重金属由于具有毒性强、不能降解、易富集、能在食物链中传递且具有致癌性等特性,对生态环境和人类健康都有潜在威胁[1]。电镀、制革、防腐和染料等工业生产过程,使大量重金属废水排入河流等水体。调查显示,中国超过80%的江河湖海均存在重金属污染,局部水体污染严重[2]。常见重金属污染物包括铅(Pb)、砷(As)、镉(Cd)、铬(Cr)、铜(Cu)和锌(Zn)等,主要来源于采矿、冶炼、化工、农业及生活垃圾等。
重金属污染废水治理已引起广泛关注。传统处理方法包括电化学法、膜处理法、化学沉淀法、蒸发浓缩法、离子交换法、化学氧化还原法以及活性炭和硅胶吸附法等[3-4]。以上方法能够在一定程度上取得较好的处理效果,但普遍存在投资高、能耗大、不易实现的问题,且大多数方法只能用于高浓度重金属废水,同时易产生二次污染,也很难有针对性地回收某一类重金属,因此在实际应用上受到了很大的限制。近年来,一种适于处理低浓度(1~100 mg·L-1)重金属废水的方法——微生物吸附法因其高效、低成本等优点已引起广泛关注。微生物吸附,即通过微生物自身或产物的化学成分或结构特性吸附去除介质中重(类)金属[5],进而通过固液相分离,从而降低水中金属离子浓度。微生物吸附成本低、效果好、时间短、可重复使用、特异性强且二次污染风险低,可行性和经济性较好[6]。目前,国内外在微生物吸附水体重金属研究中已有较多报道,该技术在成本、运行、操作管理等方面具有较好的优越性,具有较好的开发价值和应用前景[7]。然而,微生物吸附在实际应用中仍具有一定局限性,例如易受外界环境影响、吸附重金属后的微生物不易分离回收等。因此,本文综述不同微生物(细菌、真菌和藻类等)吸附重金属(Pb2+、As3+/As5+、Cd2+、Cr3+/Cr6+、Cu2+和Zn2+等)的效率与机理、吸附影响因素及吸附后重金属离子的解吸回收,并阐述影响其应用的限制性因素及提高实际应用吸附效率的技术与方法,以期为提高微生物吸附去除重金属效率及其实际应用提供理论支撑和技术参考。
1 微生物种类(Microbe species)
1949年,Rushhoft[8]利用活性污泥成功去除水中放射性元素钚(239Pu),因微生物的繁殖形成了“具有较大表面积能吸收放射性元素的胶状基质材料”,所以被认为微生物具有吸附Pu的能力,进而提出了微生物吸附的概念。自此国内外学者开始了不同微生物对不同重金属的吸附研究。1981年Strandberg等[9]发现酿酒酵母(Saccharomyces cerevisiae)和铜绿假单胞菌(Pseudomonas aeruginosa)可吸附铀(U6+);1989年Mullen等[10]发现绿脓杆菌(Pseudomonas aerations)对镧(La3+)具吸附作用。微生物吸附重金属的研究在20世纪90年代后期得到迅速发展,生物吸附材料不断被开发且生物吸附机理的理解不断深入。1991年Gloab等[11]发现链霉菌(Streptomyces)对Pb2+的吸附作用;1994年Volesky和Prasetyo[12]发现海藻(seaweed)可吸附Cd2+;1995年Volesky和May-Phillips[13]发现啤酒菌(Saccharomyces cerevisiae)可吸附水中的Zn2+、Cu2+等;1999年张建民等[14]发现脱硫弧菌(Desulfovibrio)对Cr6+的吸附作用;2008年Teclu等[15]发现硫酸盐还原菌(sulfate-reducing bacteria)对水中As3+和As5+均有吸附作用。微生物吸附去除重金属己成为一个新兴的、环境友好且不引入二次污染的技术,目前已发现的可吸附重金属的微生物众多,主要由细菌、真菌、藻类和放线菌等组成(表1)。
2 微生物吸附重金属的机理与效率(Mechanism and efficiency of microbial adsorption of heavy metals)
微生物吸附重金属是一个复杂过程。同种微生物对不同金属离子亲和力不同,不同种微生物对同一金属离子的耐受性也不同,导致微生物吸附金属机理的多样性和效率的差异性。根据被吸附离子在微生物细胞中的分布差异,分为3种:胞内吸附、胞外吸附和表面吸附[29](图1)。其中表面吸附存在于活性和非活性微生物,而胞外和胞内吸附主要存在于活性微生物。在一个吸附体系中,可能同时存在一种或多种机制。胞外吸附指利用微生物分泌的胞外聚合物(extracellular polymeric substances, EPS),例如糖蛋白、脂多糖、多聚糖以及可溶性氨基酸等,通过吸附、沉淀或络合作用去除重金属离子[30-32]。潘隆尼亚碱湖杆菌(Pannonibacter phragmitetus)吸附Cr6+时,傅里叶变换红外光谱仪(FTIR)和X射线光电子能谱(XPS)分析表明,完整细胞对Cr6+的吸附能力高于不含EPS的细胞[33]。表面吸附指在细胞表面,尤其是细胞壁组分(蛋白质、多糖和脂类等)中的化学官能团(羧基、羟基、磷酰基、酰胺基、硫酸脂基、氨基和巯基等)与金属离子相互作用后发生的吸附过程。当然,由于构象、空间位阻和其他障碍的存在,官能团并不能保证所有重金属都能被成功吸附。目前,金属离子发生表面吸附的主要机制有离子交换、表面络合、物理吸附(范德华力、静电作用)、氧化还原或无机微沉淀等[34]。离子交换指微生物表面存在着例如磷酰基、羟基、羧基和氨基等可与重金属离子结合的官能团,重金属离子与官能团的交换过程即离子交换过程。表面络合指重金属离子可与微生物细胞壁组分中官能团中的氮、硫和氧等提供的孤对电子形成络合物。静电吸附是由于微生物细胞的外表面的负电荷与带正电荷的重金属离子之间存在范德华力。氧化还原指某些本身具有氧化还原能力的微生物,可通过改变重金属离子的价态,使之变成低毒性物质。无机微沉淀是指通过化学物理的作用使重金属离子在微生物细胞壁上或细胞内形成无机沉淀物的过程。除此之外,不同微生物细胞壁的组成成分的差异导致其吸附机理的差异。如Lei等[35]认为细胞壁化学基团的离子交换作用是芽孢杆菌(Bacillus sp.)吸附去除水体重金属(Cu2+、Pb2+等)的主要机制。胞内吸附指当胞外金属离子浓度高于胞内时,金属离子可通过自由扩散方式穿过细胞壁和细胞膜进入胞内。进入细胞后,微生物通过区隔化作用将金属离子分配至代谢活动不活跃的区域(如液泡),或将金属离子与热稳定性蛋白结合,将其转变成为活动性较低的低毒形式[34]。
表1 重金属生物吸附常用微生物
Table 1 Microorganisms commonly used for biosorption of heavy metals

类别Category微生物种类Microbe species细菌Bacteria芽孢杆菌属(Bacillus sp.)[16]、假单胞菌属(Pseudomonas)[17]、微球菌属(Micrococcaceae)[18]、动胶菌(Zoogloea)[19]等真菌Fungi青霉菌(Penicillium)[20]、曲霉(Aspergillus)[21]、根霉菌(Rhizopus)[22]、酿酒酵母(Saccharomyces cerevisiae)[23]等藻类Algae褐藻(Phaeophyceae)[24]、颤藻(Oscillatoria)[25]、斜生栅藻(Scenedesmus obliquus)[26]、金鱼藻(Ceratophyllum demersum)[27]等放线菌Actinomyces链霉菌属(Streptomyces)[28]

图1 重金属微生物吸附机理
Fig. 1 Mechanisms of heavy metals by microbial sorption
微生物对重金属的吸附与微生物本身性质和重金属的种类性质有关。不同种微生物的细胞结构不同,其对同种重金属的吸附机理和吸附能力不同(表2)[36-43]。如对Pb2+的吸附中,地衣芽孢杆菌(Bacillus licheniformis)的吸附率高达96.5%,以化学吸附为主;黑曲霉吸附As时,其对As5+的吸附去除率可达90%以上,对As3+的吸附去除率可达75%,吸附机理为氧化作用和共沉淀;对Cd2+的吸附中,悬浮酵母菌(Saccharomyces cerevisiae)的吸附率高达99%,吸附位点主要为羰基、羧基和氨基等;微紫青霉(Penicillium janthinellum)吸附Cr6+时其吸附率高达87%,主要吸附位点为氨基、羧基、羰基和羟基;苏云金芽孢杆菌(Bacillus thuringiensis)吸附Cu2+时,其最高吸附率可达91.8%,以细胞表面吸附为主;对Zn2+的吸附中,塔玛曲霉(Aspergillus tamarii)的吸附率可达到99.6%,在菌株吸附过程中胺、醇和共轭C![]() C基团与吸附质形成共价键和交联网络的基体层是去除Zn2+的主要原因。
C基团与吸附质形成共价键和交联网络的基体层是去除Zn2+的主要原因。
2.1 细菌吸附重金属的机理与效率
细菌种类繁多,在土壤和水体中分布广泛且繁殖快,是最丰富且重要的微生物资源。细菌可通过多种方式吸附重金属,例如表面吸附、胞内积累及胞外沉淀作用等,从而降低重金属在土壤或水体中的活性和毒性。如表面络合已被证实是胶质芽孢杆菌(Bacillus mucilaginosus)吸附Cr6+的主要机制[44],细胞壁吸附、胞内累积是芽孢杆菌属(Bacillus sp.)吸附As3+[36],物理吸附是芽孢杆菌(Bacillus sp.)吸附Cd2+等离子[70]、离子交换是大肠杆菌(Escherichia)吸附Cd2+的[71]主要机制。细菌表面结构复杂,含有大量可结合金属离子的位点(羧基、氨基和磷酸基团等),为金属离子吸附提供多种方式。例如,革兰氏阳性细菌细胞壁中含有大量肽聚糖(N-乙酰葡萄糖胺、N-乙酰胞壁酸及氨基酸)和磷壁酸(弱酸性物质),失质子后呈电负性,通过静电吸附重金属离子[72]。革兰氏阴性细菌可利用肽聚糖外侧的脂多糖(厚度8~10 nm的类脂、核心多糖和特异性多糖)吸附重金属离子[73]。目前,已发现具备重(类)金属吸附作用的细菌众多(表2),其中,研究较多的为芽孢杆菌(Bacillus sp.),其对Pb2+、Cd2+和Cr6+等具有较为突出的吸附作用,吸附容量高达164、132和257 mg·g-1。
2.2 真菌吸附重金属的机理与效率
现存的真菌约有12万种,包括酵母菌、霉菌和大型真菌等。真菌来源广泛易得,且适合扩大培养,吸附量大、吸附后金属离子与菌体易分离[74],可极大降低重金属生物吸附剂的生产成本。真菌细胞壁富含带有负电荷的多糖和几丁质等,利于吸附重金属[75]。真菌对重金属的吸附方式主要有2种,一种是以代谢为目的的主动金属离子的吸附,即金属离子通过细胞膜进入细胞内,该过程是活细胞吸附,如白耙齿菌(Irpex lacteus)吸附Cu2+时是胞内富集[55];另一种是由细胞及其组成成分的化学补偿而引起的被动吸附结合,即通过络合、离子交换、螯合、协同、沉淀、物理吸附的方式将重金属去除,该过程在活细胞和死细胞中均可发生,例如,酵母菌(Saccharomyces)通过表面吸附过程吸附Cu2+[64],酿酒酵母(Saccharomyces cerevisiae)[71]、球孢白僵菌(Beauveria bassiana)[54]通过离子交换吸附Cd2+。由表2可知,茎点霉属(Phoma sp.)吸附Pb2+时,其最大吸附容量可达61.9 mg·g-1;黄孢原毛平革菌(Phanerochaete chrysosporium)吸附Cd2+时,最大吸附容量为71.4 mg·g-1;啤酒酵母(Saccharomyces cerevisiae)吸附Cr3+时,最大吸附量达34.9 mg·g-1。
2.3 藻类吸附重金属的机理与效率
藻类是一种可再生的天然生物质,全球已知的藻类约4万种。作为一类光合自养生物,藻类对多种重金属均表现出良好的吸附能力,具有吸附能力强、去除率高、原料易得和价格低廉等优点。由纤维素、果胶质和多糖等组成的细胞壁带有一定电荷和粘性,在吸附重金属过程中发挥重要作用。藻类表面的羟基、羧基、巯基、氨基、羰基、硫基、醛基、磷酸根和硫酸根等多种化学基团,可通过离子交换、络合及静电吸附等作用吸附重金属离子[76]。此外,藻类细胞膜是天然的具有高度选择性的半透膜,这些特性决定了其对重金属离子有很好的吸附效果。如Mata等[77]发现墨角藻(Fucus vesiculosus)细胞壁含有岩藻聚糖、藻酸盐等,通过表面络合吸附Pb2+、Cu2+和Cd2+。Sheng等[78]发现马尾藻(Scagassum)、团扇藻(Padina)对Pb2+、Zn2+、Cu2+和Cd2+的较高吸附能力主要由于细胞壁表面羟基、乙醇基和氨基的络合作用。Romera等[79]对藻类去除重金属废水的文献做了归纳和比较,发现与细菌、真菌等其他生物吸附材料相比,藻类在重金属吸附去除方面的研究比较少;且综合比较分析了20种褐藻(Phaeophyceae)、9种红藻(Rhodophyta)和8种绿藻(Chlorophyceae)对重金属的吸附能力,发现以褐藻的研究较多,且在所有情况下褐藻的吸附效果最好,其次是绿藻,平均吸附能力较低的是红藻,这些差异可以归因于每项工作的实验条件和相应细胞壁的化学成分等。由表2可知,钝顶螺旋藻(Spirulina platensis)对Cd2+、水华蓝藻(cyanobacteria)对Cu2+和鱼腥藻(Anabaena)对Zn2+的吸附率分别为95.9%、78.1%和77.3%。
3 微生物吸附影响因素(Factors affecting microbial-sorption)
3.1 微生物种类
吸附过程中,不同微生物对同一金属离子的吸附能力不同,如泡叶藻(Ascophyllum nodosum)对Zn2+的吸附量是酿酒酵母(Saccharomyces cerevisiae)的7.42倍(25.6 mg·g-1 vs. 3.45 mg·g-1),龟裂链霉菌(Streptomyces rimosus)对Cu2+的吸附量是泡叶藻的1.85倍(9.07 mg·g-1 vs. 4.89 mg·g-1),黄孢原毛平革菌(Phanerochaete chrysosporium)对Pb2+的吸附量是棒状链霉菌(Streptomy cesclavuioligerus)的近3倍(419 mg·g-1 vs. 140 mg·g-1)(表3)。
