抗生素是由微生物(包括细菌、真菌、放线菌属)或高等动植物在生活过程中所产生的具有抗病原体或其他活性的一类次级代谢产物,能干扰其他生活细胞发育功能的化学物质[1]。我国是世界上最大的抗生素生产国和使用国,2013年,我国抗生素的生产总量为24.8万t,总用量为16.2万t[2]。抗生素被广泛用于人类医疗、畜禽和水产养殖中以治疗、预防疾病和促进动物生长。但是,人类和动物摄入的抗生素不能被完全吸收代谢,大部分以原形或代谢产物的形式排出体外,进而通过污水处理厂出水、畜禽和水产养殖废水排放等方式进入水环境中[3]。目前,抗生素在江河、湖泊等地表水中普遍检出,其中最常检出的抗生素种类为磺胺类、喹诺酮类、大环内酯类和四环素类,其浓度最高可达到几百ng·L-1(表1)[4-11]。
表1 我国典型地表水体中检出的抗生素及其污染水平
Table 1 Common antibiotics and their pollution characteristic detected in the typical surface water bodies in China (ng·L-1)
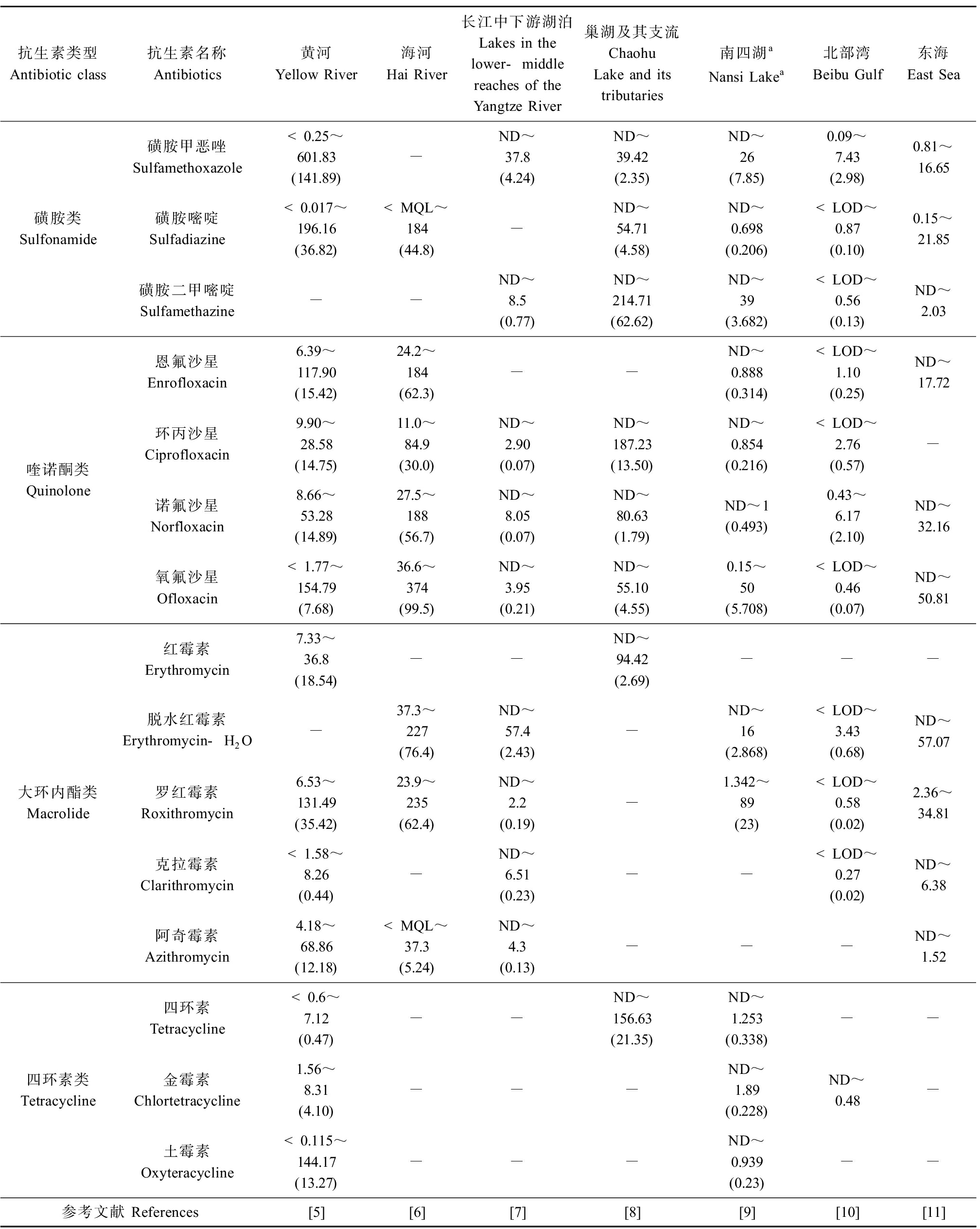
抗生素类型Antibiotic class抗生素名称Antibiotics黄河Yellow River海河Hai River长江中下游湖泊Lakes in the lower-middle reaches of the Yangtze River巢湖及其支流Chaohu Lake and its tributaries南四湖aNansi Lakea北部湾Beibu Gulf东海East Sea磺胺类Sulfonamide磺胺甲恶唑Sulfamethoxazole<0.25~601.83(141.89)-ND~37.8(4.24)ND~39.42(2.35)ND~26(7.85)0.09~7.43(2.98)0.81~16.65磺胺嘧啶Sulfadiazine<0.017~196.16(36.82) 注:ND表示未检出;LOD表示检出限;MQL表示方法定量限。 Note: ND represents not detected; LOD represents the limit of detection; MQL represents the method quantification limit.
