-8
[123] 杨倩. 微生物提高植物修复砷污染土壤的效果和机理研究[D]. 武汉: 华中农业大学, 2009: 1-98
Yang Q. The role of microorganisms in improving the phytoremediation of arsenic polluted soils and its mechanisms [D]. Wuhan: Huazhong Agricultural University, 2009: 1-98 (in Chinese)
[124] El Aafi N, Brhada F, Dary M, et al. Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant rhizobacterium Serratia sp. MSMC541 [J]. International Journal of Phytoremediation, 2012, 14(3): 261-274
[125] Dong Y, Zhu Y G, Smith F A, et al. Arbuscular mycorrhiza enhanced arsenic resistance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) plants in an arsenic-contaminated soil [J]. Environmental Pollution, 2008, 155(1): 174-181
[126] 张帅, 方晓晴, 万敏, 等. 土壤酸胁迫对2种植物生长及镉富集的影响[J]. 安徽农业科学, 2020, 48(17): 104-107, 155
Zhang S, Fang X Q, Wan M, et al. Effects of soil acid stress on the growth and cadmium accumulation of two plants [J]. Journal of Anhui Agricultural Sciences, 2020, 48(17): 104-107, 155 (in Chinese)
[127] 于永光, 赵斌. 不同pH水平下2种菌根真菌对紫云英生长的影响及其相互作用[J]. 菌物学报, 2008, 27(2): 209-216
Yu Y G, Zhao B. The interaction and effect of two species of arbuscular mycorrhizal fungi on the growth of Astragalus sinicus L. at different pH level [J]. Mycosystema, 2008, 27(2): 209-216 (in Chinese)
[128] 韦朝阳, 陈同斌. 重金属超富集植物及植物修复技术研究进展[J]. 生态学报, 2001, 21(7): 1196-1203
Wei C Y, Chen T B. Hyperaccumulators and phytoremediation of heavy metal contaminated soil: A review of studies in China and abroad [J]. Acta Ecologica Sinica, 2001, 21(7): 1196-1203 (in Chinese)
[129] 徐文静, 靳晓东, 杨秋生. 植物根际微生物的影响因素研究进展[J]. 河南农业科学, 2014, 43(5): 6-12
Xu W J, Jin X D, Yang Q S. Research progress on factors influencing plant rhizosphere microorganism [J]. Journal of Henan Agricultural Sciences, 2014, 43(5): 6-12 (in Chinese)
[130] Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization [J]. Soil Biology and Biochemistry, 2010, 42(5): 669-678
[131] Chen Y X, Wang Y P, Lin Q, et al. Effect of copper-tolerant rhizosphere bacteria on mobility of copper in soil and copper accumulation by Elsholtzia splendens [J]. Environment International, 2005, 31(6): 861-866
[132] 国辉, 毛志泉, 刘训理. 植物与微生物互作的研究进展[J]. 中国农学通报, 2011, 27(9): 28-33
Guo H, Mao Z Q, Liu X L. Research progress of interaction between plant and microorganism [J]. Chinese Agricultural Science Bulletin, 2011, 27(9): 28-33 (in Chinese)
[133] 冯洁, 陈其煐, 石磊岩. 棉花幼苗根系分泌物与枯萎病关系的研究[J]. 棉花学报, 1991, 3(1): 89-96
Feng J, Chen Q Y, Shi L Y. Studies on relation between root exudates of cotton seedling and Fusarium wilt disease [J]. Cotton Science, 1991, 3(1): 89-96 (in Chinese)
[134] 袁虹霞, 李洪连, 王烨, 等. 棉花不同抗性品种根系分泌物分析及其对黄萎病菌的影响[J]. 植物病理学报, 2002, 32(2): 127-131
Yuan H X, Li H L, Wang Y, et al. The root exudates of cotton cultivars with the different resistance and their effects on Verticillium dahliae [J]. Acta Phytopathologica Sinica, 2002, 32(2): 127-131 (in Chinese)
[135] Cavalca L, Corsini A, Canzi E, et al. Rhizobacterial communities associated with spontaneous plant species in long-term arsenic contaminated soils [J]. World Journal of Microbiology & Biotechnology, 2015, 31(5): 735-746