3.2 微生物状态
微生物状态也是吸附过程的一个重要影响因素。活性微生物可通过代谢提供的能量在胞内进行积累,非活性微生物无胞内积累能力,但其失活状态可降低重金属的毒性效应,从而促进吸附。另外,当微生物存在状态不同(游离态、载体固定态)时,其吸附效果亦不同。一般情况下,将微生物固定化可提高微生物的细胞浓度,使微生物保持着较高的生物活性,从而提高其吸附性能。如冯伟等[25]利用丝瓜络固定非活性颤藻(Oscillatoria),发现pH=5时,Pb2+初始浓度分别为70 mg·L-1和60 mg·L-1,接触时间为90 min和60 min时,固定化和游离颤藻的吸附量达到最大,且前者的吸附量增加约20.6%。
3.3 重金属离子初始浓度
在一定浓度范围内,随重金属离子初始浓度升高,吸附量和吸附效率均升高,当达到一定浓度时吸附量达到顶值,继续升高浓度,吸附量保持在一个固定值范围内波动,吸附效率则逐渐下降。郑爱芳等[55]发现白耙齿菌(Irpex lacteus)吸附Cu2+时,随初始Cu2+浓度(20~80 mg·L-1)增加,菌株吸附容量不断增加(3.87~17.2 mg·g-1),而吸附率在初始Cu2+浓度20~40 mg·L-1范围内增加,在40 mg·L-1时吸附率达到最大(27%),之后开始降低。
3.4 吸附时间
微生物吸附效率随时长升高。微生物吸附重金属过程可分为2个阶段,快速吸附阶段和慢速吸附阶段。前者是快速的表面吸附过程,通常在几十分钟内即能达到饱和吸附量的70%左右,该过程通常发生在细胞表面,主要是物理吸附。后者为慢速胞内积累过程,这一阶段重金属离子逐渐向细胞内转移,持续几小时后达到吸附平衡,与化学吸附或微生物的新陈代谢作用有关[83]。另外,活性菌株对重金属的吸附作用与其生长速度及周期有关,研究表明,活性菌株在对数生长期内去除率增长最快,在停滞期达到最大。如白耙齿菌(Irpex lacteus)吸附Cu2+时,在前1 h内,随时间增加其菌株吸附容量增加,之后随时间延长吸附量略有降低,最终吸附达到饱和,趋于稳定[55]。
表2 细菌、真菌和藻类对重金属离子的吸附效率及机理
Table 2 Heavy metals biosorption efficiency and mechanism for bacteria, fungi and algae
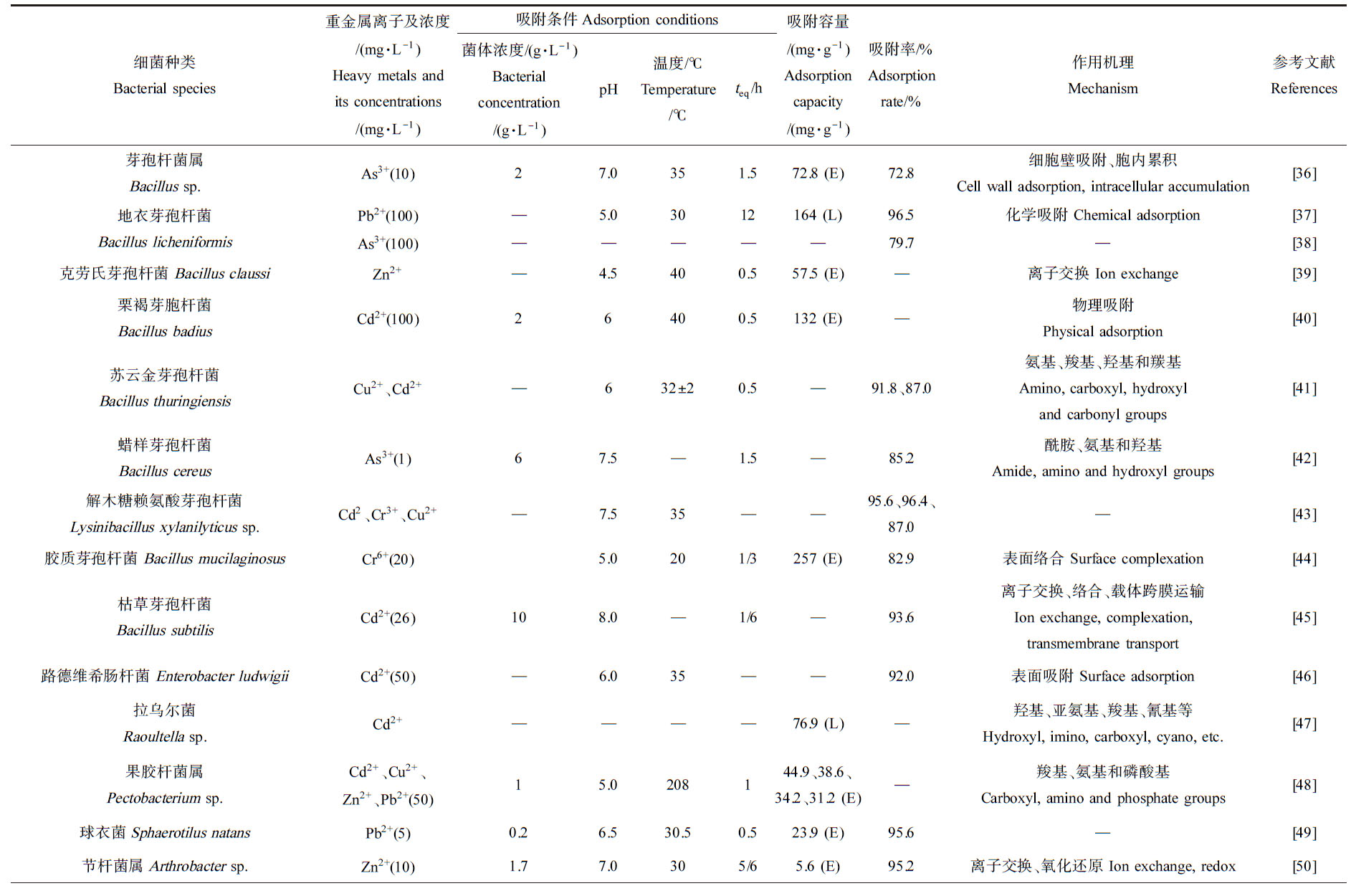
细菌种类Bacterial species重金属离子及浓度/(mg·L-1)Heavy metals and its concentrations/(mg·L-1)吸附条件Adsorption conditions菌体浓度/(g·L-1)Bacterial concentration/(g·L-1)pH温度/℃Temperature/℃teq/h吸附容量/(mg·g-1)Adsorption capacity/(mg·g-1)吸附率/%Adsorption rate/%作用机理Mechanism参考文献References芽孢杆菌属Bacillus sp.As3+ (10)27.0351.572.8 (E)72.8细胞壁吸附、胞内累积Cell wall adsorption, intracellular accumulation[36]地衣芽孢杆菌Bacillus licheniformisPb2+ (100)—5.03012164 (L)96.5化学吸附 Chemical adsorption[37]As3+ (100)—————79.7—[38]克劳氏芽孢杆菌 Bacillus claussiZn2+—4.5400.557.5 (E)—离子交换 Ion exchange[39]栗褐芽胞杆菌Bacillus badiusCd2+ (100)26400.5132 (E)—物理吸附Physical adsorption[40]苏云金芽孢杆菌Bacillus thuringiensisCu2+、Cd2+—632±20.5—91.8、87.0氨基、羧基、羟基和羰基Amino, carboxyl, hydroxyl and carbonyl groups[41]蜡样芽孢杆菌Bacillus cereusAs3+ (1)67.5—1.5—85.2酰胺、氨基和羟基Amide, amino and hydroxyl groups[42]解木糖赖氨酸芽孢杆菌Lysinibacillus xylanilyticus sp.Cd2、Cr3+、Cu2+—7.535——95.6、96.4、87.0—[43]胶质芽孢杆菌 Bacillus mucilaginosusCr6+ (20)5.0201/3257 (E)82.9表面络合 Surface complexation[44]枯草芽孢杆菌Bacillus subtilisCd2+ (26)108.0—1/6—93.6离子交换、络合、载体跨膜运输Ion exchange, complexation, transmembrane transport[45]路德维希肠杆菌 Enterobacter ludwigiiCd2+ (50)—6.035——92.0表面吸附 Surface adsorption[46]拉乌尔菌Raoultella sp.Cd2+————76.9 (L)—羟基、亚氨基、羧基、氰基等Hydroxyl, imino, carboxyl, cyano, etc.[47]果胶杆菌属Pectobacterium sp.Cd2+、Cu2+、Zn2+、Pb2+ (50)15.0208144.9、38.6、34.2、31.2 (E)—羧基、氨基和磷酸基Carboxyl, amino and phosphate groups[48]球衣菌Sphaerotilus natansPb2+ (5)0.26.530.50.523.9 (E)95.6—[49]节杆菌属Arthrobacter sp.Zn2+ (10)1.77.0305/65.6 (E)95.2离子交换、氧化还原 Ion exchange, redox[50]续表2真菌种类Fungal species重金属离子及浓度/(mg·L-1)Heavy metals and its concentrations/(mg·L-1)吸附条件Adsorption conditions菌体浓度/(g·L-1)Bacterial concentration/(g·L-1)pH温度/℃Temperature/℃teq/h吸附容量/(mg·g-1)Adsorption capacity/(mg·g-1)吸附率/%Adsorption rate/%作用机理Mechanism参考文献References塔玛曲霉Aspergillus tamariiZn2+ (25)2.56.0302/354.7 (L)99.6胺基、醇基、共轭CC基团Amine group, alcohol group, conjugated CC group[21]酱油曲霉Aspergillus sojae米曲霉Aspergillus oryzaePb2+、Cd2+—5.5301—69.8、72.360.6、81.3—[51]茎点霉Phoma sp.Pb2+ (2 000)—7.025—61.9 (E)56.3—[52]黄孢原毛平革菌Phanerochaete chrysosporiumCd2+ (25)—636971.4 (L)96.2羟基、羧基和氨基Hydroxyl, carboxyl and amino groups[53]球孢白僵菌 Beauveria bassianaCd2+ (40)—6.025——97.4离子交换 Ion exchange[54]白耙齿菌Irpex lacteusCu2+ (80)—4.015117.2 (E)—胞内富集 Intracellular accumulation[55]
续表2
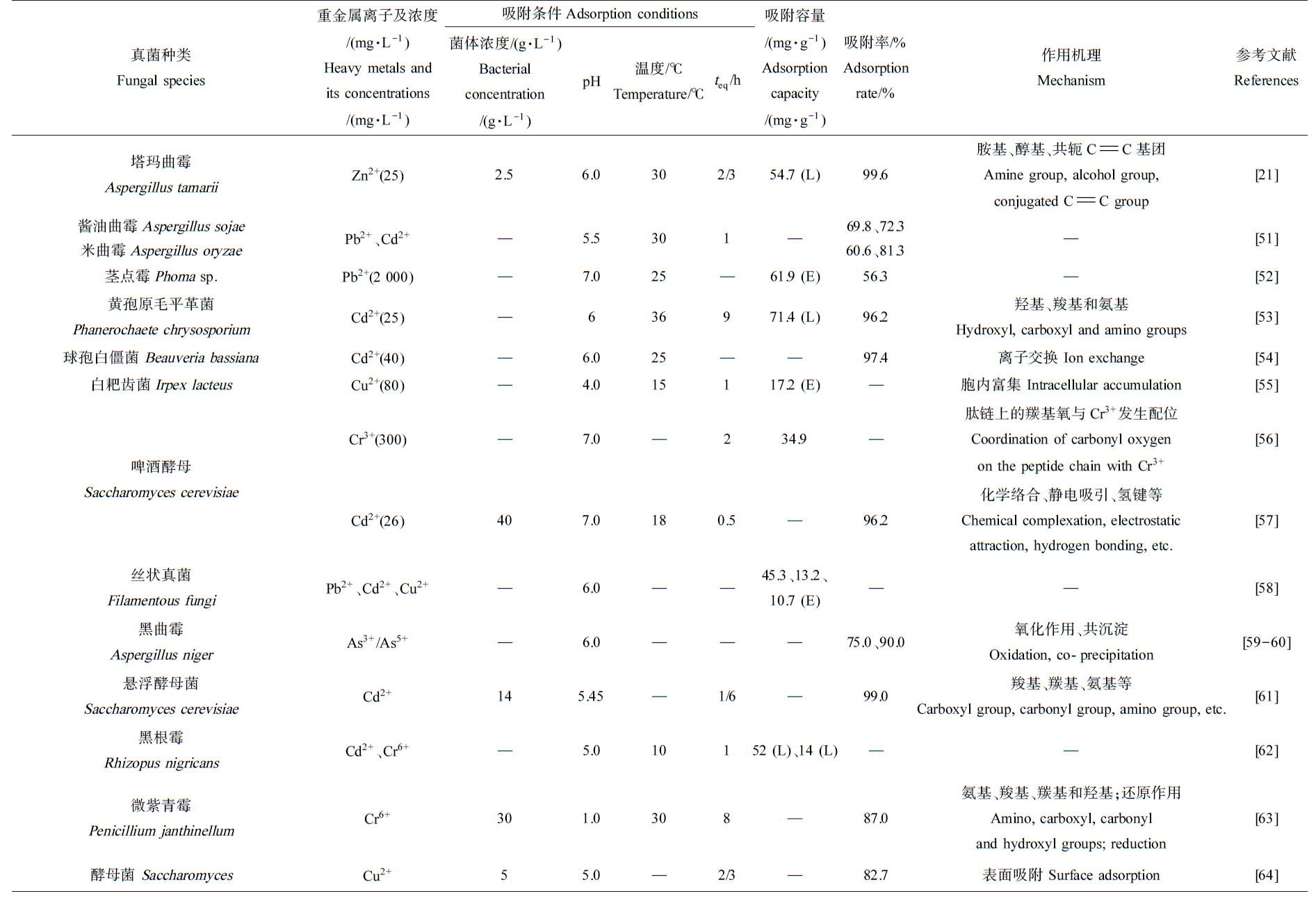
啤酒酵母Saccharomyces cerevisiaeCr3+ (300)—7.0—234.9—肽链上的羰基氧与Cr3+发生配位Coordination of carbonyl oxygen on the peptide chain with Cr3+[56]Cd2+ (26)407.0180.5—96.2化学络合、静电吸引、氢键等Chemical complexation, electrostatic attraction, hydrogen bonding, etc.[57]丝状真菌Filamentous fungiPb2+、Cd2+、Cu2+—6.0——45.3、13.2、10.7 (E)——[58]黑曲霉Aspergillus nigerAs3+/As5+—6.0———75.0、90.0氧化作用、共沉淀Oxidation, co-precipitation[59-60]悬浮酵母菌Saccharomyces cerevisiaeCd2+145.45—1/6—99.0羧基、羰基、氨基等Carboxyl group, carbonyl group, amino group, etc.[61]黑根霉Rhizopus nigricansCd2+、Cr6+—5.010152 (L)、14 (L)——[62]微紫青霉Penicillium janthinellumCr6+301.0308—87.0氨基、羧基、羰基和羟基;还原作用Amino, carboxyl, carbonyl and hydroxyl groups; reduction[63]酵母菌 SaccharomycesCu2+55.0—2/3—82.7表面吸附 Surface adsorption[64]续表2藻类Algae重金属离子及浓度/(mg·L-1)Heavy metals and its concentrations/(mg·L-1)吸附条件Adsorption conditions菌体浓度/(g·L-1)Bacterial concentration/(g·L-1)pH温度/℃Temperature/℃teq/h吸附容量/(mg·g-1)Adsorption capacity/(mg·g-1)吸附率/%Adsorption rate/%作用机理Mechanism参考文献References颤藻(Oscillatoria)Pb2+ (70)—5—1.587.8±1.51 (L)—离子交换、络合作用Ion exchange, complexation[25]钝顶螺旋藻(Spirulina platensis)Cd2+—7—0.598.0 (L)95.9—[65]水华蓝藻(Cyanobacteria)Cu2+—4——7.81 (E)78.1—[66]鱼腥藻(Anabaena)Zn2+0.629———4.86 (E)77.3—[67]双歧褐藻(Bifurcaria bifurcata)多棱褐藻(Saccorhiza polyschides)产岩藻多糖(Pelvetia caniculata)结球藻(Ascophyllum nodosum)楔基海带(Laminaria ochroleuca)Cd2+—4.5——74±3 (L)95±3 (L)73±2 (L)79±2 (L)64±3 (L)—羧基Carboxyl group[68]
续表2

海带(Laminaria japonica)裙带菜(Undaria pinnatifida)条斑紫菜(Porphyra yezoensis)Cu2+——20—89.6 (L)62.5 (L)10.7 (L)—羧基Carboxyl group—[69]
注:teq表示平衡时间;E表示实验数值;L表示模型数值。
Note: teq indicates equilibrium time; E represents the experimental value; L represents the model value.
表3 不同微生物对重金属的吸附能力对比
Table 3 Comparison of biosorption capacity of heavy metals by different microbes

金属离子Heavy metals吸附量/(mg·g-1)Adsorption capacity/(mg·g-1)参考文献ReferencesZn2+泡叶藻(Ascophyllum nodosum)(25.6)>产黄青霉(Penicillium chrysogenum)(19.2)>墨角藻(Fucus vesiculo-sus)(17.3)>龟裂链霉菌(Streptomyces rimosus)(6.63)>酿酒酵母(Saccharomyces cerevisiae)(3.45)[80]Cu2+龟裂链霉菌(Streptomyces rimosus)(9.07)>产黄青霉(Penicillium chrysogenum)(8.62)>墨角藻(Fucus vesic-ulosus)(7.37)>酿酒酵母(Saccharomyces cerevisiae)(4.93)>泡叶藻(Ascophyllum nodosum)(4.89)[80]Pb2+黄孢原毛平革菌(Phanerochaete chrysosporium)(419)>黑根霉(Rhizopus nigricans)(403)>绛红小单孢菌(Micromonospora purpurea)(280)>酿酒酵母(Saccharomyces cerevisiae)(211)>土曲霉(Aspergillus terreus)(201)>伊纽小单孢菌(Micromonospora inyoensis)(159)>棒状链霉菌(Streptomy cesclavuioligerus)(140)[81]Cd2+/Cu2+迟缓芽孢杆菌(Bacillus lentus)(≈30)>米曲霉(Aspergillus oryzae)>酿酒酵母(Saccharomyces cerevisiae)(<5)[82]