水环境中的抗生素残留可能对非靶标生物产生不利影响,造成抗性基因的扩散传播,严重威胁生态安全和人类健康[12]。微藻是水生态系统的初级生产者,对于整个生态系统的平衡和稳定具有重要作用。但是,由于微藻对污染物胁迫的敏感性较强,其可能是最容易受到抗生素影响的一类水生生物[13]。研究表明,抗生素对微藻具有生态毒性效应,如诱导氧化胁迫、干扰叶绿素合成和抑制微藻生长等[12-13];同时,微藻对抗生素也具有一定的抗性,其可通过生物降解、生物吸附和生物积累等方式消除水中的抗生素,表现出一定的解毒特征[14-15]。微藻对抗生素的生态毒性响应及其消除作用可能决定了抗生素的生态风险。因此,本文系统综述了抗生素对微藻的生态毒性效应和微藻对抗生素的消除作用,并初步分析了两者之间的交互作用机制,旨在为我国地表水环境中抗生素的生态风险评估和控制提供科学依据。
1 抗生素对微藻的生态毒性效应(Ecotoxicological effects of antibiotics on microalgae)
1.1 抗生素对微藻生长的影响
抗生素对微藻生长的半数抑制浓度(EC50)是其生态毒性评估和分级的重要依据。图1展示了不同种类抗生素对不同微藻的EC50值[16-36]。在抗生素的生态毒性研究中,常用的微藻有近头状伪蹄形藻(Pseudokirchneriella subcapitata)、近头状尖胞藻(Raphidocelis subcapitata)、小球藻(Chlorella vulgaris)、羊角月牙藻(Selenastrum capricornutum)、斜生栅藻(Scenedesmus obliquus)和蛋白核小球藻(Chlorella pyrenoidosa)。不同微藻对抗生素胁迫的敏感性不同。在各种受试微藻中,蛋白核小球藻的敏感性相对较弱,而斜生栅藻和近头状伪蹄形藻的敏感性相对较强。如磺胺二甲氧嘧啶对蛋白核小球藻的72 h-EC50为10.05 mg·L-1,对斜生栅藻的72 h-EC50为1.23 mg·L-1[17]。金霉素对蛋白核小球藻的96 h-EC50为35.1 mg·L-1[36],对近头状伪蹄形藻的96 h-EC50为1.19 mg·L-1[26]。
不同种类的抗生素对微藻的EC50值也有明显差异。由图1可知,大环内酯类抗生素的生态毒性最高,除罗红霉素对尖状栅藻(Scenedesmus acuminatus)的96 h-EC50为2.876 mg·L-1外[31],其他大环内酯类抗生素对微藻生长的EC50值均<1 mg·L-1。根据原国家环境保护总局《新化学物质危害评估导则》(HJ/T 154—2004)中的毒性分级标准[37],大环内酯类抗生素对微藻具有极高毒性。磺胺类和四环素类抗生素对微藻生长的平均EC50值为3.48 mg·L-1和6.68 mg·L-1。大部分磺胺类和四环素类抗生素对微藻生长的EC50值在1~10 mg·L-1范围内,说明大部分磺胺类和四环素类抗生素对绿藻具有高毒性。喹诺酮类抗生素对微藻的EC50值在1~100 mg·L-1范围内,对微藻具有中-高毒性。
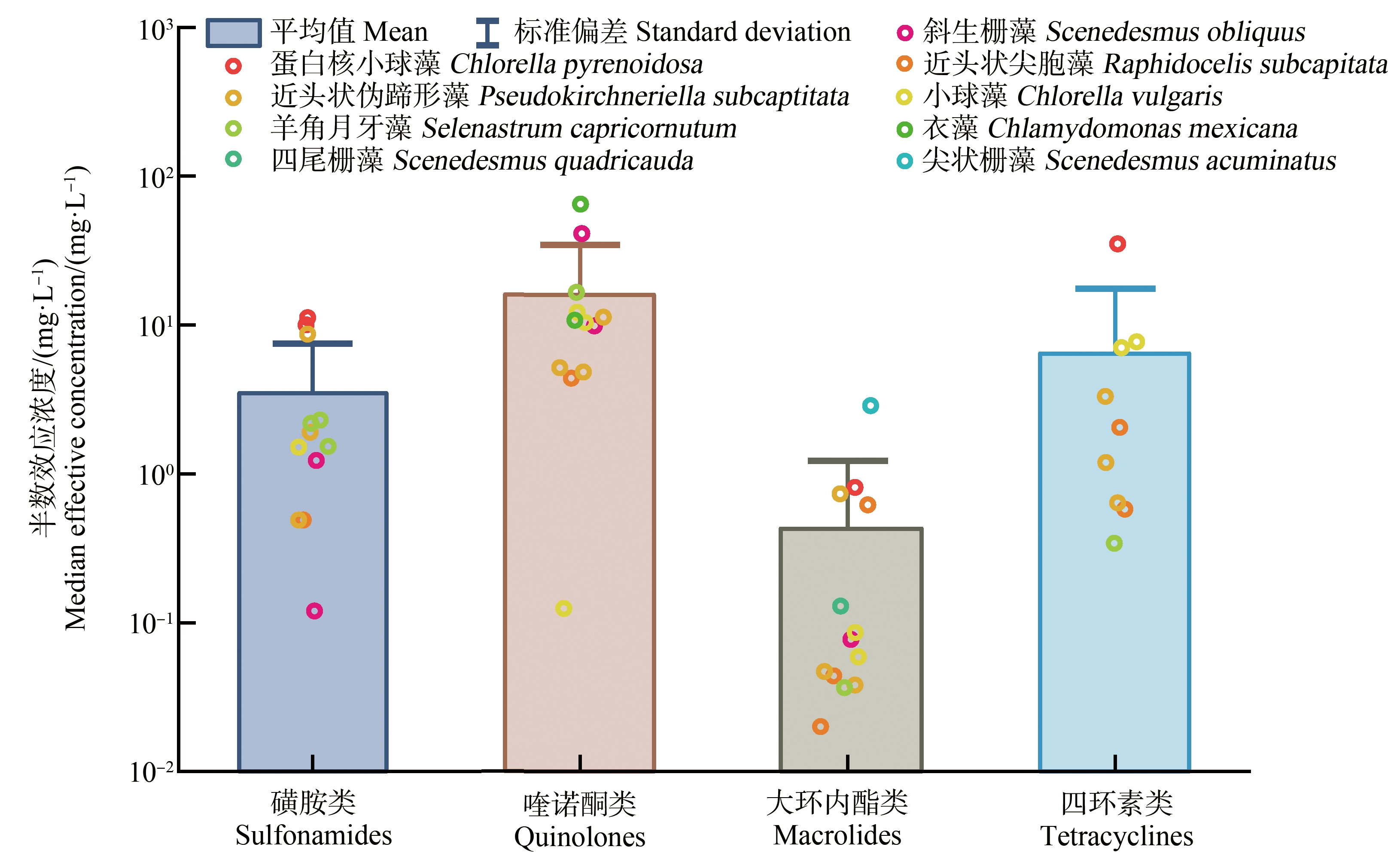
图1 抗生素对微藻的EC50值
Fig. 1 The EC50 values of antibiotics to microalgae
总体而言,抗生素对微藻的EC50值往往要比其环境检出浓度(ng·L-1~μg·L-1级别)高出1个或数个数量级,因此环境中的抗生素一般不会引起急性毒性效应。但是,一方面,低浓度抗生素暴露可能具有促进微藻生长的Hormesis效应[38],另一方面,抗生素长期低浓度暴露也会抑制微藻的生长并产生氧化胁迫[30,39],因此,低浓度抗生素对微藻的生态毒性效应还有待于深入的研究探讨。
1.2 抗生素对微藻细胞超微结构的影响
抗生素胁迫可能会影响微藻细胞的超微结构,干扰微藻细胞的正常生命活动。Xu等[35]研究发现5 mg·L-1的四环素导致小球藻的细胞膜渗透性显著增强,10 mg·L-1的四环素导致小球藻细胞质壁分离和类囊体变形,使光合作用受阻。Chen等[40]研究发现,磺胺类抗生素导致小球藻细胞中叶绿体形状不规则、类囊体功能紊乱,而且线粒体的尺寸明显增大。Xu等[41]也发现磺胺甲恶唑造成了斜生栅藻细胞壁与叶绿体严重受损、线粒体数量和体积明显增加。线粒体的扩增可能跟微藻应对逆境条件下的能量需求增加有关,因此线粒体数量和体积的增加可能是微藻应对抗生素胁迫的一种防御机制[40-41]。
1.3 抗生素对微藻光合系统的影响
叶绿素和类胡萝卜素等光合色素在微藻的光合作用过程中发挥着重要作用。多数研究表明,高浓度的抗生素胁迫抑制了微藻叶绿素和类胡萝卜素的合成,导致光合色素含量降低[30,38,42]。但是,也有研究发现抗生素胁迫导致光合色素含量升高。如Mao等[38]发现低浓度的阿奇霉素(0.5~1 μg·L-1)通过Hormesis效应促进了微藻的叶绿素b和类胡萝卜素的合成。Xiong等[29,43]研究发现,60 mg·L-1和100 mg·L-1的环丙沙星分别使衣藻的叶绿素含量增加了38%和19%,使类胡萝卜素含量显著增加了32%和16%;0.15~0.35 mg·L-1的磺胺甲恶唑和磺胺二甲嘧啶联合胁迫导致斜生栅藻的类胡萝卜素显著升高。这说明类胡萝卜素可作为抗氧化剂消除细胞中积累的活性氧物种(ROS),是微藻应对抗生素胁迫的一种防御机制。
叶绿素荧光参数,如光系统Ⅱ(PSII)的最大光化学效率(ΦP0)和用于电子传递的量子产额(ΦE0)等参数也常用来评价抗生素对微藻光合系统的影响。张红波等[44]研究发现,链霉素导致念珠藻(Nostoc)的可变荧光(Fv)与ΦP0、ΦE0值均随链霉素浓度升高而显著下降,说明链霉素降低了念珠藻PSⅡ用于电子传递的能量,抑制了PSⅡ的供体侧电子传递,从而抑制了初级光反应和氧化还原反应,阻断了电子从![]() 流向光系统Ⅰ(PSⅠ)。相似地,庆大霉素也会抑制PSⅡ受体侧电子传递,使PSⅡ功能受损;而且,高浓度的庆大霉素还会使斜生栅藻类囊体膜上反应中心的捕光能力不足,导致用于固定CO2和其他途径的ATP产量下降[45]。总体上,抗生素对微藻光合作用的影响是其抑制微藻生长的主要原因。
流向光系统Ⅰ(PSⅠ)。相似地,庆大霉素也会抑制PSⅡ受体侧电子传递,使PSⅡ功能受损;而且,高浓度的庆大霉素还会使斜生栅藻类囊体膜上反应中心的捕光能力不足,导致用于固定CO2和其他途径的ATP产量下降[45]。总体上,抗生素对微藻光合作用的影响是其抑制微藻生长的主要原因。
1.4 抗生素对微藻抗氧化系统的影响
抗生素胁迫可能诱导微藻体内产生过量的ROS。正常水平的ROS作为信号分子调控细胞的代谢过程;而过量的ROS可导致对细胞成分如脂质、蛋白质、甚至DNA的氧化损伤[46]。同时,微藻也可通过自身的非酶系统(谷胱甘肽(GSH)、类胡萝卜素等)和酶系统(超氧化物歧化酶(SOD),过氧化氢酶(CAT)等)的抗氧化防御机制,在一定程度上消除ROS的氧化胁迫[25]。因此,常用抗氧化系统,特别是抗氧化酶活性的变化来评价抗生素对微藻的氧化胁迫。
在各种抗氧化酶中,SOD可将超氧阴离子![]() 转化成H2O2和O2;CAT可进一步催化H2O2分解成H2O和O2;谷胱甘肽-S-转移酶(GST)可催化污染物的亲电基团与GSH发生结合反应从而解毒。研究表明,较低浓度抗生素的氧化胁迫通常会诱导微藻抗氧化酶活性的提高[18,22,30,32,38,47]。但是,较高浓度的抗生素可能会抑制抗氧化酶的活性[48]。如Nie等[48]发现,暴露在0~0.06 mg·L-1的红霉素中时,近头状伪蹄形藻中的SOD、CAT和谷胱甘肽过氧化物(GPX)活性都显著升高,但是更高浓度的红霉素则导致这些酶活性降低。
转化成H2O2和O2;CAT可进一步催化H2O2分解成H2O和O2;谷胱甘肽-S-转移酶(GST)可催化污染物的亲电基团与GSH发生结合反应从而解毒。研究表明,较低浓度抗生素的氧化胁迫通常会诱导微藻抗氧化酶活性的提高[18,22,30,32,38,47]。但是,较高浓度的抗生素可能会抑制抗氧化酶的活性[48]。如Nie等[48]发现,暴露在0~0.06 mg·L-1的红霉素中时,近头状伪蹄形藻中的SOD、CAT和谷胱甘肽过氧化物(GPX)活性都显著升高,但是更高浓度的红霉素则导致这些酶活性降低。
丙二醛(MDA)是ROS引起的不饱和脂质的氧化产物,常用来评价抗生素对微藻的氧化损伤。多数情况下,高浓度的抗生素胁迫导致细胞ROS水平升高,进而导致MDA的积累和含量的升高。如Xiong等[29]发现60~100 mg·L-1的环丙沙星导致微藻的MDA含量升高了152%和295%。Han等[47]发现0.09 mg·L-1的罗红霉素导致近头状尖胞藻的MDA水平升高了591%。MDA含量的升高表明抗生素已引起了微藻的结构和功能损伤。
1.5 抗生素对微藻的基因表达的影响
