微囊藻毒素(MCs)是淡水水体中最常见、研究最多的一种蓝藻毒素,可由微囊藻属(Microcystis)、长胞藻属(Dolichospermum)、浮丝藻属(Planktothrix)、颤藻属(Oscillatoria)、念珠藻属(Nostoc)和束丝藻属(Aphanizomenon)等藻类在细胞内合成[1-2]。MCs毒性效应严重[3],能够通过抑制丝氨酸/苏氨酸蛋白磷酸酶活性、氧化应激、脂质过氧化作用、基因损伤和细胞凋亡等机制损伤肝细胞从而导致生物肝内出血甚至死亡[4]。1996年,巴西医院发生因使用被藻毒素污染的水造成60例患者死亡的严重事故,引起了世界各国对有毒蓝藻水华和MCs污染的进一步关注[5-6]。为了避免类似事件的发生,我国与世界卫生组织(WHO)均规定MC-LR在饮用水中的浓度限值为1 μg·L-1(生活饮用水水质卫生规范GB/T 5750—2001,地表水环境质量标准GB 3838—2002)。
MCs结构种类较多,得益于质谱技术的发展,近年来已知的MCs异构体数量迅速增加,现已报道了246种构型[7]。不同异构体的毒性存在较大差异,MC-LR的半数致死量(LD50)(小鼠腹腔注射)为50 μg·kg-1(以单位体质量计),与它在结构上仅一个L-氨基酸差异的MC-RR的LD50为500~800 μg·kg-1[8],而[(6Z)-Adda5]MC-LR的LD50可高于1 200 μg·kg-1[9]。一般来说,自然水体中MCs由多种异构体组成[10-11],仅凭总MCs或MC-LR浓度无法准确判断一个区域的MCs实际毒性和污染情况,因此,了解不同MCs异构体在水体的分布特征对准确进行MCs毒性风险评价至关重要。
目前,国内外学者已对MCs异构体产生的调控机制进行了一系列探究,发现除遗传因子调控外,温度、光照、营养元素、风力和水力条件等环境因子也能不同程度影响藻类生长代谢,进而影响MCs的产生[10, 12-15]。但针对异构体的研究多集中于原位调查数据的统计分析,且分析结果在不同研究中常存在较大差异。因此,关于MCs异构体产生驱动机制尚无法确定,识别MCs异构体产生的关键影响因子、探究其调控机制仍是研究热点。