3.5 共存离子
共存离子通过竞争吸附位点降低目标金属离子的吸附。相反,某些共存离子对目标金属离子吸附具有促进作用。例如,沟戈登氏菌(Gordona amarae)吸附Pb2+过程中添加K+、Na+、Ca2+和Mg2+可抑制吸附,原因是与Pb2+共同竞争细胞表面的电负性吸附位点,而![]() 则促进吸附,
则促进吸附,![]() 使吸附率由88%提高至93%[84],原因是
使吸附率由88%提高至93%[84],原因是![]() 破坏了细胞膜导致通路增加,从而增加细胞膜传输量。
破坏了细胞膜导致通路增加,从而增加细胞膜传输量。
3.6 环境因素
(1)pH。通常认为pH是影响微生物吸附最显著的因素。pH对金属离子的化学特性、细胞壁表面官能团的活性和金属离子间的竞争等均有影响。它直接影响有机或无机配体的络合、氨离子的水解、氧化还原反应和沉淀反应等。当环境pH较低时,溶液中H3O+浓度升高,微生物表面可被H3O+占据,由于静电斥力导致金属离子难以与微生物表面的吸附位点结合。随pH升高,细胞表面暴露出相对较多的羧基、磷酸基和氨基等电负性基团,可与正电性金属离子结合[85],吸附效率逐步增强。而随pH的继续升高,溶液中金属离子与水电离出的OH-结合形成氢氧化物[86],从而导致微生物吸附量降低。当pH超过金属离子沉淀反应的上限数值时,重金属水解形成不溶性/难溶性沉淀,导致其生物吸附效率降低。研究表明,不同微生物在同一pH条件下对重金属的吸附效率存在差异,但具有相似规律,即吸附曲线表现为:随pH升高,吸附量呈先增加后降低的趋势[6](图2)。徐韶足等[47]通过研究拉乌尔菌(Raoultella sp.)对Cd2+的吸附,发现随着pH增加,菌株对Cd2+的吸附量逐渐增加,在pH=6.0时吸附量达到最大(61.6 mg·g-1),之后随着pH继续增加,吸附量下降。郑爱芳等[55]亦发现白耙齿菌(Irpex lacteus)吸附Cu2+时,随pH升高其吸附容量增加,在达到最大吸附容量(pH 4.0,5.2 mg·g-1)后开始下降。
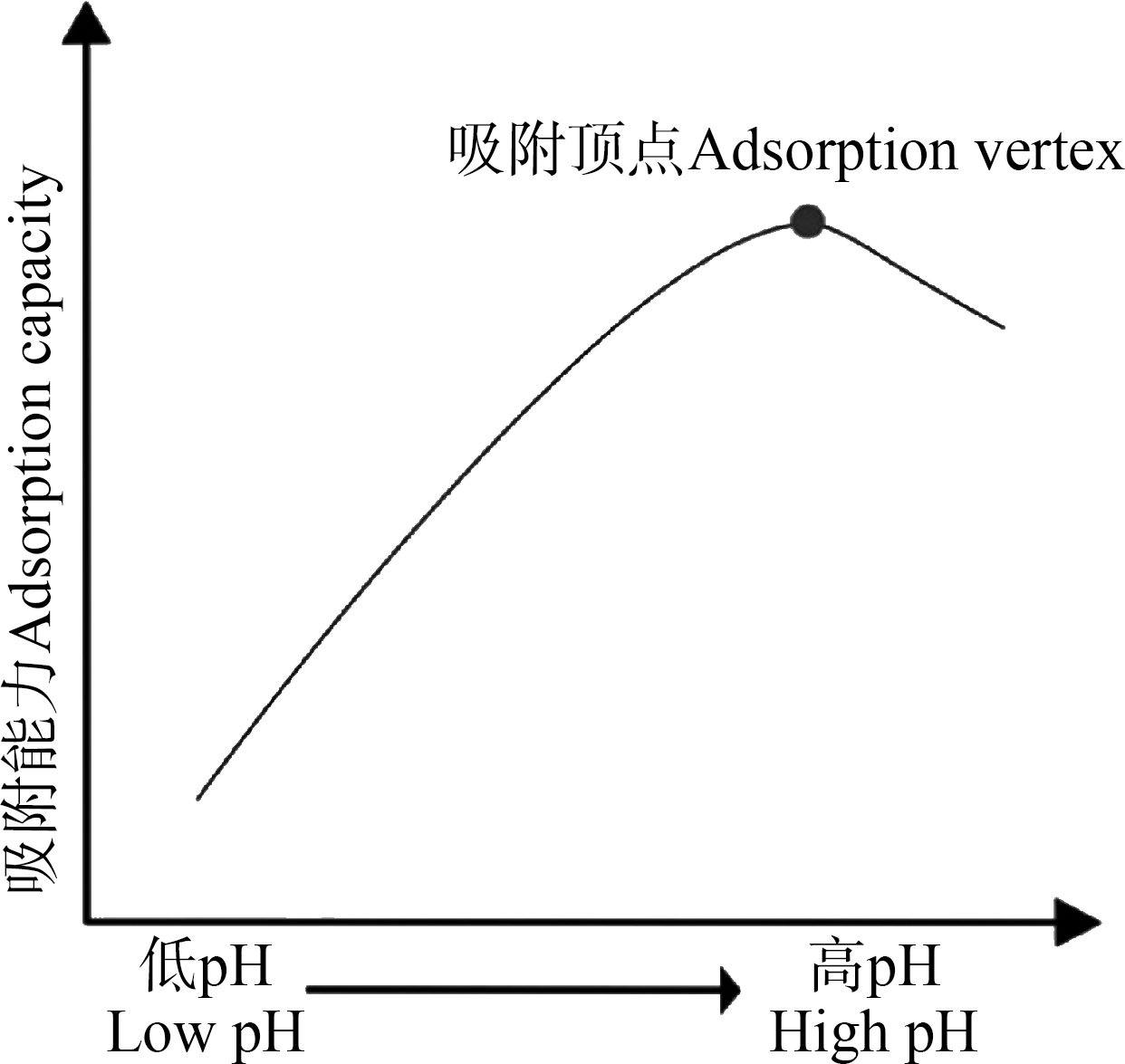
图2 pH对微生物吸附重金属的影响[6]
Fig. 2 The effect of pH on microbial-sorption of heavy metals[6]
(2)温度。温度可影响溶液的物理化学性质和微生物的生理代谢活动,当温度过高时,可使菌体代谢减缓以及代谢产物失活;当温度过低时,可抑制代谢产物的活性,同时代谢减慢[83],进而影响其对重金属离子的吸附作用。在适宜温度范围(20~35 ℃)内,微生物代谢活动随温度升高而增强,吸附能力随之提高。如余甜甜等[54]的研究表明,在10~40 ℃范围内,球孢白僵菌(Beauveria bassiana)吸附Cd2+吸附率呈现先上升后下降的趋势,25 ℃时吸附率达最大值60.1%。然而,鉴于增温会导致吸附成本增加,所以一般不建议采用高温操作。可用来吸附金属离子的微生物主要为中温性微生物。也有研究表明,当温度在适合细菌繁殖的范围内时,温度对吸附过程无明显影响。如余晨兴等[49]发现球衣菌(Sphaerotilus natans)对Pb2+的吸附不依赖温度,在适宜条件下,其吸附率可达95.5%,吸附量为239 mg·g-1。
4 吸附后重金属离子的解吸(Desorption of heavy metals after biosorption)
微生物吸附重金属后,需将吸附的重金属进行解吸,避免二次污染的同时可实现贵重金属回收。解吸方法较多,化学试剂洗涤是常用方法之一。常用解吸剂有盐酸、硫酸、硝酸、乙酸、EDTA、硫脲和碳酸盐等。不同解吸剂对不同重金属的解吸效率存在差异(表4),如硝酸对Pb2+的解吸率比Na2EDTA对Pb2+的解吸率更高(95% vs. 90%),而Na2EDTA对Ni2+的解吸率仅为21.1%、硝酸钠对Hg2+的解吸率仅为3.65%;盐酸对Mn2+的解吸率为92%,而草酸对Cu2+的解吸率为42.2%。因此在实际应用过程中需根据不同的吸附体系选择解吸速度快和效率高的解吸剂。
5 微生物吸附材料改性及固定化(Modification and immobilization of microbial adsorbents)
5.1 微生物吸附材料的改性
一般来说,未经处理的微生物其吸附能力很低。可通过增加吸附剂表面的官能团数量和增加吸附位点来提高吸附能力,包括物理、化学或生物方法(图3)。常用物理方法包括加热、干燥、冻融和粉碎等;化学方法包括利用试剂(如酸、碱、有机溶剂和无机盐等)对微生物进行处理;生物方法包括培养液优化、基因工程等(表5)[31]。
表4 微生物吸附重金属后的解吸(解吸剂种类与浓度、反应条件与解吸率)
Table 4 Desorption of heavy metals after biosorption (desorption agents and concentration, reaction condition and desorption efficiency)

重金属离子Heavy metals解吸剂Desorption agent反应条件Reaction condition解吸剂浓度/(mol·L-1)Concentration of desorbent/(mol·L-1)金属离子浓度/(mg·L-1)Concentration of heavy metals/(mg·L-1)温度/℃Temperature/℃解吸时间/hDesorption time/h解吸率/%Desorption rate/%参考文献ReferencesPb2+硝酸 HNO30.00175301295[87]Na2EDTA0.1——390[88]盐酸 HCl0.5—300.593[37]Cd2+Cd2+Cu2+Zn2+盐酸 HCl0.1——2.5硝酸 HNO30.1———9089.888.590.4[40][48]Cu2+盐酸 HCl0.1——2481[89]Mn2+盐酸 HCl0.1——892[90]U6+碳酸钠 NaNO30.117—0.2597[91]Pt4+EDTA0.1———90.7[92]铼(Re3+)碳酸钠 NaNO30.11030285.7草酸钾 K2C2O4————80.3柠檬酸钠Trisodium citrate dihydrate————92.3EDTA0.0011030290[93]
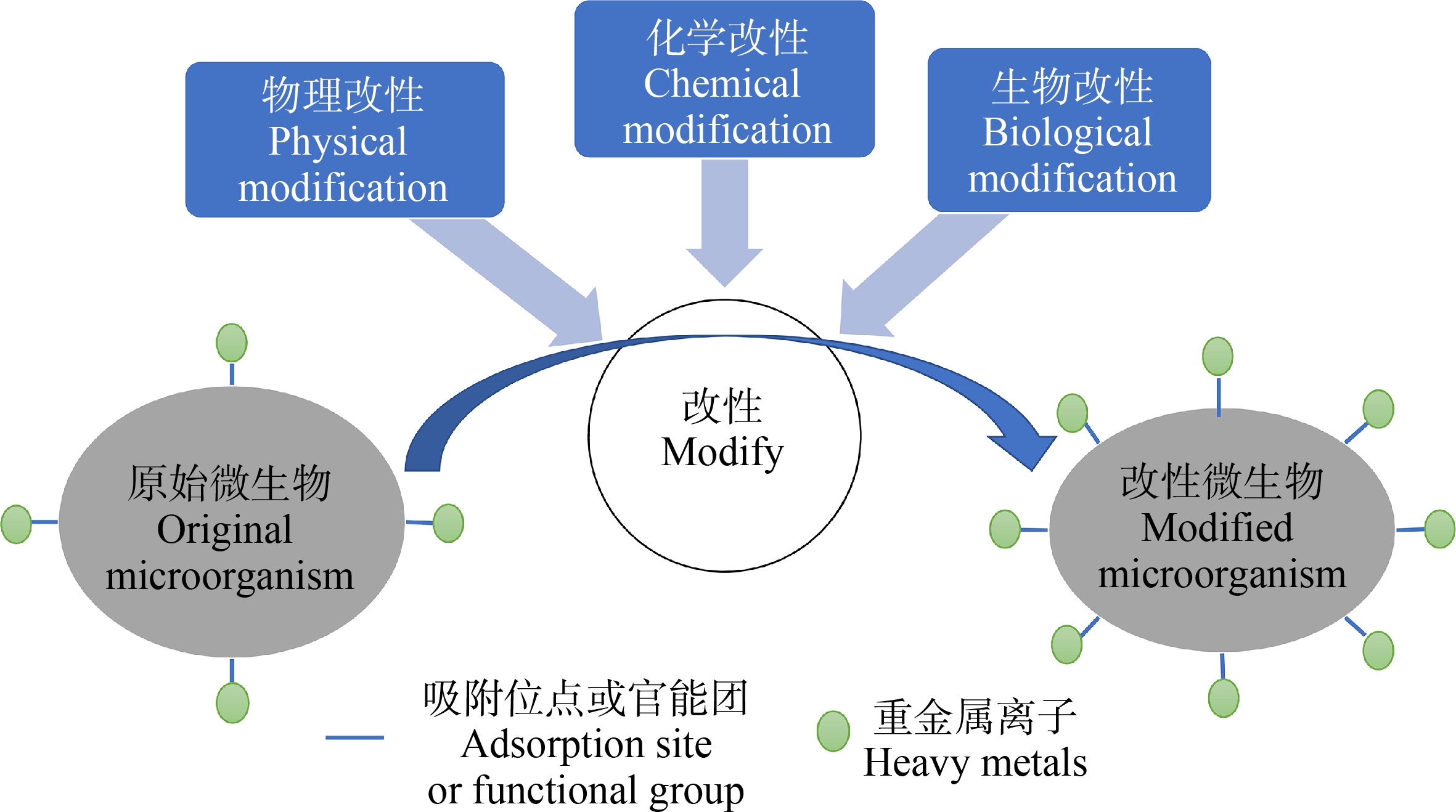
图3 微生物材料改性方法
Fig. 3 Methods for microbial adsorbents modification
表5 微生物吸附剂改性方法[31]
Table 5 Modification method of microbial adsorbents[31]
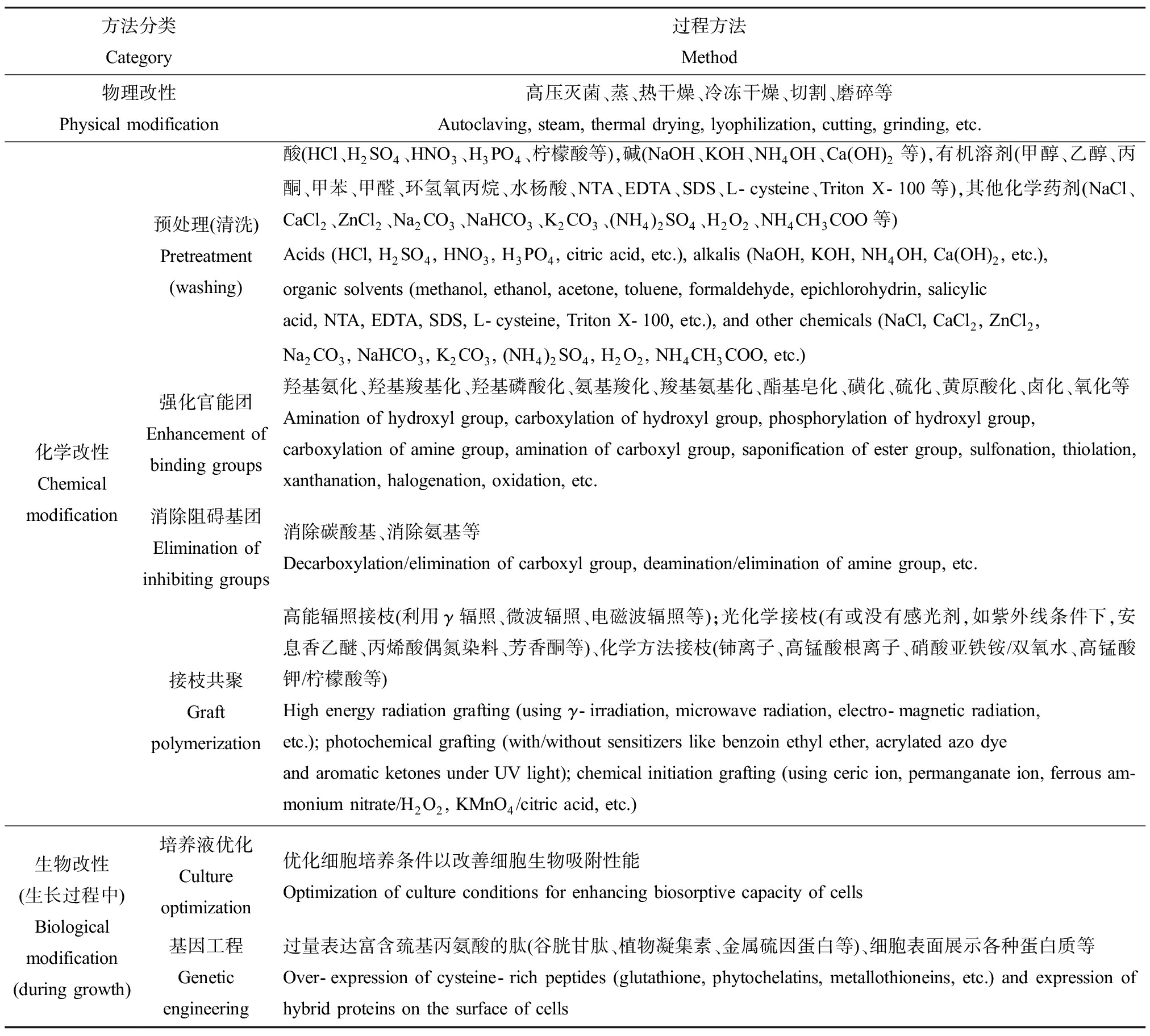
方法分类Category过程方法Method物理改性Physical modification高压灭菌、蒸、热干燥、冷冻干燥、切割、磨碎等Autoclaving, steam, thermal drying, lyophilization, cutting, grinding, etc.化学改性Chemical modification预处理(清洗)Pretreatment(washing)酸(HCl、H2SO4、HNO3、H3PO4、柠檬酸等),碱(NaOH、KOH、NH4OH、Ca(OH)2等),有机溶剂(甲醇、乙醇、丙酮、甲苯、甲醛、环氢氧丙烷、水杨酸、NTA、EDTA、SDS、L-cysteine、Triton X-100等),其他化学药剂(NaCl、CaCl2、ZnCl2、Na2CO3、NaHCO3、K2CO3、(NH4)2SO4、H2O2、NH4CH3COO等)Acids (HCl, H2SO4, HNO3, H3PO4, citric acid, etc.), alkalis (NaOH, KOH, NH4OH, Ca(OH)2, etc.),organic solvents (methanol, ethanol, acetone, toluene, formaldehyde, epichlorohydrin, salicylicacid, NTA, EDTA, SDS, L-cysteine, Triton X-100, etc.), and other chemicals (NaCl, CaCl2, ZnCl2,Na2CO3, NaHCO3, K2CO3, (NH4)2SO4, H2O2, NH4CH3COO, etc.)强化官能团Enhancement ofbinding groups羟基氨化、羟基羧基化、羟基磷酸化、氨基羧化、羧基氨基化、酯基皂化、磺化、硫化、黄原酸化、卤化、氧化等Amination of hydroxyl group, carboxylation of hydroxyl group, phosphorylation of hydroxyl group,carboxylation of amine group, amination of carboxyl group, saponification of ester group, sulfonation, thiolation, xanthanation, halogenation, oxidation, etc.消除阻碍基团Elimination ofinhibiting groups消除碳酸基、消除氨基等Decarboxylation/elimination of carboxyl group, deamination/elimination of amine group, etc.接枝共聚Graft polymerization高能辐照接枝(利用γ辐照、微波辐照、电磁波辐照等);光化学接枝(有或没有感光剂,如紫外线条件下,安息香乙醚、丙烯酸偶氮染料、芳香酮等)、化学方法接枝(铈离子、高锰酸根离子、硝酸亚铁铵/双氧水、高锰酸钾/柠檬酸等)High energy radiation grafting (using γ-irradiation, microwave radiation, electro-magnetic radiation,etc.); photochemical grafting (with/without sensitizers like benzoin ethyl ether, acrylated azo dyeand aromatic ketones under UV light); chemical initiation grafting (using ceric ion, permanganate ion, ferrous am-monium nitrate/H2O2, KMnO4/citric acid, etc.)生物改性(生长过程中)Biological modification (during growth)培养液优化Culture optimization优化细胞培养条件以改善细胞生物吸附性能Optimization of culture conditions for enhancing biosorptive capacity of cells基因工程Genetic engineering过量表达富含巯基丙氨酸的肽(谷胱甘肽、植物凝集素、金属硫因蛋白等)、细胞表面展示各种蛋白质等Over-expression of cysteine-rich peptides (glutathione, phytochelatins, metallothioneins, etc.) and expression of hybrid proteins on the surface of cells
将微生物使用物理方法(如高温、高压、射线照射和冷冻等)灭活后,得到的微生物材料仍可对重金属进行吸附,灭活后的微生物可降低环境因素对其吸附效果的影响,甚至部分灭活后的微生物的吸附效果优于活体微生物,原因可能是高温(或高压、冷冻等)会破坏微生物的细胞结构,细胞内与金属结合的物质暴露出来,从而金属结合位点增加[94]。例如,Velásquez和Dussan[95]研究球形芽孢杆菌(Bacillus sphaericus)对Cr6+的吸附时,发现死菌株较活菌株其吸附率提高19.5%(44.5% vs. 25.0%)。Mohapatra等[96]用厦门芽孢杆菌(Bacillus xiamenensis)作为吸附剂去除水溶液中Pb2+,结果发现活性菌株与高压灭活菌株对Pb2+的吸附量分别为207.4 mg·g-1和216.8 mg·g-1,表明灭活使菌株的吸附量略有提高。死细胞吸附主要是通过具有配位能力的基团,如巯基、羧基和羟基等与金属离子形成离子键或共价键结合重金属离子[97]。利用物理方法改性微生物材料,方法简单且成本低,但其效果通常不及化学改性。
化学改性指利用各种试剂(酸、碱、有机溶剂、无机盐等有机物或无机物)处理微生物,增加或提高微生物表面的重金属结合位点暴露率,从而增强微生物吸附效率的方法,主要包括预处理、增加结合位点和结合位点修饰3种方式。在各种方法中,化学预处理(洗法)因其简单、高效而受到青睐。在许多情况下,洗法可以提高微生物对金属阳离子的吸附能力,然而,一些化学物质可导致微生物质量损失(结构损伤),较多研究根据改性生物吸附剂对目标金属离子的去除率对改性方法的效率进行评估,未考虑微生物生物量[31]。如Mungasavalli等[98]利用十六烷基三甲基溴化铵对黑曲霉(Aspergillus niger)进行预处理后,发现经过处理后的菌体对Cr6+的吸附率由30%提升至63%。戊二醛交联胱氨酸处理酿酒酵母(Saccharomyces cerevisiae)后,其对Pb2+和Cd2+的吸附容量分别由19 mg·g-1和3.9 mg·g-1提高到45.9 mg·g-1和11.6 mg·g-1[99]。氯化铵改性的拉乌尔菌(Raoultella sp.)对Cd2+的吸附容量较原始菌株增加了47.3%(66.4 mg·g-1 vs. 45 mg·g-1)[47]。