相对于生长和生理生化指标而言,基因表达水平的变化是更敏感的生物标志物。而且,基于基因表达水平变化分析是研究并揭示抗生素对微藻的毒性作用机制的重要手段。目前,有关抗生素对微藻的毒性作用机制尚不完全清楚。有限的研究表明,抗生素可能是通过影响微藻的光合作用、DNA复制和修复相关基因的表达而抑制微藻生长[49-52]。如Guo等[50]基于转录组学研究了磺胺甲恶唑对近头状尖胞藻的生态毒性作用,发现微藻的差异表达基因主要集中在DNA复制和修复、光合作用和转录通路中,说明其主要毒性作用机制为抑制DNA的复制和转录过程。Xu等[41]也发现磺胺甲恶唑影响了斜生栅藻的非编码RNA代谢过程,导致细胞核结构受损,说明磺胺甲恶唑具有基因毒性。罗红霉素显著下调了蛋白核小球藻的卟啉与叶绿素代谢、DNA修复相关基因的表达,从而抑制了蛋白核小球藻的生长[51]。Guo等[52]也发现罗红霉素抑制了近头状尖胞藻的碱基切除修复相关基因的表达,可能造成微藻的DNA损伤。
2 微藻对抗生素的消除作用及其机制(Removal of antibiotics by microalgae and the mechanism)
2.1 微藻对抗生素的消除效果及影响因素
表2列出了不同微藻对抗生素的去除效果和反应动力学[53-64]。由表2可知,微藻对抗生素的消除效果主要受微藻与抗生素的种类和浓度以及培养条件等因素的影响。
表2 微藻对抗生素的去除作用
Table 2 The removal efficiency of antibiotics by microalgae
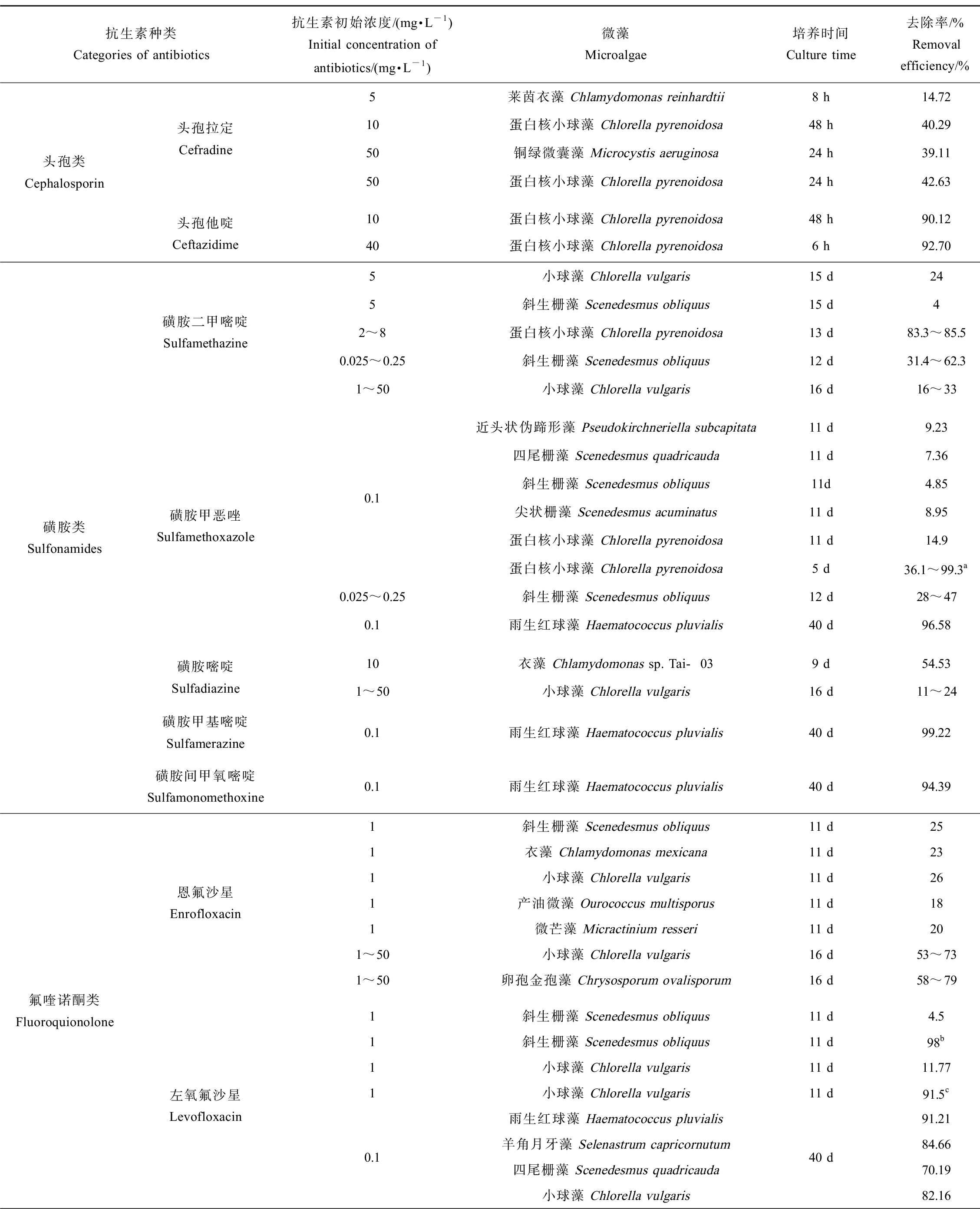
抗生素种类Categories of antibiotics抗生素初始浓度/(mg·L-1)Initial concentration of antibiotics/(mg·L-1)微藻Microalgae培养时间Culture time去除率/%Removal efficiency/%反应速率常数/d-1Reaction rate constant/d-1参考文献 References头孢类Cephalosporin头孢拉定Cefradine5莱茵衣藻 Chlamydomonas reinhardtii8 h14.720.312[53]10蛋白核小球藻 Chlorella pyrenoidosa48 h40.290.1128[54]50铜绿微囊藻 Microcystis aeruginosa24 h39.11-[55]50蛋白核小球藻 Chlorella pyrenoidosa24 h42.63-[55]头孢他啶Ceftazidime10蛋白核小球藻 Chlorella pyrenoidosa48 h90.122.148[54]40蛋白核小球藻 Chlorella pyrenoidosa6 h92.70-[56]磺胺类Sulfonamides磺胺二甲嘧啶Sulfamethazine5小球藻 Chlorella vulgaris15 d240.0141[57]5斜生栅藻 Scenedesmus obliquus15 d40.00482~8蛋白核小球藻 Chlorella pyrenoidosa13 d83.3~85.5-[22]0.025~0.25斜生栅藻 Scenedesmus obliquus12 d31.4~62.30.037~0.093[43]1~50小球藻 Chlorella vulgaris16 d16~33-[42]磺胺甲恶唑Sulfamethoxazole0.1近头状伪蹄形藻 Pseudokirchneriella subcapitata11 d9.23-四尾栅藻 Scenedesmus quadricauda11 d7.36-斜生栅藻 Scenedesmus obliquus11d4.85-尖状栅藻 Scenedesmus acuminatus11 d8.95-蛋白核小球藻 Chlorella pyrenoidosa11 d14.9-蛋白核小球藻 Chlorella pyrenoidosa5 d36.1~99.3a0.0107~0.9811[58]0.025~0.25斜生栅藻 Scenedesmus obliquus12 d28~470.035~0.061[43]0.1雨生红球藻 Haematococcus pluvialis40 d96.580.073[59]磺胺嘧啶Sulfadiazine10衣藻 Chlamydomonas sp. Tai-039 d54.530.0857[15]1~50小球藻 Chlorella vulgaris16 d11~24-[42]磺胺甲基嘧啶Sulfamerazine0.1雨生红球藻 Haematococcus pluvialis40 d99.220.083[59]磺胺间甲氧嘧啶Sulfamonomethoxine0.1雨生红球藻 Haematococcus pluvialis40 d94.390.089[59]氟喹诺酮类Fluoroquionolone恩氟沙星Enrofloxacin1斜生栅藻 Scenedesmus obliquus11 d250.02541衣藻 Chlamydomonas mexicana11 d230.02241小球藻 Chlorella vulgaris11 d260.027[25]1产油微藻 Ourococcus multisporus11 d180.01861微芒藻 Micractinium resseri11 d200.01861~50小球藻 Chlorella vulgaris16 d53~73-[42]1~50卵孢金孢藻 Chrysosporum ovalisporum16 d58~79-左氧氟沙星Levofloxacin1斜生栅藻 Scenedesmus obliquus11 d4.50.005[60]1斜生栅藻 Scenedesmus obliquus11 d98b0.1371小球藻 Chlorella vulgaris11 d11.770.011[61]1小球藻 Chlorella vulgaris11 d91.5c0.2570.1雨生红球藻 Haematococcus pluvialis羊角月牙藻 Selenastrum capricornutum四尾栅藻 Scenedesmus quadricauda小球藻 Chlorella vulgaris40 d91.210.08884.660.07170.190.10682.160.042[59]
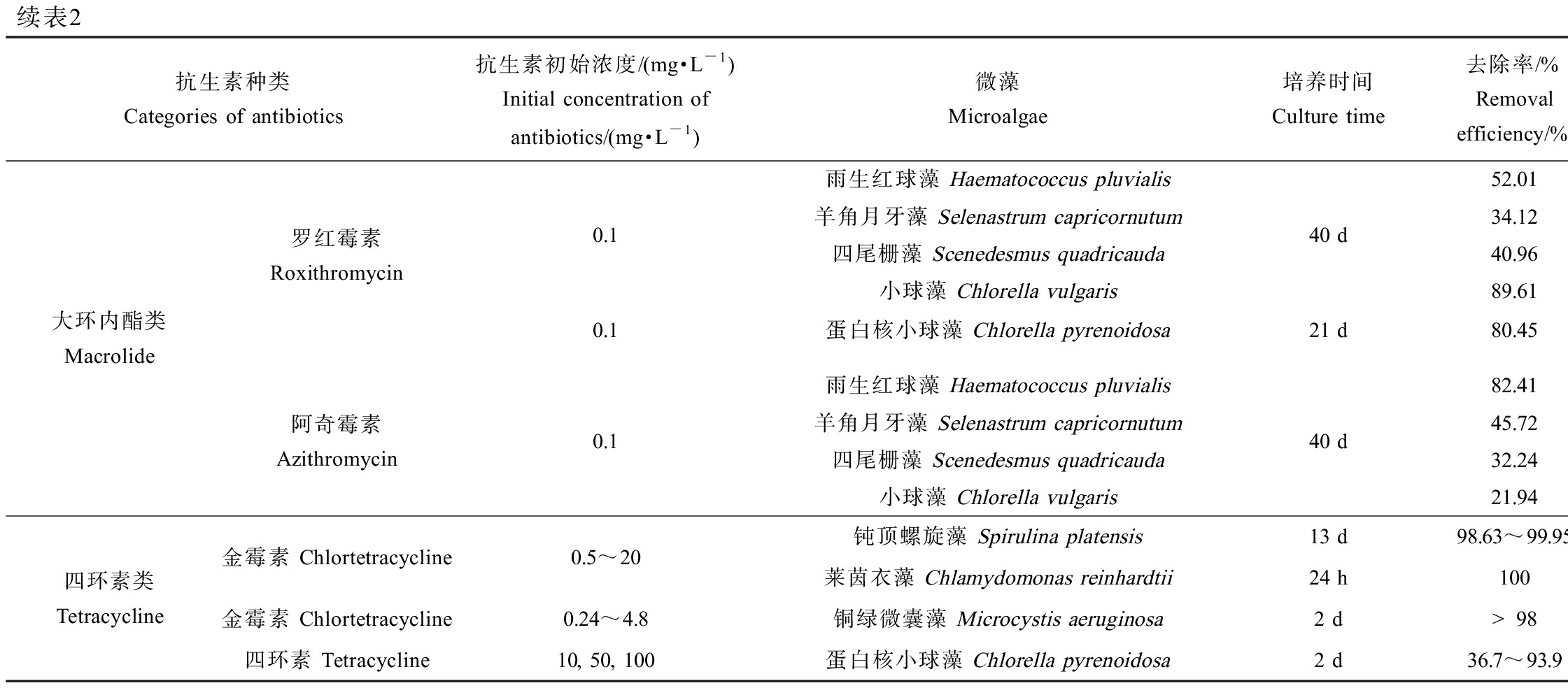
续表2抗生素种类Categories of antibiotics抗生素初始浓度/(mg·L-1)Initial concentration of antibiotics/(mg·L-1)微藻Microalgae培养时间Culture time去除率/%Removal efficiency/%反应速率常数/d-1Reaction rate constant/d-1参考文献 References大环内酯类Macrolide罗红霉素Roxithromycin0.1雨生红球藻 Haematococcus pluvialis羊角月牙藻 Selenastrum capricornutum四尾栅藻 Scenedesmus quadricauda小球藻 Chlorella vulgaris40 d52.010.04234.120.00440.960.00589.610.021[59]0.1蛋白核小球藻 Chlorella pyrenoidosa21 d80.450.058[30]阿奇霉素Azithromycin0.1雨生红球藻 Haematococcus pluvialis羊角月牙藻 Selenastrum capricornutum四尾栅藻 Scenedesmus quadricauda小球藻 Chlorella vulgaris40 d82.410.05745.720.02332.240.01521.940.026[59]四环素类Tetracycline金霉素 Chlortetracycline0.5~20金霉素 Chlortetracycline0.24~4.8四环素 Tetracycline10, 50, 100钝顶螺旋藻 Spirulina platensis13 d98.63~99.95-莱茵衣藻 Chlamydomonas reinhardtii24 h1006.522铜绿微囊藻 Microcystis aeruginosa2 d>980.17, 0.077, 0.086蛋白核小球藻 Chlorella pyrenoidosa2 d36.7~93.90.036, 0.022, 0.007[62][63][64]
注:a 添加1~8 mmol·L-1乙酸钠;b 添加171 mmol·L-1 NaCl;c 添加1% NaCl。
Note: a with addition of 1~8 mmol·L-1 sodium acetate; b with addition of 171 mmol·L-1 NaCl; c with addition of 1% NaCl.
微藻对抗生素的消除作用主要取决于微藻的种类。研究表明,蛋白核小球藻对抗生素普遍具有较高的去除效果。除了对磺胺甲恶唑和头孢拉定的去除率为14.90%和40.29%外[54,58],蛋白核小球藻对其他抗生素的去除率的均在80%以上[22,30,54,56],这可能与其对抗生素的抗性较强有关。另一方面,微藻的细胞密度也会影响到其对抗生素的去除作用。在一定范围内,增加微藻细胞浓度有利于抗生素的降解,因为更多的微藻细胞可能提供了更多的活性位点或者生成了更多的活性物种从而促进了抗生素的光降解;但是,过高的藻细胞密度则可能导致屏蔽效应,从而对抗生素的降解造成不利影响[56]。