综上所述,针对MCs异构体研究的国内外现状,本文主要从MCs的基本概况、异构体分布、遗传因子和环境因子对MCs异构体调控的影响等几个方面进行概述,重点介绍了MCs异构体合成的调控机制方面的重要研究,并对相关领域进行了展望。这对正确认识MCs异构体产生及转化过程以及评价MCs综合毒性和健康风险具有重要意义,并可为湖泊蓝藻水华次生危害的防护与治理提供理论依据。
1 自然水MCs的基本概况(Basic profiles of MCs)
1.1 MCs的结构特征
MCs为单环七肽结构,多肽中2种可变L-氨基酸的替换形成了多种MCs异构体[7, 16](图1),其中最常见的是MC-LR、MC-RR和MC-YR[17]。环状结构使得MCs化学性质稳定,纯化的MCs具有耐热性,加热煮沸都不能将其毒素破坏,能够抵抗极端pH(pH=1,T=40 ℃时开始分解)、阳光和常见酶[18]。所以过滤、混凝和沉淀等传统水处理工艺对MCs的去除效果较低[19-20],处理过程中还可能会破坏藻细胞,使细胞内的毒素释放到水体中,反而增加了水中MCs浓度[21]。
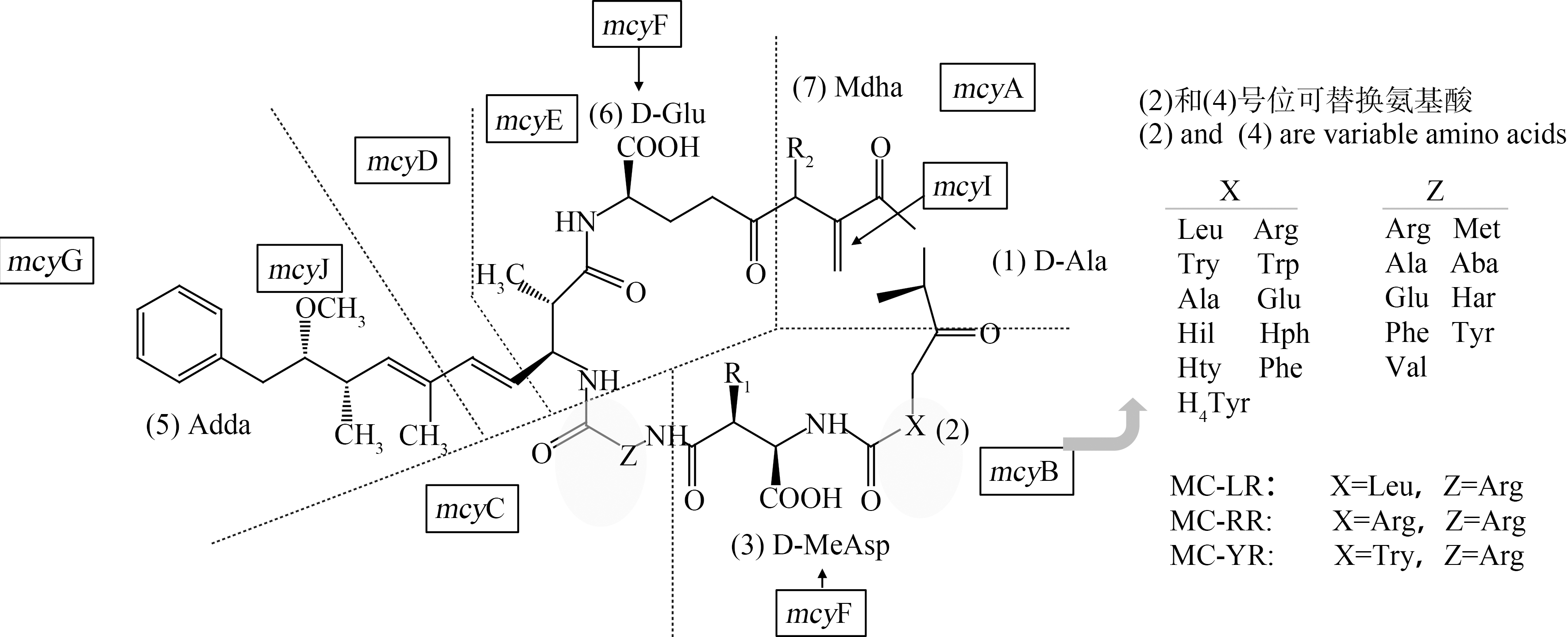
图1 微囊藻毒素的一般结构
注:X、Z为可变L-氨基酸,mcyA-J为微囊藻毒素合成酶基因簇的10个开放阅读域。
Fig. 1 Structure of microcystin
Note: X and Z are variable L-amino acids,mcyA-J are 10 open reading frames of microcystin synthase gene cluster.
1.2 产毒基因mcy及MCs合成过程
已有研究表明,并非所有微囊藻都能产毒,产毒菌株和无毒菌株差异是由一种或多种疑似微囊藻毒素合成酶基因(mcy)造成的[22],欧洲9个国家分离出的所有无毒蓝藻菌株均显示丢失了90%的mcy基因簇[23]。2000年,Tillett等[4]在铜绿微囊藻(Microcystis aeruginosa)PCC 7806的染色体中分离出了mcy基因并进行了测序分析,发现mcy基因簇是一段55 kb的DNA,由mcyA-C和mcyD-J这2个转录操纵子组成(图2(a))。中央启动子位于mcyA和mcyD之间,而mcyE、mcyF、mcyG、mcyH、mcyI和mcyJ可能具有单独的启动子[24]。