生长条件影响微生物细胞表面性质,从而影响生物吸附量[100]。因此,可在微生物生长过程中对生物体进行改性。生物改性通过改善微生物培养条件(如制备絮凝型微生物等)、基因工程菌(如细胞表面展示各种蛋白质等)等提高微生物的吸附能力。但在实际应用中这些方法在存在操作复杂、成本高等缺点,因此运用较少。
综上所述,由于微生物表面具有大量官能团,可通过不同方法引入更多的具有金属离子结合能力的官能团,从而提高其对重金属的吸附效率。但值得注意的是,各种改性方法均增加吸附剂的生产成本[31],且具有潜在生态环境风险,例如过程中使用有毒化学药剂、有害微生物等,其中基因工程菌争议较大[101];部分改性过程较为复杂;部分化学药剂可能会破坏生物本身结构,降低生物质量,导致生物吸附量降低[102]。因此,寻求经济、环境友好及操作简单的改性方法是微生物改性需关注的问题。
5.2 微生物固定化
微生物固定化技术指采用物理或化学的方法将微生物通过吸附、共价结合、截留及包埋等方式固定在特定材料中,再将整体加入到反应体系的技术[103-104]。相较于常规的微生物技术,该技术具有成本低、效果好、可重复利用、利于固液分离等优点,在实际应用中更有优势[105]。自20世纪60年代被提出以后,微生物固定化技术发展迅速,广泛应用于环保[106]、医药[107]和食品[108]等领域。该技术大致可分为3类:吸附法、包埋法和交联法,但由于交联法所用交联剂较为昂贵且降解效率偏低,因此常用的微生物固定化方法以包埋法和吸附法为主。微生物固定化载体的选择是该技术极为重要的一个方面,载体的作用是为微生物提供一个适宜生存的微环境,合适的载体不仅要能使微生物稳定地固定在载体上,还要具有一定的渗透性,使重金属离子容易通过载体间隙渗入微生物表面[94]。目前,传统的微生物固定化载体材料(无机载体材料、有机载体材料等)已被大量研究与应用,但单一有机、无机材料具有一定的局限性,不能满足实际处理过程中复杂的环境条件,因此近年来复合载体材料开始成为一种热点。同时,制备各种新型载体材料如改性载体材料、磁性材料、大孔聚合物载体材料等已成为当前研究热点,部分载体材料固定/负载微生物对重金属的吸附去除效果如表6所示。
6 实际应用中的常见问题与展望(Problems and prospects during practical application)
微生物吸附是由学科交叉而产生的一种新技术,因其成本和技术难度较低、环境友好,故具有较好的应用前景。但由于实验室研究的适用性是有限的,很少有报道评估其在工业规模的可行性。要使微生物吸附从实验室转移到现场,成本较高,其实际应用仍存在局限性。
表6 固定化微生物对金属离子的吸附率
Table 6 Biosorption efficiency of heavy metals by immobilized microbes
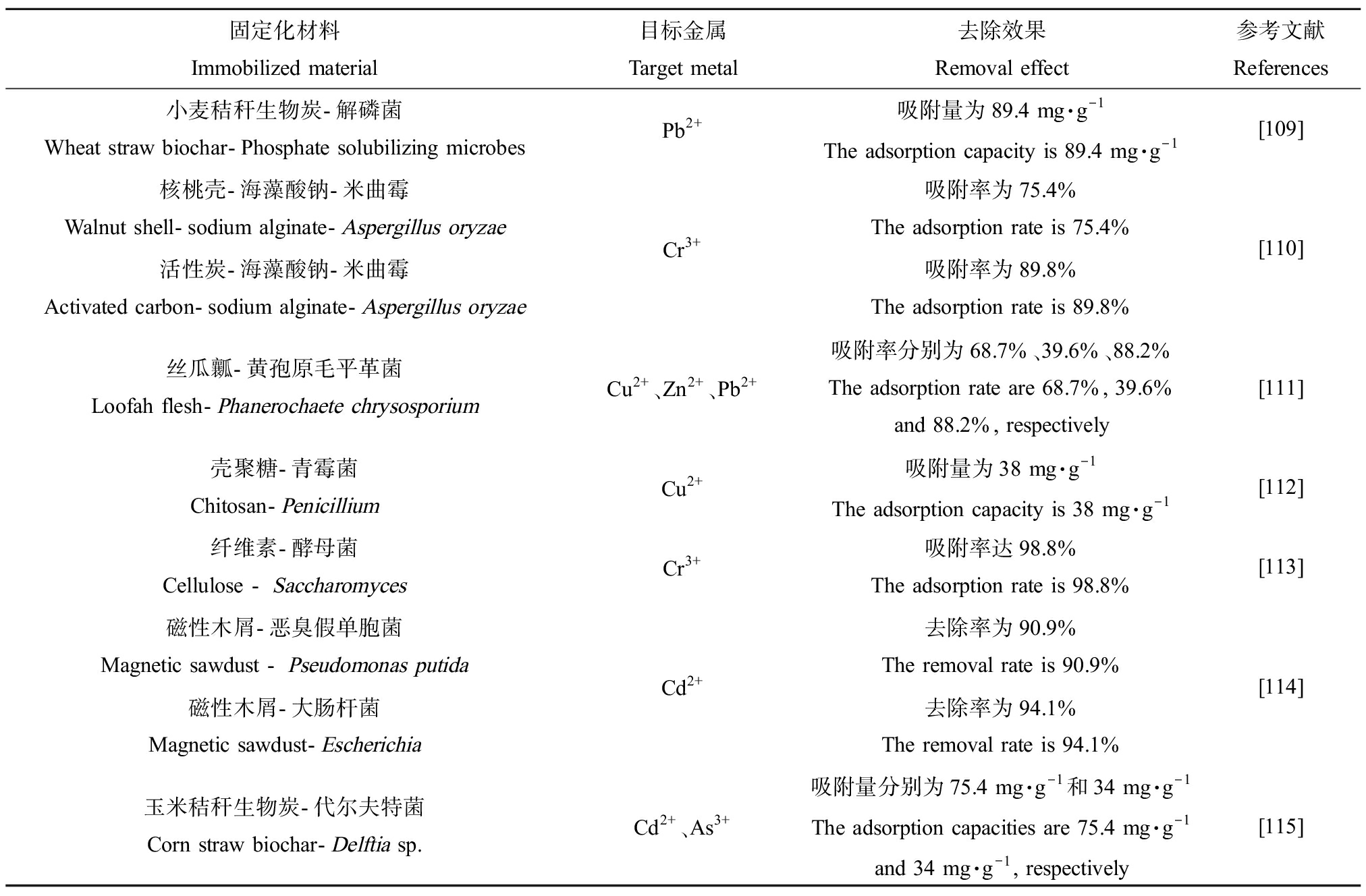
固定化材料Immobilized material目标金属Target metal去除效果Removal effect参考文献References小麦秸秆生物炭-解磷菌Wheat straw biochar-Phosphate solubilizing microbesPb2+吸附量为89.4 mg·g-1The adsorption capacity is 89.4 mg·g-1[109]核桃壳-海藻酸钠-米曲霉Walnut shell-sodium alginate-Aspergillus oryzae活性炭-海藻酸钠-米曲霉Activated carbon-sodium alginate-Aspergillus oryzaeCr3+吸附率为75.4%The adsorption rate is 75.4%吸附率为89.8%The adsorption rate is 89.8%[110]丝瓜瓤-黄孢原毛平革菌Loofah flesh-Phanerochaete chrysosporiumCu2+、Zn2+、Pb2+吸附率分别为68.7%、39.6%、88.2%The adsorption rate are 68.7%, 39.6% and 88.2%, respectively[111]壳聚糖-青霉菌Chitosan-PenicilliumCu2+吸附量为38 mg·g-1The adsorption capacity is 38 mg·g-1[112]纤维素-酵母菌Cellulose - SaccharomycesCr3+吸附率达98.8%The adsorption rate is 98.8%[113]磁性木屑-恶臭假单胞菌Magnetic sawdust - Pseudomonas putida磁性木屑-大肠杆菌Magnetic sawdust-EscherichiaCd2+去除率为90.9%The removal rate is 90.9%去除率为94.1%The removal rate is 94.1%[114]玉米秸秆生物炭-代尔夫特菌Corn straw biochar-Delftia sp.Cd2+、As3+吸附量分别为75.4 mg·g-1和34 mg·g-1The adsorption capacities are 75.4 mg·g-1and 34 mg·g-1, respectively[115]
因为重金属对一般的微生物都有很大的毒害作用,最主要的问题就是微生物菌种的选择。同时,由于不同的环境条件(如温度、pH、水中其他物质和成份)等因素都会对微生物的生长繁殖、生物活性等产生影响,进而影响吸附效果,一旦环境条件改变,微生物吸附过程极易受到限制。因此筛选和寻找出耐重金属且重金属富集能力强的微生物是重中之重。近年来,部分学者采取基因工程手段定向改变微生物的基因特性,将对某些重金属有较强吸附力的络合蛋白(肽)在微生物细胞表面或体内表达,以此来提高微生物对重金属离子的吸附效应[116]。赵清等[117]通过DNA技术中的体外重组方法,将一种抗As基因片段,接入一种喜温硫杆菌(Thiobacillus caldus)体内,成功繁殖出一种抗As冶金工程菌,这种细菌可以处理高As废水。另外,使用化学方法将生物吸附剂与纳米颗粒结合,可形成纳米生物吸附剂[118]。2种母材(纳米粒子和生物吸附剂材料)结合在一起,形成了一种具有多重增强性能的新型材料。目前,该技术用于其他材料较多,运用于微生物较少。如Arshadi等[119]报道了用水生植物满江红(Azolla filiculoides)修饰制备纳米零价铁,用于去除水中的Pb2+和Hg2+。Nguyen Thanh等[120]报道了珠光体纳米复合材料与铁和锰纳米材料相比,在去除As(Ⅴ)方面性能有所提高。纳米-微生物复合材料的应用是去除水中重金属的一种新的有效途径。
为确保微生物吸附剂使用安全,需对吸附剂的生物安全性进行分析和测试,一般包括急性毒性实验、生殖毒性实验和致畸敏感期毒性实验[121]。微生物吸附剂生物安全性分析中,由于微生物吸附剂多用来处理污染水体,对水生生态环境影响明显,故常用鱼类急性毒性测试进行评估。同时鱼类对水环境的变化反应较为灵敏,当水体中存在一定浓度的外源物质时,可引起一系列生理生化反应,包括行为异常、生理功能紊乱、组织细胞病变甚至死亡。因此,可在一定条件下,通过将鱼类暴露于特定种类、浓度的微生物吸附剂,在一定周期内观测实验鱼类的反应现象及死亡率,以短期暴露效应评估受试菌的安全性。
另外,微生物比表面积较小、其表面活性位点常被有机质掩盖[96];浮游微生物通常颗粒较小、机械强度差,运行时吸附剂易流失,在固液分离时可能产生菌体膨胀等问题[99];菌种选育比较耗时;微生物在水溶液中不易进行分离与回收[122]。微生物吸附剂对重金属的特异性选择也是微生物吸附存在的一个问题,较难找到一种对多种重金属离子均耐受的微生物。且吸附容量和选择性不够高,难以应用于实际的水处理工程。此外,由于微生物吸附剂官能团的多样性及功能差异行,稳定程度及吸附效果的不可预见性也是限制其应用一个因素。因此,阻碍了微生物吸附法的发展和推广应用。在后续研究中,可重点关注以下几方面:(1)利用分子生物学、基因工程学等筛选高效耐重金属微生物及构建基因工程菌;(2)开发新型高效固定化生物反应器,降低环境对微生物的干扰和影响;(3)设计高比表面积的微生物吸附剂,如微生物复合材料的制备;(4)吸附后贵重金属的选择性高效回收。
[1] Fu F L, Wang Q. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407-418
[2] Bilgin A, Konanç M U. Evaluation of surface water quality and heavy metal pollution of Coruh River Basin (Turkey) by multivariate statistical methods [J]. Environmental Earth Sciences, 2016, 75(12): 1029
[3] 王建龙, 韩英健, 钱易. 微生物吸附金属离子的研究进展[J]. 微生物学通报, 2000, 27(6): 449-452
[4] 张玉刚, 龙新宪, 陈雪梅. 微生物处理重金属废水的研究进展[J]. 环境科学与技术, 2008, 31(6): 58-63
Zhang Y G, Long X X, Chen X M. Recent advances in research of treating heavy-metal containing wastewater by microorganism [J]. Environmental Science & Technology, 2008, 31(6): 58-63 (in Chinese)
[5] 熊婧. 乳酸菌对重金属镉的耐受性和吸附机制研究[D]. 广州: 暨南大学, 2015: 9
Xiong J. Mechanism of cadmium resistance of lactic acid bacteria [D]. Guangzhou: Jinan University, 2015: 9 (in Chinese)
[6] 江娜, 宁增平, 郭圆, 等. 环境pH对微生物生物膜吸附重金属的影响研究进展[J]. 地球与环境, 2021, 49(2): 216-226
Jiang N, Ning Z P, Guo Y, et al. Research progress of the effect of pH on the adsorption of heavy metals by microbial biofilms [J]. Earth and Environment, 2021, 49(2): 216-226 (in Chinese)
[7] Rangabhashiyam S, Balasubramanian P. Characteristics, performances, equilibrium and kinetic modeling aspects of heavy metal removal using algae [J]. Bioresource Technology Reports, 2019, 5: 261-279
[8] Rushhoft C C. The possibilities of disposal of radioactive wastes by biological treatment methods [J]. Sewage Works Journal, 1949, 21(5): 877-883
[9] Strandberg G W, Shumate S E, Parrott J R. Microbial cells as biosorbents for heavy metals: Accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa [J]. Applied and Environmental Microbiology, 1981, 41(1): 237-245
[10] Mullen M D, Wolf D C, Ferris F G, et al. Bacterial sorption of heavy metals [J]. Applied and Environmental Microbiology, 1989, 55(12): 3143-3149
[11] Golab Z, Orlowska B, Smith R W. Biosorption of lead and uranium by Streptomyces sp. [J]. Water, Air, and Soil Pollution, 1991, 60(1): 99-106
[12] Volesky B, Prasetyo I. Cadmium removal in a biosorption column [J]. Biotechnology and Bioengineering, 1994, 43(11): 1010-1015
[13] Volesky B, May-Phillips H A. Biosorption of heavy metals by Saccharomyces cerevisiae [J]. Applied Microbiology and Biotechnology, 1995, 42(5): 797-806