微藻对不同种类抗生素的去除效果也跟微藻种类有关。Kiki等[59]研究了4种微藻对3类10种抗生素的去除作用,结果表明,雨生红球藻(Haematococcus pluvialis)和羊角月牙藻对磺胺类抗生素的去除作用要高于喹诺酮类和大环内酯类,而四尾栅藻(Scenedesmus quadricauda)和小球藻对喹诺酮类和大环内酯类的去除效果要优于磺胺类抗生素。
微藻对抗生素的消除作用也受到培养条件,如温度、光照强度、pH、共存基质等的影响[53,55,58,61]。温度和光照强度等因素主要通过影响微藻的生长从而影响抗生素的去除。如Du等[55]发现,铜绿微囊藻对头孢拉定和阿莫西林的去除效果在光照强度为5 500和8 500 lx时达到最大值,而进一步增大光照强度则会抑制抗生素的去除。有机质和营养元素的添加可以强化微藻对抗生素的消除作用[29,58,65]。如Xiong等[58]发现,培养11 d时,蛋白核小球藻对磺胺甲恶唑的去除率为14.9%,但是当加入2~4 mmol·L-1的乙酸钠时,磺胺甲恶唑的去除率可达到93.1%~99.3%。此外,有研究表明,NaCl也会促进微藻对抗生素的降解,这可能是因为NaCl增加了细胞膜的渗透性,使抗生素更易于穿过细胞膜进入到微藻内部,有利于胞内酶系统对抗生素的催化降解作用[52-53]。
2.2 微藻对抗生素的消除机制
在含藻水体中,抗生素主要通过非生物降解(光降解、水解等)和生物降解而消除。微藻对抗生素的消除机制主要包括藻源有机质介导抗生素的光降解、生物吸附、生物富集和生物降解(图2)。

图2 微藻对抗生素的去除机制
注:ROS表示活性氧物种;3EOM*表示三重态的胞外有机物。
Fig. 2 Removal mechanism of antibiotics by microalgae
Note: ROS means reactive oxygen species; 3EOM*means triplet state extracellular organic matter.
2.2.1 光降解
在太阳光照下,抗生素可以发生直接光解和间接光解。直接光解是指抗生素直接吸收太阳光能,从而发生化学键的断裂或重排。抗生素的直接光解速率主要取决于其结构特征和对太阳光的吸收特性。如磺胺甲恶唑和环丙沙星对太阳光有较强的吸收,直接光解速率较快,而大环内酯类抗生素对太阳光的吸收较弱,直接光解速率较慢[66]。抗生素的间接光解是指水体中的溶解性有机质(DOM)等吸收光后可生成三重态有机物(3DOM*)、羟基自由基(·OH)、单态氧(1O2)等活性中间体(RIs),这些RIs与抗生素反应使其降解[67]。间接光解是水体中抗生素消除的重要途径。
近期研究发现,微藻分泌产生的胞外有机物(EOM)能够促进抗生素的光降解[68-69]。Tian等[69]发现,微藻促进了金霉素的光降解,且促进效果随微藻浓度增加而增加。在微藻细胞浓度为2×109个·L-1时,金霉素的光降解速率常数(0.0246 min-1)是其在无藻体系中光降解速率常数的2倍。这主要是由于微藻产生的EOM在光照下产生了3EOM*、·OH和1O2,其中3EOM*对金霉素光降解的贡献可达到93%,而·OH和1O2的贡献仅为7%。进一步研究发现,EOM的光化学活性与其羰基含量成正比,即EOM中羰基含量越高,其对抗生素光降解的促进作用越强[70]。
2.2.2 生物吸附
微藻对抗生素的生物吸附主要是通过细胞壁表面或胞外聚合物(EPS)的氢键结合、电荷吸引、孔隙填充、分配和疏水作用等实现的[71]。微藻对抗生素的吸附作用可通过灭活的死亡藻细胞进行评价[72]。研究发现,有些微藻对抗生素的吸附作用是其从水体中去除的主要机制[73-74]。如Hena等[73]研究发现,EPS的吸附作用可使5 μmol·L-1甲硝唑的去除率达到100%。但更多情况下,生物吸附对于抗生素的去除贡献较小[30, 59-61]。如Kiki等[59]报道,生物吸附对小球藻去除磺胺类抗生素的贡献仅为1%~4%,对大环内酯类抗生素的贡献为2%~4%。尽管生物吸附对微藻去除抗生素的贡献较小,其对于抗生素的生物降解仍然非常重要。因为生物吸附可能是抗生素进入细胞的首要步骤,之后,吸附在微藻上的抗生素穿过细胞壁和细胞膜进入细胞,进而发生生物富集和生物降解[56]。
2.2.3 生物富集
生物富集是指抗生素进入微藻细胞并储存于细胞内,这可能导致抗生素通过食物链进入更高营养级生物体内,产生更高的生态和健康风险。和生物吸附一样,多数情况下,生物富集对抗生素去除的贡献较小,这可能是因为进入到细胞内部的抗生素更容易被酶代谢去除[75]。微藻对抗生素的生物富集与抗生素的疏水性密切相关。抗生素的辛醇水分配系数(Kow)越大,其越容易在生物体内发生生物富集。如磺胺类抗生素的Kow为0.14~0.91,其在雨生红球藻、羊角月牙藻、四尾栅藻和小球藻中均没有明显的生物富集效应,而大环内酯类抗生素的Kow为3.0~4.02,其在雨生红球藻、羊角月牙藻和四尾栅藻中的生物富集贡献为2%~3%,在小球藻中的生物富集贡献为4%~5%[59]。
2.2.4 生物降解
生物降解是指抗生素在微藻胞内和胞外酶的作用下被分解转化的过程,这是微藻消除抗生素的主要途径[25,59]。目前微藻对抗生素生物降解的研究主要集中在胞内降解。一般而言,抗生素首先在Ⅰ相阶段酶如细胞色素P450等的作用下发生氧化、还原和水解等反应,转化成亲水性的化合物;之后,这些转化产物在Ⅱ相阶段酶如GST的作用下发生结合反应,导致环氧化合物的环断裂从而使其毒性降低[75-76]。同时,Ⅱ相阶段的结合产物的亲水性更强,有利于其向体外的排出。
抗生素的生物降解可分为生长代谢和共代谢。生长代谢指微藻以抗生素作为唯一碳源和能量来源,通过特异酶的作用,使抗生素降解转化;共代谢指在有额外碳源和能量来源输入的条件下,通过非特异酶的作用,使抗生素降解[71]。这2种代谢模式在微藻对抗生素的生物降解过程中可能是同时存在的。近期研究发现,乙酸钠作为外加碳源,能够促进小球藻对阿莫西林和蛋白核小球藻对磺胺甲恶唑的共代谢[58, 77]。
3 抗生素的生态毒性和消除的交互作用(Interactive effects of the ecotoxicity and removal of antibiotics)
抗生素对微藻的生态毒性效应和微藻对抗生素的消除作用之间可能存在着复杂的交互作用,但是,由于不同研究所采用的抗生素种类和浓度、微藻的种类和浓度,以及培养条件的不同,尚难以通过比较现有的研究得出明确的结论。根据现有的研究推测,抗生素胁迫能诱导微藻对抗生素的降解[21,30]。如经过200 mg·L-1左氧氟沙星驯化11 d后的小球藻,对左氧氟沙星的去除率提高了16%。蛋白核小球藻在罗红霉素中暴露14 d后,对罗红霉素的生物降解作用显著增强[30]。Zhao等[63]发现,莱茵衣藻体内的ROS水平与其对金霉素的降解效果成正比,而抗生素的生物降解过程中Ⅰ相和Ⅱ相阶段酶发挥着重要作用[76]。因此,抗生素胁迫诱导微藻细胞内产生的ROS可能作为信号传导分子,使微藻细胞内代谢酶活性升高,从而促进微藻对抗生素的降解。另一方面,Hom-Diaz等[78]研究发现微藻生长速率越高,其对抗生素的去除效率越高。Xiong等[43]研究发现磺胺二甲嘧啶和磺胺甲恶唑对斜生栅藻的EC50值分别为1.23 mg·L-1和0.12 mg·L-1,斜生栅藻对2.5 mg·L-1的磺胺二甲嘧啶和磺胺甲恶唑的去除率分别为62.3%和46.8%,这可能说明同种微藻对抗生素的去除率与抗生素的毒性成反比。因此,抗生素浓度过高或毒性过大时可能会抑制微藻的生物降解作用。
4 结语与展望(Conclusion and prospect)
抗生素在环境中的暴露以及由此引起的生态风险和对人类健康的威胁已受到广泛关注。已有的研究表明,抗生素胁迫通常会抑制微藻生长、干扰叶绿素合成,并造成氧化胁迫和损伤;同时,微藻也可通过生物降解等方式消除抗生素,表现出一定的解毒效应。抗生素的生态毒性效应与微藻对抗生素的消除作用决定了其生态风险。但目前相关的研究还存在一些不足,以后应加强如下2个方面的研究。
(1)目前有关抗生素生态毒性效应的研究主要集中在单一抗生素且暴露浓度往往远高于环境浓度,而环境中抗生素多呈现出“混合性、低浓度和长期性”的污染特征。尽管现有研究表明,环境中的抗生素不太可能引起急性毒性效应,但抗生素的长期暴露和联合毒性效应可能具有较高的生态风险,因此,有必要开展长期暴露下,低浓度抗生素的联合毒性效应研究。
(2)目前有关微藻对抗生素的消除方面的研究主要集中在微藻种类的筛选和抗生素去除途径的分析,而对于抗生素的生物降解机制,如抗生素降解过程中起关键作用的酶,以及抗生素对这些酶的诱导作用等尚不清楚。因此,有必要借助于转录组学和代谢组学等技术,深入研究微藻对抗生素的生物降解机制,以及抗生素的生态毒性与微藻对其生物降解的交互作用,为准确评估环境中抗生素的生态风险提供科学依据。