根据Tillett等[4]推测,Adda侧链合成是形成MC-LR的第1步,mcyG首先启动,活化苯丙素为底物,并在mcyG操纵子PKS模块的作用下与丙二酰辅酶A(Malonyl-CoA)、还原性辅酶Ⅱ(NADPH)、H+和2个S-腺苷酸硫氨酸(SAM)进行反应,完成第1个丙二酰辅酶A延伸,生成中间产物①。由mcyD基因完成Adda合成的第2阶段,在mcyG基因的合成产物上进行第2个丙二酰基团的延长和修饰,生成中间产物②;mcyE基因完成第3个丙二酰基团的添加、延长和修饰,并负责将D-谷氨酸结合到Adda链上,生成中间产物③(图2(b))。在mcyE、mcyA、mcyB、mcyI和mcyC的作用下,Adda尾部依次延伸L-Glu、L-Ala、L-Ser、L-Leu、D-MeAs和L-Arg,逐步形成七肽,然后环化形成MC-LR。当mcyB和mcyC中A2、A4均激活氨基酸L-Arg时,在中间产物⑦和⑨上分别延伸L-Arg,在最终合成为MC-RR,当mcyB和mcyC中的A2、A4分别激活L-Try和L-Arg时,在中间产物⑦和⑨上分别延伸L-Try和L-Arg,最终合成MC-YR(图2(c))。
1.3 MCs的检测方法
MCs在自然水体中的浓度仅为μg·L-1,甚至ng·L-1级别,常规的化学方法往往难以快速、准确地对其进行检测。高效液相色谱法(HPLC)是世界卫生组织所推荐的MCs检测方法,也是最常用的方法,可以对多种构型的MCs进行同步检测[26]。在传统HPLC基础上发展的UPLC/U-HPLC结合质谱提高了检测MCs的灵敏度和速度[10, 27-28]。然而,目前仍有多种MCs异构体未分离纯化出标准样品,考虑到检测效率、成本和实际样品浓度,通常检测MC-LR、MC-RR和MC-YR这3种含量较高且毒性较大的异构体[18, 29]。酶联免疫法(ELISA)也是常见的检测方法之一[30-32],其试剂盒已经商品化。但酶联反应干扰因素较多,容易出现假阳性的结果,而且此方法只针对MC-LR一种构型,对其他MCs异构体往往需要不同的标准品和试剂盒[33-34]。另外,分子生物学检测法是建立在mcy基因和实时荧光定量PCR(quantitative real-time PCR, qPCR)基础上的一种新兴检测方法,通过检测产毒藻基因丰度表征MCs浓度。Via-Ordorika等[35]、Otten等[36]的研究证明产毒基因mcyA、mcyB、mcyE基因与MCs浓度有着显著正相关关系。然而也有研究指出,产毒基因丰度与毒素浓度之间没有明显的相关性[37]。qPCR技术在自然水样检测过程中受诸多因素的影响,温度、光照、营养盐等都可能影响mcy表达。可见,产毒基因丰度与MCs浓度的关系还需进一步研究。