[14] 张建民, 宗刚, 朱宝瑜, 等. 生物处理电镀铬废水的研究[J]. 工业水处理, 1999, 19(5): 21-22
[15] Teclu D, Tivchev G, Laing M, et al. Bioremoval of arsenic species from contaminated waters by sulphate-reducing bacteria [J]. Water Research, 2008, 42(19): 4885-4893
[16] Hua J Q, Zhang R, Chen R P, et al. Energy-saving preparation of a bioflocculant under high-salt condition by using strain Bacillus sp. and the interaction mechanism towards heavy metals [J]. Chemosphere, 2021, 267: 129324
[17] Wang X, Li D P, Gao P, et al. Analysis of biosorption and biotransformation mechanism of Pseudomonas chengduensis strain MBR under Cd(Ⅱ) stress from genomic perspective [J]. Ecotoxicology and Environmental Safety, 2020, 198: 110655
[18] Günan Yücel H, Aksu Z, Usta T, et al. Novel application of isolated Micrococcus luteus and Bacillus pumilus for Li+ ion biosorption: A comparative study [J]. Preparative Biochemistry & Biotechnology, 2021, 51(9): 892-900
[19] Chang Q, Ali A, Su J F, et al. Simultaneous removal of nitrate, manganese, and tetracycline by Zoogloea sp. MFQ7: Adsorption mechanism of tetracycline by biological precipitation [J]. Bioresource Technology, 2021, 340: 125690
[20] Xu X Y, Hao R X, Xu H, et al. Removal mechanism of Pb(Ⅱ) by Penicillium polonicum: Immobilization, adsorption, and bioaccumulation [J]. Scientific Reports, 2020, 10(1): 9079
[21] Saravanan A, Jeevanantham S, Senthil Kumar P, et al. Enhanced Zn(Ⅱ) ion adsorption on surface modified mixed biomass - Borassus flabellifer and Aspergillus tamarii: Equilibrium, kinetics and thermodynamics study [J]. Industrial Crops and Products, 2020, 153: 112613
[22] Naeimi B, Foroutan R, Ahmadi B, et al. Pb(Ⅱ) and Cd(Ⅱ) removal from aqueous solution, shipyard wastewater, and landfill leachate by modified Rhizopus oryzae biomass [J]. Materials Research Express, 2018, 5(4): 045501
[23] de Rossi A, Rigueto C V T, Dettmer A, et al. Synthesis, characterization, and application of Saccharomyces cerevisiae/alginate composites beads for adsorption of heavy metals [J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 104009
[24] Figueira M M, Volesky B, Ciminelli V S T, et al. Biosorption of metals in brown seaweed biomass [J]. Water Research, 2000, 34(1): 196-204
[25] 冯伟, 王雪青, 张译丹, 等. 丝瓜络固定化非活性颤藻对Pb2+的吸附特性[J]. 环境化学, 2020, 39(4): 1129-1136
Feng W, Wang X Q, Zhang Y D, et al. Biosorption characteristics of non-living Oscillatoria lutea immobilized in loofa sponge for removal of Pb2+ [J]. Environmental Chemistry, 2020, 39(4): 1129-1136 (in Chinese)
[26] 罗晓暄, 魏群, 廖运生, 等. 活性微藻对镉去除及其解吸剂的优选研究[J]. 水处理技术, 2021, 47(3): 12-15
Luo X X, Wei Q, Liao Y S, et al. The study of cadmium (Cd) removal by living algae with optimal selection of Cd desorption reagents in wastewater treatment [J]. Technology of Water Treatment, 2021, 47(3): 12-15 (in Chinese)
[27] 宋旭, 林陶, 夏品华, 等. 沉水植物附植生物膜藻类组成及重金属累积特征[J]. 湖泊科学, 2019, 31(5): 1268-1278
Song X, Lin T, Xia P H, et al. Algae composition and accumulation characteristics of heavy metals in epiphytic bioflioms of submerged macrophytes [J]. Journal of Lake Sciences, 2019, 31(5): 1268-1278 (in Chinese)
[28] Hamdan A M, Abd-El-Mageed H, Ghanem N. Biological treatment of hazardous heavy metals by Streptomyces rochei ANH for sustainable water management in agriculture [J]. Scientific Reports, 2021, 11: 9314
[29] Pavel K, Martina M, Tomas M. Microbial Biosorption of Metals [M]. Berlin: Springer Science Business Media B.V., 2011: 320
[30] Quintelas C, Pereira R, Kaplan E, et al. Removal of Ni(Ⅱ) from aqueous solutions by an Arthrobacter viscosus biofilm supported on zeolite: From laboratory to pilot scale [J]. Bioresource Technology, 2013, 142: 368-374
[31] Park D, Yun Y S, Park J M. The past, present, and future trends of biosorption [J]. Biotechnology and Bioprocess Engineering, 2010, 15(1): 86-102
[32] Carpio I E, Machado-Santelli G, Sakata S K, et al. Copper removal using a heavy-metal resistant microbial consortium in a fixed-bed reactor [J]. Water Research, 2014, 62: 156-166
[33] Wang Y Y, Liu Y D, Zheng K X, et al. The role of extracellular polymeric substances (EPS) in the reduction of Cr(Ⅵ) by Pannonibacter phragmitetus BB [J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106163
[34] Wang J L, Chen C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review [J]. Biotechnology Advances, 2006, 24(5): 427-451
[35] Lei D Y, Liu Z, Peng Y H, et al. Biosorption of copper, lead and nickel on immobilized Bacillus coagulans using experimental design methodologies [J]. Annals of Microbiology, 2013, 64(3): 1371-1384
[36] 麻淳雅, 朱健, 汤思敏, 等. 耐砷芽孢杆菌对As3+的吸附性能与机制研究[J]. 环境科学学报, 2020, 40(8): 2758-2770
Ma C Y, Zhu J, Tang S M, et al. Study on adsorption properties and mechanism of As3+ by an arsenic-resistant Bacillus strain [J]. Acta Scientiae Circumstantiae, 2020, 40(8): 2758-2770 (in Chinese)
[37] 张金帆, 杜春艳, 文晓凤, 等. 固定化地衣芽孢杆菌对Pb2+的吸附特性研究[J]. 环境科学与技术, 2018, 41(11): 87-92
Zhang J F, Du C Y, Wen X F, et al. A novel biosorbent prepared by fixed Bacillus licheniformis for Pb2+ removal from wastewater [J]. Environmental Science & Technology, 2018, 41(11): 87-92 (in Chinese)
[38] 望子龙, 罗学刚, 司慧, 等. 锰、砷对地衣芽孢杆菌铀富集的影响[J]. 生物技术通报, 2018, 34(6): 164-171
Wang Z L, Luo X G, Si H, et al. Effects of manganese and arsenic on uranium enrichment of Bacillus licheniformis [J]. Biotechnology Bulletin, 2018, 34(6): 164-171 (in Chinese)
[39] 范瑞梅, 张保国, 张洪勋, 等. 克劳氏芽孢杆菌(Bacillus clausii S-4)吸附Zn2+的研究[J]. 环境工程学报, 2007, 1(8): 44-47
Fan R M, Zhang B G, Zhang H X, et al. Study on adsorption of Zn2+ by Bacillus clausii S-4 [J]. Chinese Journal of Environmental Engineering, 2007, 1(8): 44-47 (in Chinese)
[40] Vishan I, Saha B, Sivaprakasam S, et al. Evaluation of Cd(Ⅱ) biosorption in aqueous solution by using lyophilized biomass of novel bacterial strain Bacillus badius AK: Biosorption kinetics, thermodynamics and mechanism [J]. Environmental Technology & Innovation, 2019, 14: 100323