[1] 徐永刚, 宇万太, 马强, 等. 环境中抗生素及其生态毒性效应研究进展[J]. 生态毒理学报, 2015, 10(3): 11-27
Xu Y G, Yu W T, Ma Q, et al. The antibiotic in environment and its ecotoxicity: A review [J]. Asian Journal of Ecotoxicology, 2015, 10(3): 11-27 (in Chinese)
[2] Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782
[3] 陈宇, 许亚南, 庞燕. 抗生素赋存、来源及风险评估研究进展[J]. 环境工程技术学报, 2021, 11(3): 562-570
Chen Y, Xu Y N, Pang Y. Advances in research on the occurrence, source and risk assessment of antibiotics [J]. Journal of Environmental Engineering Technology, 2021, 11(3): 562-570 (in Chinese)
[4] 李威, 李佳熙, 李吉平, 等. 我国不同环境介质中的抗生素污染特征研究进展[J]. 南京林业大学学报(自然科学版), 2020, 44(1): 205-214
Li W, Li J X, Li J P, et al. Pollution characteristics of antibiotics in different environment media in China: A review [J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2020, 44(1): 205-214 (in Chinese)
[5] Wang L F, Wang Y F, Li H, et al. Occurrence, source apportionment and source-specific risk assessment of antibiotics in a typical tributary of the Yellow River Basin [J]. Journal of Environmental Management, 2022, 305: 114382
[6] Lei K, Zhu Y, Chen W, et al. Spatial and seasonal variation of antibiotics in river waters in the Haihe River Catchment in China and ecotoxicological risk assessment [J]. Environmental International, 2019, 130: 104919
[7] Zhou L J, Li J, Zhang Y D, et al. Trends in the occurrence and risk assessment of antibiotics in shallow lakes in the lower-middle reaches of the Yangtze River Basin, China [J]. Ecotoxicology and Environmental Safety, 2019, 183: 109511
[8] Zhou Q Q, Liu G J, Arif M, et al. Occurrence and risk assessment of antibiotics in the surface water of Chaohu Lake and its tributaries in China [J]. The Science of the Total Environment, 2022, 807(Pt 3): 151040
[9] Zhang G D, Liu X H, Lu S Y, et al. Occurrence of typical antibiotics in Nansi Lake’s inflowing rivers and antibiotic source contribution to Nansi Lake based on principal component analysis-multiple linear regression model [J]. Chemosphere, 2020, 242: 125269
[10] Wu Q, Xiao S K, Pan C G, et al. Occurrence, source apportionment and risk assessment of antibiotics in water and sediment from the subtropical Beibu Gulf, South China [J]. Science of the Total Environment, 2022, 806: 150439
[11] Li F F, Chen L J, Chen W D, et al. Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening [J]. Marine Pollution Bulletin, 2020, 151: 110810
[12] Välitalo P, Kruglova A, Mikola A, et al. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review [J]. International Journal of Hygiene and Environmental Health, 2017, 220(3): 558-569
[13] 方媛瑗, 丁惠君. 抗生素的生态毒性效应研究进展[J]. 环境科学与技术, 2018, 41(5): 102-110
Fang Y Y, Ding H J. Advance in ecological toxicity of antibiotics [J]. Environmental Science & Technology, 2018, 41(5): 102-110 (in Chinese)
[14] Liu R B, Li S Q, Tu Y F, et al. Capabilities and mechanisms of microalgae on removing micropollutants from wastewater: A review [J]. Journal of Environmental Management, 2021, 285: 112149
[15] Xie P, Chen C, Zhang C F, et al. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae [J]. Water Research, 2020, 172: 115475
[16] Xiong J Q, Kim S J, Kurade M B, et al. Combined effects of sulfamethazine and sulfamethoxazole on a freshwater microalga, Scenedesmus obliquus: Toxicity, biodegradation, and metabolic fate [J]. Journal of Hazardous Materials, 2019, 370: 138-146
[17] Eguchi K, Nagase H, Ozawa M, et al. Evaluation of antimicrobial agents for veterinary use in the ecotoxicity test using microalgae [J]. Chemosphere, 2004, 57(11): 1733-1738
[18] Zhang Y B, He D, Chang F, et al. Combined effects of sulfamethoxazole and erythromycin on a freshwater microalga, Raphidocelis subcapitata: Toxicity and oxidative stress [J]. Antibiotics, 2021, 10(5): 576
[19] Yang L H, Ying G G, Su H C, et al. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata [J]. Environmental Toxicology and Chemistry, 2008, 27(5): 1201-1208