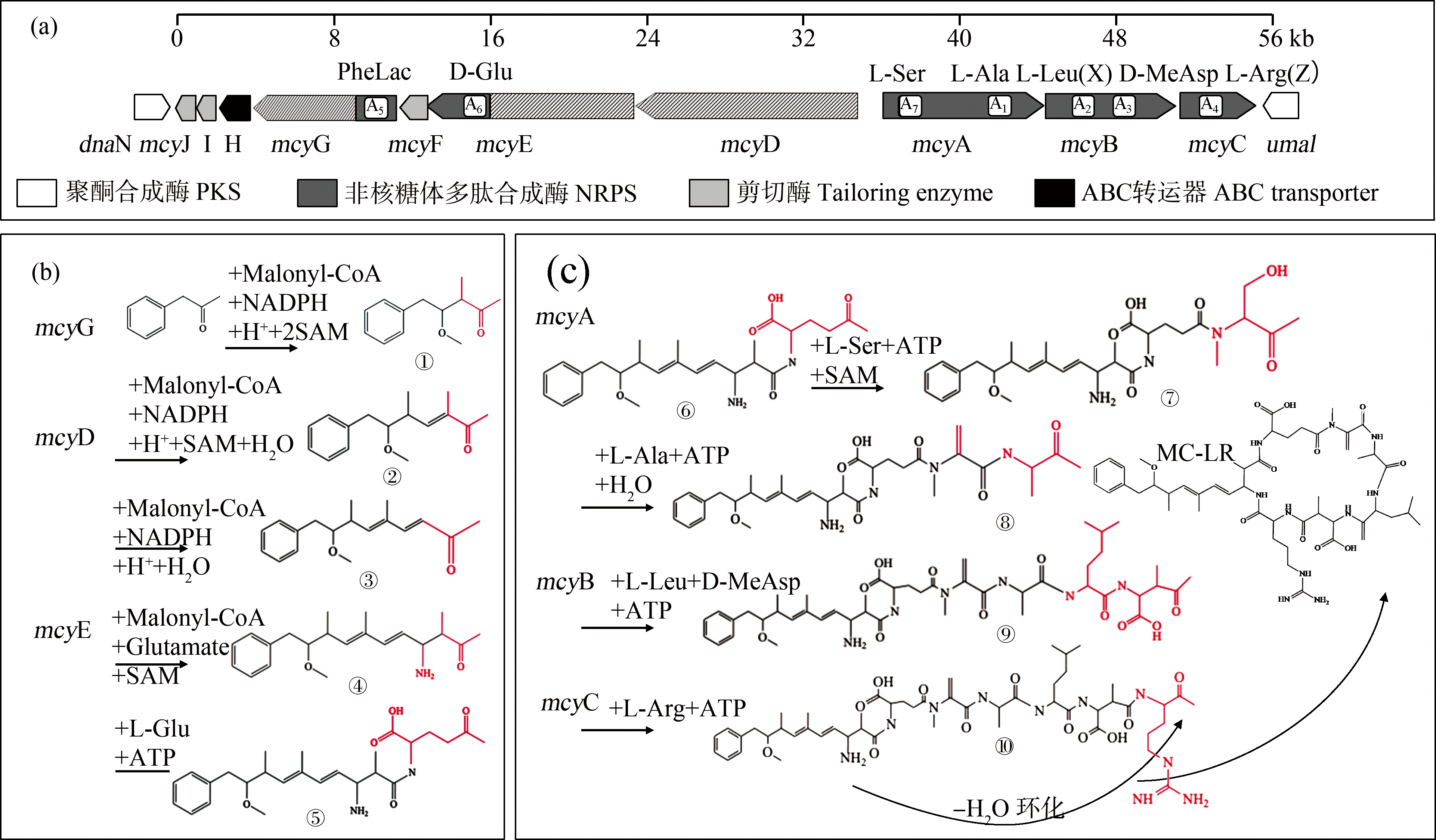
图2 mcy基因簇结构及MCs可能的生物合成途径示意图(改自文献[4, 25])
注:(a) mcy基因簇结构图,(b) MCs Adda侧链的合成过程,(c) MCs肽链的合成和环化过程;KS表示β-酮脂酰合成酶,AT表示乙酰基转移酶,CM表示C-甲基化转移酶,DH表示脱水酶,KR表示酮酯酰还原酶,ACP表示乙酰基转运蛋白,AMT表示氨基转移酶,C表示缩合酶,A表示氨基酸腺苷化酶,PCP表示肽酰基载体蛋白,NMT表示N-甲基转移酶,EP表示消旋酶,TE表示硫酯酶。
Fig. 2 Schematic of microcystin biosynthesis gene cluster and predicted microcystin biosynthesis (Modified from literature [4, 25])
Note: (a) Structures of mcy gene clusters, (b) Synthesis process of Adda side chain of MCs (c) Synthesis and cyclization process of peptide chains of MCs; KS means β-ketoacyl synthase, AT means acyltransferase, CM means C-methyltransferase, DH means dehydratase, KR means ketoacyl reductase, ACP means acyl carrier protein, AMT means aminotransferase, C means condensation, A means aminoacyl adenylation, PCP means peptidylcarrier protein, NMT means N-methyltransferase, EP means epimerization, and TE means thioesterase.