[41] Oves M, Khan M S, Zaidi A. Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil [J]. Saudi Journal of Biological Sciences, 2013, 20(2): 121-129
[42] Giri A K, Patel R K, Mahapatra S S. Artificial neural network (ANN) approach for modelling of arsenic (Ⅲ) biosorption from aqueous solution by living cells of Bacillus cereus biomass [J]. Chemical Engineering Journal, 2011, 178: 15-25
[43] 林梵宇, 王润萍, 易志伟, 等. 海洋解木糖赖氨酸芽孢杆菌JZ008对重金属Cd2+、Cr3+和Cu2+的吸附作用[J]. 应用海洋学学报, 2018, 37(3): 387-394
Lin F Y, Wang R P, Yi Z W, et al. Biosorption of heavy metals cadmium, chromium, copper by marine bacterium Lysinibacillus xylanilyticus strain JZ008 [J]. Journal of Applied Oceanography, 2018, 37(3): 387-394 (in Chinese)
[44] 高玉振, 赵林, 刘德飞, 等. 胶质芽孢杆菌对重金属Cr(Ⅵ)的吸附[J]. 河南科技大学学报: 自然科学版, 2015, 36(4): 78-81, 9
Gao Y Z, Zhao L, Liu D F, et al. Adsorption of Cr(Ⅵ) by colloid Bacillus [J]. Journal of Henan University of Science & Technology: Natural Science, 2015, 36(4): 78-81, 9 (in Chinese)
[45] 代淑娟, 王玉娟, 魏德洲, 等. 枯草芽孢杆菌对电镀废水中镉的吸附[J]. 有色金属, 2010, 62(3): 156-159
Dai S J, Wang Y J, Wei D Z, et al. Biosorption of cadmium from cadmium-containing electroplating wastewater by Bacillus subtilis [J]. Nonferrous Metals, 2010, 62(3): 156-159 (in Chinese)
[46] 徐淑霞, 杜文涛, 王晓雅, 等. 1株耐Cd细菌的分离、鉴定及其吸附特性研究[J]. 河南农业科学, 2017, 46(5): 71-76, 83
Xu S X, Du W T, Wang X Y, et al. Isolation, identification and adsorption characteristics of a Cd-tolerant bacterium [J]. Journal of Henan Agricultural Sciences, 2017, 46(5): 71-76, 83 (in Chinese)
[47] 徐韶足, 王瑶, 毕文龙, 等. 一种改性微生物吸附剂的制备及其对镉离子的吸附特性[J]. 环境科学学报, 2021, 41(4): 1342-1350
Xu S Z, Wang Y, Bi W L, et al. Preparation of a modified microbial adsorbent and its adsorption characteristics for Cd2+ [J]. Acta Scientiae Circumstantiae, 2021, 41(4): 1342-1350 (in Chinese)
[48] Liu T, Hou J H, Wang J B, et al. Biosorption of heavy metals from aqueous solution by the novel biosorbent Pectobacterium sp. ND2 [J]. Environmental Progress & Sustainable Energy, 2018, 37(3): 968-974
[49] 佘晨兴, 许旭萍, 沈雪贤, 等. 球衣菌对重金属离子的耐受性及其吸附能力[J]. 应用与环境生物学报, 2005, 11(1): 90-92
She C X, Xu X P, Shen X X, et al. Biosorption and tolerance of Sphaerotilus natans to heavy metal ion [J]. Chinese Journal of Applied and Environmental Biology, 2005, 11(1): 90-92 (in Chinese)
[50] 王鹤. 一株重金属耐受菌的分离鉴定及其生物吸附Zn2+特性与机理研究[D]. 厦门: 集美大学, 2016: 25-41
Wang H. Isolation and identification of a heavy metal resistance strain and its characterization and mechanism of zinc biosorption [D]. Xiamen: Jimei University, 2016: 25-41 (in Chinese)
[51] 黄民生, 施华丽, 郑乐平. 曲霉对水中重金属的吸附去除[J]. 化工装备技术, 2001, 22(4): 17-21
[52] 赵琪琪, 李海红, 安凤秋. 抗铅真菌的筛选、鉴定及其对Pb2+的吸附特性[J]. 西南农业学报, 2020, 33(2): 401-407
Zhao Q Q, Li H H, An F Q. Isolation and identification of lead-resistant fungus and its adsorption characteristics to Pb2+ [J]. Southwest China Journal of Agricultural Sciences, 2020, 33(2): 401-407 (in Chinese)
[53] Noormohamadi H R, Fat’hi M R, Ghaedi M, et al. Potentiality of white-rot fungi in biosorption of nickel and cadmium: Modeling optimization and kinetics study [J]. Chemosphere, 2019, 216: 124-130
[54] 余甜甜, 张丽杰, 马争发, 等. 高抗镉球孢白僵菌筛选及其吸附性能研究[J]. 重庆理工大学学报: 自然科学, 2021, 35(1): 214-221
Yu T T, Zhang L J, Ma Z F, et al. Screening of a strain of Beauveria bassiana with high resistance to cadmium and its adsorption property [J]. Journal of Chongqing University of Technology: Natural Science, 2021, 35(1): 214-221 (in Chinese)
[55] 郑爱芳, 吴甘霖, 李伟娟, 等. 一株野生抗铜大型真菌对铜离子的吸附作用[J]. 生物学杂志, 2020, 37(1): 72-76
Zheng A F, Wu G L, Li W J, et al. The copper ion biosorption of a wild macrofungi with copper resistance [J]. Journal of Biology, 2020, 37(1): 72-76 (in Chinese)
[56] 王翀. 啤酒酵母对重金属Cr(Ⅲ)生物转化及生物吸附的研究[D]. 西安: 西北大学, 2005: 26-31
Wang C. Studies of Cr(Ⅲ) biotransformation and biosorption by brewer’s yeast [D]. Xi’an: Northwest University, 2005: 26-31 (in Chinese)
[57] 代淑娟, 魏德洲, 白丽梅, 等. 生物吸附-沉降法去除电镀废水中镉[J]. 中国有色金属学报, 2008, 18(10): 1945-1950
Dai S J, Wei D Z, Bai L M, et al. Removing cadmium from cadmium-containing electroplating wastewater by biosorption-sedimentation [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(10): 1945-1950 (in Chinese)
[58] Say R. Biosorption of cadmium(Ⅱ), lead(Ⅱ) and copper(Ⅱ) with the filamentous fungus Phanerochaete chrysosporium [J]. Bioresource Technology, 2001, 76(1): 67-70
[59] Pokhrel D, Viraraghavan T. Arsenic removal from an aqueous solution by a modified fungal biomass [J]. Water Research, 2006, 40(3): 549-552
[60] 许生辉. 嗜酸氧化亚铁硫杆菌对不同环境条件中砷的生物吸附及其应用研究[D]. 兰州: 兰州大学, 2018: 16
Xu S H. Biosorption of arsenic in different environmental conditions using Acidithiobacillus ferrooxidans and its application [D]. Lanzhou: Lanzhou University, 2018: 16 (in Chinese)
[61] 苏秀娟, 朱一民, 沈岩柏, 等. 悬浮酵母菌对重金属Cd2+的吸附研究[J]. 东北大学学报, 2005, 26(11): 90-93
Su X J, Zhu Y M, Shen Y B, et al. Effect of biosorption of Cd2+ by suspended saccharomycete biomass [J]. Journal of Northeastern University, 2005, 26(11): 90-93 (in Chinese)
[62] 张亚娟. 黑根霉菌对重金属废水的生物吸附及其固定化技术[J]. 环保科技, 2009, 15(3): 30-34, 42
Zhang Y J. Studies on biosorption used in heavy metal contaminated wastewater treatment and immobilized of Rhizopus stolonifer [J]. Environmental Protection and Technology, 2009, 15(3): 30-34, 42 (in Chinese)
[63] 芦亚男. 海洋微紫青霉菌P1的Cr(Ⅵ)吸附特性及全基因组学的研究[D]. 桂林: 桂林理工大学, 2020: 25-37
Lu Y N. A research of Cr(Ⅵ) adsorption characteristics and whole genomics of Penicillium janthinellum P1 [D]. Guilin: Guilin University of Technology, 2020: 25-37 (in Chinese)
[64] 肖宁, 陈强, 裴浩言, 等. 酵母菌Y17吸附Cu2+的影响因素及吸附机理研究[J]. 微生物学通报, 2008, 35(5): 772-776
Xiao N, Chen Q, Pei H Y, et al. Influence factors and absorbing mechanics of copper ion by yeast strain Y17 [J]. Microbiology, 2008, 35(5): 772-776 (in Chinese)
[65] Rangsayator N, Upatham E S, Kruatrachue M, et al. Phytoremediation potential of Spirulina (Arthrospira) platensis: Biosorption and toxicity studies of cadmium [J]. Environmental Pollution, 2002, 119(1): 45-53
[66] 王坎, 李政, 熊晶, 等. 水华蓝藻生物质对Cu2+的吸附动力学及吸附平衡研究[J]. 环境科学与技术, 2011, 34(S2): 47-50