[20] Kovalakova P, Cizmas L, Feng M B, et al. Oxidation of antibiotics by ferrate(Ⅵ) in water: Evaluation of their removal efficiency and toxicity changes [J]. Chemosphere, 2021, 277: 130365
[21] Borecka M, Bia k-Bielińska A, Haliński
k-Bielińska A, Haliński  P, et al. The influence of salinity on the toxicity of selected sulfonamides and trimethoprim towards the green algae Chlorella vulgaris [J]. Journal of Hazardous Materials, 2016, 308: 179-186
P, et al. The influence of salinity on the toxicity of selected sulfonamides and trimethoprim towards the green algae Chlorella vulgaris [J]. Journal of Hazardous Materials, 2016, 308: 179-186
[22] Sun M, Lin H, Guo W, et al. Bioaccumulation and biodegradation of sulfamethazine in Chlorella pyrenoidosa [J]. Journal of Ocean University of China, 2017, 16(6): 1167-1174
[23] Rico A, Zhao W K, Gillissen F, et al. Effects of temperature, genetic variation and species competition on the sensitivity of algae populations to the antibiotic enrofloxacin [J]. Ecotoxicology and Environmental Safety, 2018, 148: 228-236
[24] Wang G X, Zhang Q, Li J L, et al. Combined effects of erythromycin and enrofloxacin on antioxidant enzymes and photosynthesis-related gene transcription in Chlorella vulgaris [J]. Aquatic Toxicology, 2019, 212: 138-145
[25] Xiong J Q, Kurade M B, Jeon B H. Ecotoxicological effects of enrofloxacin and its removal by monoculture of microalgal species and their consortium [J]. Environmental Pollution, 2017, 226: 486-493
[26] Carusso S, Juárez A B, Moretton J, et al. Effects of three veterinary antibiotics and their binary mixtures on two green alga species [J]. Chemosphere, 2018, 194: 821-827
[27] Magdaleno A, Saenz M E, Juárez A B, et al. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata [J]. Ecotoxicology and Environmental Safety, 2015, 113: 72-78
[28] Martins N, Pereira R, Abrantes N, et al. Ecotoxicological effects of ciprofloxacin on freshwater species: Data integration and derivation of toxicity thresholds for risk assessment [J]. Ecotoxicology, 2012, 21(4): 1167-1176
[29] Xiong J Q, Kurade M B, Kim J R, et al. Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas mexicana [J]. Journal of Hazardous Materials, 2017, 323(Pt A): 212-219
[30] Li J P, Min Z F, Li W, et al. Interactive effects of roxithromycin and freshwater microalgae, Chlorella pyrenoidosa: Toxicity and removal mechanism [J]. Ecotoxicology and Environmental Safety, 2020, 191: 110156
[31] Xiong Q, Hu L X, Liu Y S, et al. New insight into the toxic effects of chloramphenicol and roxithromycin to algae using FTIR spectroscopy [J]. Aquatic Toxicology, 2019, 207: 197-207
[32] Guo J H, Peng J L, Lei Y, et al. Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris [J]. Aquatic Toxicology, 2020, 219: 105376
[33] Machado M D, Soares E V. Impact of erythromycin on a non-target organism: Cellular effects on the freshwater microalga Pseudokirchneriella subcapitata [J]. Aquatic Toxicology, 2019, 208: 179-186
[34] González-Pleiter M, Gonzalo S, Rodea-Palomares I, et al. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment [J]. Water Research, 2013, 47(6): 2050-2064
[35] Xu D M, Xiao Y P, Pan H, et al. Toxic effects of tetracycline and its degradation products on freshwater green algae [J]. Ecotoxicology and Environmental Safety, 2019, 174: 43-47
[36] Lu L, Wu Y X, Ding H J, et al. The combined and second exposure effect of copper (Ⅱ) and chlortetracycline on fresh water algae, Chlorella pyrenoidosa and Microcystis aeruginosa [J]. Environmental Toxicology and Pharmacology, 2015, 40(1): 140-148
[37] 中华人民共和国国家环境保护总局. 新化学物质危害评估导则: HJ/T 154—2004[S]. 北京: 中国环境科学出版社, 2004
[38] Mao Y F, Yu Y, Ma Z X, et al. Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress [J]. Ecotoxicology and Environmental Safety, 2021, 222: 112496
[39] Niu Z G, Xu W A, Na J, et al. How long-term exposure of environmentally relevant antibiotics may stimulate the growth of Prorocentrum lima: A probable positive factor for red tides [J]. Environmental Pollution, 2019, 255(Pt 1): 113149