2 自然水体中MCs分布及其异构体组成(Distribution and congeners composition of MCs in natural water body)
据报道,MCs在世界范围(除南极洲外)的水体中均有分布,257个国家和地区中有108个国家暴发过微囊藻水华,79个国家出现过MCs污染[9]。我国的太湖、巢湖、滇池和洱海等许多淡水湖泊也存在较为严重的蓝藻水华和MCs污染问题。
综合已有调查结果,各地区水域中MCs异构体的组成存在明显差异(表1)。中国鄱阳湖[10]、日本Suwa湖[38]、土耳其Sapanca湖[39]中主要异构体为MC-RR,而德国Neukirchener湖[39]、希腊Zaravina湖[40]中主要异构体为MC-LR。2013—2014年太湖中检出的MC-LR和MC-RR占比相近,分别占40%和39%[11],这与法国一些湖泊的调查结果相似。MC-FR和MC-WR在我国水体中检出较少,但它们却是新西兰的Horowhenua水体中的主要MCs异构体,浓度达13.86 μg·L-1和12.90 μg·L-1[41]。另外,水体中总MCs及异构体的组成和浓度往往会因采样季节的不同而存在差异。从季节分布特征看,总MCs浓度通常表现出夏季最高,秋季降低,冬季最低,随后春季又升高的变化趋势[11-12, 42]。在以微囊藻为优势产毒蓝藻的希腊Pamvotis湖中,MC-LR表现出春夏升高、秋冬降低的变化趋势,而MC-RR表现出秋冬占比上升(86%和71%),春夏占比下降(34%和47%)的趋势,MC-YR仅在冬季检出,占比为25%[40]。
表1 国内外MCs分布及其主要异构体类型
Table 1 MCs distribution and the main microcystin congeners all over the world
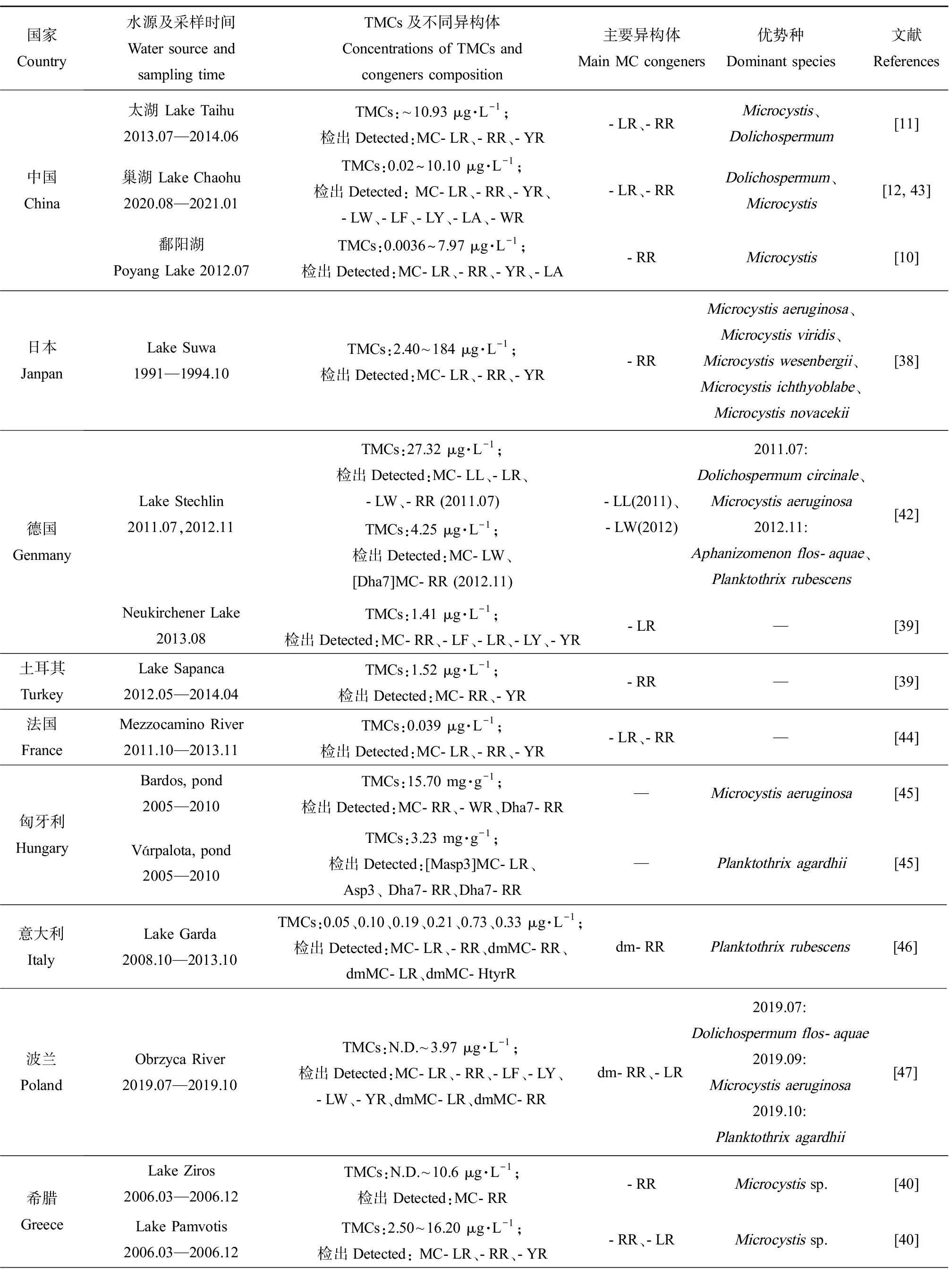
国家 Country水源及采样时间Water source and sampling timeTMCs及不同异构体 Concentrations of TMCs and congeners composition主要异构体Main MC congeners优势种Dominant species文献References中国China太湖 Lake Taihu2013.07—2014.06TMCs:~10.93 μg·L-1;检出Detected:MC-LR、-RR、-YR-LR、-RRMicrocystis、Dolichospermum[11]巢湖 Lake Chaohu2020.08—2021.01TMCs:0.02~10.10 μg·L-1;检出Detected: MC-LR、-RR、-YR、-LW、-LF、-LY、-LA、-WR-LR、-RRDolichospermum、Microcystis[12, 43]鄱阳湖 Poyang Lake 2012.07TMCs:0.0036~7.97 μg·L-1;检出Detected:MC-LR、-RR、-YR、-LA-RRMicrocystis[10]日本JanpanLake Suwa1991—1994.10TMCs:2.40~184 μg·L-1;检出Detected:MC-LR、-RR、-YR-RRMicrocystis aeruginosa、Microcystis viridis、Microcystis wesenbergii、Microcystis ichthyoblabe、Microcystis novacekii[38]德国 GenmanyLake Stechlin2011.07,2012.11TMCs:27.32 μg·L-1;检出Detected:MC-LL、-LR、-LW、-RR (2011.07)TMCs:4.25 