Wang K, Li Z, Xiong J, et al. Kinetics and equilibrium of Cu2+ biosorption by cyanobacterial bloom biomass [J]. Environmental Science & Technology, 2011, 34(S2): 47-50 (in Chinese)
[67] 孙东红, 于红凤, 邹宁. 鱼腥藻对重金属污水中Zn2+的吸附研究[J]. 安徽农业科学, 2015, 43(36): 164-166
Sun D H, Yu H F, Zou N. Adsorption of Zn2+ from electroplating wastewater by Anabaena sp. [J]. Journal of Anhui Agricultural Sciences, 2015, 43(36): 164-166 (in Chinese)
[68] Lodeiro P, Cordero B, Barriada J L, et al. Biosorption of cadmium by biomass of brown marine macroalgae [J]. Bioresource Technology, 2005, 96(16): 1796-1803
[69] 周洪英, 李娜, 王学松, 等. 3种常见海藻对废水中铜的生物吸附性能[J]. 江苏农业科学, 2012, 40(1): 309-312
[70] 林海, 李真, 董颖博, 等. 修复钒镉复合污染水体的菌株分离及性能[J]. 中南大学学报: 自然科学版, 2021, 52(5): 1418-1426
Lin H, Li Z, Dong Y B, et al. Isolation and characterization of bacteria for vanadium and cadmium polluted water remediation [J]. Journal of Central South University: Science and Technology, 2021, 52(5): 1418-1426 (in Chinese)
[71] 黄浩杰. 三种微生物菌种对镉的吸附作用及机理研究[D]. 太原: 山西大学, 2020: 33-34
Huang H J. Comparison of adsorption capacity and adsorption mechanism of three microorganisms to cadmium [D]. Taiyuan: Shanxi University, 2020: 33-34 (in Chinese)
[72] 余雪梅. 耐镉芽孢杆菌对Cd2+的吸附特性及其机理研究[D]. 成都: 成都理工大学, 2019: 7
Yu X M. Characterization and mechanism of Cd2+ adsorption by cadmium-tolerant Bacillus sp. PFYN01 [D]. Chengdu: Chengdu University of Technology, 2019: 7 (in Chinese)
[73] 孙嘉龙, 李梅, 曾德华. 微生物对重金属的吸附、转化作用[J]. 贵州农业科学, 2007, 35(5): 147-150
Sun J L, Li M, Zeng D H. Study on adsorption and transformation of heavy metals by microbes [J]. Guizhou Agricultural Sciences, 2007, 35(5): 147-150 (in Chinese)
[74] Padmavathy V, Vasudevan P, Dhingra S C. Biosorption of nickel(Ⅱ) ions on Baker’s yeast [J]. Process Biochemistry, 2003, 38(10): 1389-1395
[75] Ledin M. Accumulation of metals by microorganisms—Processes and importance for soil systems [J]. Earth-Science Reviews, 2000, 51(1-4): 1-31
[76] Bayramoglu G, Arica M Y. Preparation of a composite biosorbent using Scenedesmus quadricauda biomass and alginate/polyvinyl alcohol for removal of Cu(Ⅱ) and Cd(Ⅱ) ions: Isotherms, kinetics, and thermodynamic studies [J]. Water, Air, & Soil Pollution, 2011, 221(1): 391-403
[77] Mata Y N, Blázquez M L, Ballester A, et al. Studies on sorption, desorption, regeneration and reuse of sugar-beet pectin gels for heavy metal removal [J]. Journal of Hazardous Materials, 2010, 178(1-3): 243-248
[78] Sheng P X, Ting Y P, Chen J P, et al. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms [J]. Journal of Colloid and Interface Science, 2004, 275(1): 131-141
[79] Romera E, González F, Ballester A, et al. Biosorption with algae: A statistical review [J]. Critical Reviews in Biotechnology, 2006, 26(4): 223-235
[80] Bakkaloglu I, Butter T J, Evison L M, et al. Screening of various types biomass for removal and recovery of heavy metals (Zn, Cu, Ni) by biosorption, sedimentation and desorption [J]. Water Science and Technology, 1998, 38(6): 269-277
[81] Kogej A, Pavko A. Comparison of Rhizopus nigricans in a pelleted growth form with some other types of waste microbial biomass as biosorbents for metal ions [J]. World Journal of Microbiology & Biotechnology, 2001, 17(7): 677-685
[82] Vianna L, Andrade M, Nicoli J R. Screening of waste biomass from Saccharomyces cerevisiae, Aspergillus oryzae and Bacillus lentus fermentations for removal of Cu, Zn and Cd by biosorption [J]. World Journal of Microbiology & Biotechnology, 2000, 16(5): 437-440
[83] 唐多利. 明胶废水中耐钙微生物的筛选、鉴定及其钙吸附研究[D]. 兰州: 兰州理工大学, 2020: 5-7
Tang D L. Screening, identification and calcium adsorption of calcium-resistant microorganisms in gelatin wastewater [D]. Lanzhou: Lanzhou University of Technology, 2020: 5-7 (in Chinese)
[84] 周东琴, 魏德洲. 沟戈登氏菌对重金属的生物吸附-浮选和解吸性能[J]. 环境科学, 2006, 27(5): 960-964
Zhou D Q, Wei D Z. Biosorptive-flotation and desorption operation of heavy metals from wastewater effluents by Gordona amarae [J]. Environmental Science, 2006, 27(5): 960-964 (in Chinese)
[85] 张梦梅, 刘书亮. 乳酸菌吸附重金属的影响因素、机理及应用研究进展[J]. 食品科学, 2018, 39(15): 316-322
Zhang M M, Liu S L. Progress in research on the biosorption of heavy metals by lactic acid bacteria: Influencing factors, mechanism and application [J]. Food Science, 2018, 39(15): 316-322 (in Chinese)
[86] Ghosh A, Ghosh Dastidar M, Sreekrishnan T R. Recent advances in bioremediation of heavy metals and metal complex dyes: Review [J]. Journal of Environmental Engineering, 2016, 142(9): 1-14
[87] 李进. 铅锌尾砂矿耐性微生物的筛选及其吸附机理研究[D]. 长沙: 中南林业科技大学, 2015: 51
Li J. Screening of tolerant microorganism from lead-zinc tailing and its biosorption mechanism [D]. Changsha: Central South University of Forestry & Technology, 2015: 51 (in Chinese)
[88] 邹春艳, 连宾, 臧淑艳, 等. 细菌-矿物复合吸附剂对重金属离子的吸附与解吸作用[J]. 安全与环境学报, 2011, 11(2): 43-46
Zou C Y, Lian B, Zang S Y, et al. Adsorption and desorption of heavy metal ions by bacteria-contained mineral composite adsorbents [J]. Journal of Safety and Environment, 2011, 11(2): 43-46 (in Chinese)
[89] 孙士顺. 重金属铜抗性细菌的筛选鉴定、吸附特性与机理研究[D]. 长春: 东北师范大学, 2016: 39-40
Sun S S. Copper-resistant bacterium isolation, biosorption characteristics and mechanism [D]. Changchun: Northeast Normal University, 2016: 39-40 (in Chinese)
[90] 范蕾, 宋秋芳, 杨峥, 等. 吸附重金属材料的解吸附实验研究[C]// 中国环境科学学会, 中南大学, 中南林业科技大学, 湖南农业大学. 第十届重金属污染防治技术及风险评价研讨会论文集. 长沙: 中国环境科学学会, 2020: 4
[91] 黄民生, 郑乐平, 朱莉. 微生物对重金属的吸附与解吸[J]. 化工装备技术, 2000, 21(2): 17-22
[92] Ma H W, Liao X P, Liu X, et al. Recovery of platinum(Ⅳ) and palladium(Ⅱ) by bayberry tannin immobilized collagen fiber membrane from water solution [J]. Journal of Membrane Science, 2006, 278(1-2): 373-380
[93] 尹敬群. 风化壳淋积型稀土矿浸出液中稀土离子的微生物吸附研究[D]. 南昌: 南昌大学, 2012: 35-36
Yin J Q. Study on microbe adsorption of rare earths from the leach liquid of the weathered crust elution-deposited rare earth ore [D]. Nanchang: Nanchang University, 2012: 35-36 (in Chinese)
[94] 刘金香, 葛玉杰, 谢水波, 等. 改性微生物吸附剂在重金属废水处理中的应用进展[J]. 微生物学通报, 2020, 47(3): 941-951