[40] Chen S, Wang L Q, Feng W B, et al. Sulfonamides-induced oxidative stress in freshwater microalga Chlorella vulgaris: Evaluation of growth, photosynthesis, antioxidants, ultrastructure, and nucleic acids [J]. Scientific Reports, 2020, 10(1): 8243
[41] Xu D M, Xie Y T, Li J. Toxic effects and molecular mechanisms of sulfamethoxazole on Scenedesmus obliquus [J]. Ecotoxicology and Environmental Safety, 2022, 232: 113258
[42] Chen S, Zhang W, Li J Y, et al. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum) [J]. Environmental Pollution, 2020, 263: 114554
[43] Xiong J Q, Govindwar S, Kurade M B, et al. Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus [J]. Chemosphere, 2019, 218: 551-558
[44] 张红波, 董聪聪, 杨燕君, 等. 基于叶绿素荧光探讨链霉素对念珠藻生长及光合毒性效应[J]. 水生生物学报, 2019, 43(3): 664-669
Zhang H B, Dong C C, Yang Y J, et al. The toxic effect of streptomycin on the growth and photosynthesis of Nostoc using the chlorophyll fluorescence analysis [J]. Acta Hydrobiologica Sinica, 2019, 43(3): 664-669 (in Chinese)
[45] 许萍萍, 涂晓杰, 成凤凤, 等. 庆大霉素对斜生栅藻生长与光合活性的影响[J]. 环境科学与技术, 2021, 44(8): 146-153
Xu P P, Tu X J, Cheng F F, et al. Toxic effects of gentamicin on growth and activity of photosynthetic system Ⅱ of Scenedesmus obliquus [J]. Environmental Science & Technology, 2021, 44(8): 146-153 (in Chinese)
[46] Xiong J Q, Kurade M B, Abou-Shanab R A, et al. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate [J]. Bioresource Technology, 2016, 205: 183-190
[47] Han Q Z, Zheng Y, Qi Q J, et al. Involvement of oxidative stress in the sensitivity of two algal species exposed to roxithromycin [J]. Ecotoxicology, 2020, 29(5): 625-633
[48] Nie X P, Liu B Y, Yu H J, et al. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata [J]. Environmental Pollution, 2013, 172: 23-32
[49] Zhang Q, Bai Y, Chen Z, et al. Lincomycin-induced transcriptional alterations in the green alga Raphidocelis subcapitata [J]. Applied Sciences, 2020, 10(23): 8565
[50] Guo J H, Zhang Y B, Mo J Z, et al. Sulfamethoxazole-altered transcriptomein green alga Raphidocelis subcapitata suggests inhibition of translation and DNA damage repair [J]. Frontiers in Microbiology, 2021, 12: 541451
[51] Li J P, Li W, Min Z F, et al. Physiological, biochemical and transcription effects of roxithromycin before and after phototransformation in Chlorella pyrenoidosa [J]. Aquatic Toxicology, 2021, 238: 105911
[52] Guo J H, Bai Y, Chen Z, et al. Transcriptomic analysis suggests the inhibition of DNA damage repair in green alga Raphidocelis subcapitata exposed to roxithromycin [J]. Ecotoxicology and Environmental Safety, 2020, 201: 110737
[53] Jiang R X, Wei Y R, Sun J Y, et al. Degradation of cefradine in alga-containing water environment: A mechanism and kinetic study [J]. Environmental Science and Pollution Research International, 2019, 26(9): 9184-9192
[54] 杜迎翔, 冯云庆, 项钟润, 等. 蛋白核小球藻去除2种头孢类抗生素的研究[J]. 环境科学与技术, 2015, 38(10): 105-111
Du Y X, Feng Y Q, Xiang Z R, et al. Removal of two cephalosporins in Chlorella pyrenoidosa [J]. Environmental Science & Technology, 2015, 38(10): 105-111 (in Chinese)
[55] Du Y X, Wang J, Li H T, et al. The dual function of the algal treatment: Antibiotic elimination combined with CO2 fixation [J]. Chemosphere, 2018, 211: 192-201
[56] Yu Y, Zhou Y Y, Wang Z L, et al. Investigation of the removal mechanism of antibiotic ceftazidime by green algae and subsequent microbic impact assessment [J]. Scientific Reports, 2017, 7(1): 4168
[57] Chen Q H, Zhang L, Han Y H, et al. Degradation and metabolic pathways of sulfamethazine and enrofloxacin in Chlorella vulgaris and Scenedesmus obliquus treatment systems [J]. Environmental Science and Pollution Research International, 2020, 27(22): 28198-28208
[58] Xiong Q, Liu Y S, Hu L X, et al. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa [J]. Water Research, 2020, 175: 115656
[59] Kiki C, Rashid A, Wang Y W, et al. Dissipation of antibiotics by microalgae: Kinetics, identification of transformation products and pathways [J]. Journal of Hazardous Materials, 2020, 387: 121985
[60] Xiong J Q, Kurade M B, Patil D V, et al. Biodegradation and metabolic fate of levofloxacin via a freshwater green alga, Scenedesmus obliquus in synthetic saline wastewater [J]. Algal Research, 2017, 25: 54-61
[61] Xiong J Q, Kurade M B, Jeon B H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris [J]. Chemical Engineering Journal, 2017, 313: 1251-1257
[62] Zhou T, Cao L P, Zhang Q, et al. Effect of chlortetracycline on the growth and intracellular components of Spirulina platensis and its biodegradation pathway [J]. Journal of Hazardous Materials, 2021, 413: 125310
[63] Zhao F, Zhang D, Xu C Y, et al. The enhanced degradation and detoxification of chlortetracycline by Chlamydomonas reinhardtii [J]. Ecotoxicology and Environmental Safety, 2020, 196: 110552
[64] Pan M M, Lyu T, Zhan L M, et al. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism [J]. Water Research, 2021, 190: 116735
[65] 周楠, 陈建秋, 王晓, 等. 氮磷营养调控对微藻去除抗生素的增效作用研究[J]. 水处理技术, 2021, 47(3): 32-37
Zhou N, Chen J Q, Wang X, et al. Study on the synergistic effect of nitrogen and phosphorus nutrition regulation on the antibiotic removal by microalgae [J]. Technology of Water Treatment, 2021, 47(3): 32-37 (in Chinese)
[66] Batchu S R, Panditi V R, O’Shea K E, et al. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate [J]. The Science of the Total Environment, 2014, 470-471: 299-310
[67] Yan S W, Song W H. Photo-transformation of pharmaceutically active compounds in the aqueous environment: A review [J]. Environmental Science: Processes & Impacts, 2014, 16(4): 697-720
[68] Wei L X, Li H X, Lu J F. Algae-induced photodegradation of antibiotics: A review [J]. Environmental Pollution, 2021, 272: 115589
[69] Tian Y J, Zou J R, Feng L, et al. Chlorella vulgaris enhance the photodegradation of chlortetracycline in aqueous solution via extracellular organic matters (EOMs): Role of triplet state EOMs [J]. Water Research, 2019, 149: 35-41
[70] Tian Y J, Wei L X, Yin Z, et al. Photosensitization mechanism of algogenic extracellular organic matters (EOMs) in the photo-transformation of chlortetracycline: Role of chemical constituents and structure [J]. Water Research, 2019, 164: 114940
[71] Leng L J, Wei L, Xiong Q, et al. Use of microalgae based technology for the removal of antibiotics from wastewater: A review [J]. Chemosphere, 2020, 238: 124680
[72] Santaeufemia S, Torres E, Mera R, et al. Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum [J]. Journal of Hazardous Materials, 2016, 320: 315-325
[73] Hena S, Gutierrez L, Croué J P. Removal of metronidazole from aqueous media by C. vulgaris [J]. Journal of Hazardous Materials, 2020, 384: 121400
[74] Cao J S, Jiang R X, Wang J Q, et al. Study on the interaction mechanism between cefradine and Chlamydomonas reinhardtii in water solutions under dark condition [J]. Ecotoxicology and Environmental Safety, 2018, 159: 56-62
[75] 钟雪晴, 朱雅莉, 王玉娇, 等. 含抗生素废水的微藻处理技术及其进展[J]. 化工进展, 2021, 40(4): 2308-2317
Zhong X Q, Zhu Y L, Wang Y J, et al. Progress on antibiotic wastewater treatment by microalgae [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2308-2317 (in Chinese)
[76] Xiong J Q, Kurade M B, Jeon B H. Can microalgae remove pharmaceutical contaminants from water? [J]. Trends in Biotechnology, 2018, 36(1): 30-44
[77] Zhang C J, Zhang Q F, Dong S S, et al. Could co-substrate sodium acetate simultaneously promote Chlorella to degrade amoxicillin and produce bioresources? [J]. Journal of Hazardous Materials, 2021, 417: 126147
[78] Hom-Diaz A, Jaén-Gil A, Rodríguez-Mozaz S, et al. Insights into removal of antibiotics by selected microalgae (Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta and Pseudokirchneriella subcapitata) [J]. Algal Research, 2022, 61: 102560