Liu J X, Ge Y J, Xie S B, et al. Application progress of modified microbial adsorbents for the treatment of heavy metal wastewater [J]. Microbiology China, 2020, 47(3): 941-951 (in Chinese)
[95] Velásquez L, Dussan J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus [J]. Journal of Hazardous Materials, 2009, 167(1-3): 713-716
[96] Mohapatra R K, Parhi P K, Pandey S, et al. Active and passive biosorption of Pb(Ⅱ) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies [J]. Journal of Environmental Management, 2019, 247: 121-134
[97] 刘树丽. 四种微生物吸附剂的制备及其对废水中重金属的去除特性与机理研究[D]. 昆明: 昆明理工大学, 2019: 14
Liu S L. Preparation of four microbial adsorbents and their removal characteristics and mechanisms for heavy metals from wastewater [D]. Kunming: Kunming University of Science and Technology, 2019: 14 (in Chinese)
[98] Mungasavalli D P, Viraraghavan T, Jin Y C. Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: Batch and column studies [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 301(1-3): 214-223
[99] Yu J X, Tong M, Sun X M, et al. Cystine-modified biomass for Cd(Ⅱ) and Pb(Ⅱ) biosorption [J]. Journal of Hazardous Materials, 2007, 143(1-2): 277-284
[100] Mehta S K, Gaur J P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects [J]. Critical Reviews in Biotechnology, 2005, 25(3): 113-152
[101] Fomina M, Gadd G M. Biosorption: Current perspectives on concept, definition and application [J]. Bioresource Technology, 2014, 160: 3-14
[102] Wang J L, Chen C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides [J]. Bioresource Technology, 2014, 160: 129-141
[103] Bouabidi Z B, El-Naas M H, Zhang Z E. Immobilization of microbial cells for the biotreatment of wastewater: A review [J]. Environmental Chemistry Letters, 2019, 17(1): 241-257
[104] Partovinia A, Rasekh B. Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments [J]. Critical Reviews in Environmental Science and Technology, 2018, 48(1): 1-38
[105] 杨宗政, 许文帅, 吴志国, 等. 微生物固定化及其在环境污染治理中的应用研究进展[J]. 微生物学通报, 2020, 47(12): 4278-4292
Yang Z Z, Xu W S, Wu Z G, et al. Microbial immobilization in environmental pollution treatment: A review [J]. Microbiology China, 2020, 47(12): 4278-4292 (in Chinese)
[106] Noori M, Sadeghi S. Novel immobilization of Pseudomonas aeruginosa on graphene oxide and its applications to biodegradation of phenol existing in industrial wastewaters [J]. Journal of Water Chemistry and Technology, 2019, 41(6): 363-370
[107] Hassan M E, Yang Q Y, Xiao Z G, et al. Impact of immobilization technology in industrial and pharmaceutical applications [J]. 3 Biotech, 2019, 9(12): 440
[108] Ogawa M, Bisson L F, García-Martínez T, et al. New insights on yeast and filamentous fungus adhesion in a natural co-immobilization system: Proposed advances and applications in wine industry [J]. Applied Microbiology and Biotechnology, 2019, 103(12): 4723-4731
[109] 张杰, 朱晓丽, 尚小清, 等. 生物炭固定化解磷菌对Pb2+的吸附特性[J]. 环境污染与防治, 2019, 41(4): 387-392
Zhang J, Zhu X L, Shang X Q, et al. Adsorption characteristics of Pb2+ on biochar immobilized phosphate-solubilizing bacteria [J]. Environmental Pollution & Control, 2019, 41(4): 387-392 (in Chinese)
[110] 赵锐. 耐Cr(Ⅲ)微生物的固定化及吸附Cr(Ⅲ)的特性研究[D]. 西安: 陕西科技大学, 2019: 41-47
Zhao R. Study on immobilization and adsorption of Cr(Ⅲ) by Cr(Ⅲ) resistant microorganisms [D]. Xi’an: Shaanxi University of Science & Technology, 2019: 41-47 (in Chinese)
[111] Iqbal M, Edyvean R G J. Biosorption of lead, copper and zinc ions on loofa sponge immobilized biomass of Phanerochaete chrysosporium [J]. Minerals Engineering, 2004, 17(2): 217-223
[112] Zhang X, Su H J, Tan T W, et al. Study of thermodynamics and dynamics of removing Cu(Ⅱ) by biosorption membrane of Penicillium biomass [J]. Journal of Hazardous Materials, 2011, 193: 1-9
[113] Sathvika T, Manasi, Rajesh V, et al. Microwave assisted immobilization of yeast in cellulose biopolymer as a green adsorbent for the sequestration of chromium [J]. Chemical Engineering Journal, 2015, 279: 38-46
[114] 芦炳炎. 磁性微生物复合材料的制备及其对水体中重金属吸附效果[D]. 湘潭: 湘潭大学, 2014: 25-27
Lu B Y. Preparation of magnetic absorbents with microorganism and their characteristics on the heavy metals wastewater treatment [D]. Xiangtan: Xiangtan University, 2014: 25-27 (in Chinese)
[115] 刘玉玲, 朱虎成, 彭鸥, 等. 玉米秸秆生物炭固化细菌对镉砷吸附[J]. 环境科学, 2020, 41(9): 4322-4332
Liu Y L, Zhu H C, Peng O, et al. Adsorption of cadmium and arsenic by corn stalk biochar solidified microorganism [J]. Environmental Science, 2020, 41(9): 4322-4332 (in Chinese)
[116] Isani G, Carpenè E. Metallothioneins, unconventional proteins from unconventional animals: A long journey from nematodes to mammals [J]. Biomolecules, 2014, 4(2): 435-457
[117] 赵清, 刘相梅, 边疆, 等. 抗砷载体的构建及在喜温硫杆菌中的接合转移[J]. 生物技术, 2005, 15(3): 19-21
Zhao Q, Liu X M, Bian J, et al. Construction of the arsenic resistance plasmids and the conjugative transfer to Thiobacillus caldus [J]. Biotechnology, 2005, 15(3): 19-21 (in Chinese)
[118] Boubakri S, Djebbi M A, Bouaziz Z, et al. Nanoscale zero-valent iron functionalized Posidonia oceanica marine biomass for heavy metal removal from water [J]. Environmental Science and Pollution Research International, 2017, 24(36): 27879-27896
[119] Arshadi M, Abdolmaleki M K, Mousavinia F, et al. Nano modification of NZVI with an aquatic plant Azolla filiculoides to remove Pb(Ⅱ) and Hg(Ⅱ) from water: Aging time and mechanism study [J]. Journal of Colloid and Interface Science, 2017, 486: 296-308
[120] Nguyen Thanh D, Singh M, Ulbrich P, et al. Perlite incorporating γ-Fe2O3 and α-MnO2 nanomaterials: Preparation and evaluation of a new adsorbent for As(Ⅴ) removal [J]. Separation and Purification Technology, 2011, 82: 93-101
[121] 尹华, 陈烁娜, 叶锦韶. 微生物吸附剂[M]. 北京: 科学出版社, 2015: 19
[122] 刘金香, 熊芬, 谢水波, 等. 纳米Fe3O4/黑曲霉磁性微球对U(Ⅵ)的吸附性能及机制[J]. 复合材料学报, 2017, 34(12): 2826-2833
Liu J X, Xiong F, Xie S B, et al. Adsorption characteristic and mechanism of uranium(Ⅵ) by nano Fe3O4/Aspergillus niger magnetic microspheres [J]. Acta Materiae Compositae Sinica, 2017, 34(12): 2826-2833 (in Chinese)