μg·L-1;检出Detected:MC-LW、 [Dha7]MC-RR (2012.11)-LL(2011)、-LW(2012)2011.07: Dolichospermum circinale、Microcystis aeruginosa2012.11: Aphanizomenon flos-aquae、Planktothrix rubescens [42]Neukirchener Lake2013.08TMCs:1.41 μg·L-1;检出Detected:MC-RR、-LF、-LR、-LY、-YR-LR—[39]土耳其TurkeyLake Sapanca2012.05—2014.04TMCs:1.52 μg·L-1;检出Detected:MC-RR、-YR-RR—[39]法国FranceMezzocamino River2011.10—2013.11TMCs:0.039 μg·L-1;检出Detected:MC-LR、-RR、-YR-LR、-RR—[44]匈牙利HungaryBardos, pond2005—2010TMCs:15.70 mg·g-1;检出Detected:MC-RR、-WR、Dha7-RR—Microcystis aeruginosa[45]Várpalota, pond2005—2010TMCs:3.23 mg·g-1;检出Detected:[Masp3]MC-LR、Asp3、 Dha7-RR、Dha7-RR—Planktothrix agardhii[45]意大利ItalyLake Garda2008.10—2013.10TMCs:0.05、0.10、0.19、0.21、0.73、0.33 μg·L-1;检出Detected:MC-LR、-RR、dmMC-RR、dmMC-LR、dmMC-HtyrRdm-RRPlanktothrix rubescens[46]波兰PolandObrzyca River2019.07—2019.10TMCs:N.D.~3.97 μg·L-1;检出Detected:MC-LR、-RR、-LF、-LY、-LW、-YR、dmMC-LR、dmMC-RRdm-RR、-LR2019.07: Dolichospermum flos-aquae2019.09: Microcystis aeruginosa2019.10: Planktothrix agardhii[47]希腊GreeceLake Ziros2006.03—2006.12TMCs:N.D.~10.6 μg·L-1;检出Detected:MC-RR-RRMicrocystis sp.[40]Lake Pamvotis2006.03—2006.12TMCs:2.50~16.20 μg·L-1;检出Detected: MC-LR、-RR、-YR-RR、-LR Microcystis sp.[40]
续表1

国家 Country水源及采样时间Water source and sampling timeTMCs及不同异构体 Concentrations of TMCs and congeners composition主要异构体Main MC congeners优势种Dominant species文献References美国USASt Lucie Estuary2005.07TMCs:45 501.00 μg·L-1;检出Detected:MC-LR、-RR、-LF、[DAsp3]-LR、-LY-LRMicrocystis aeruginosa[48]St. Lucie River2016.07TMCs:5 328.50 μg·L-1;检出Detected:MC-LR、-RR、-YR、-LA、-WR、-LF、-LT,-LR、-LA Microcystis aeruginosa[48]新西兰New ZealandHorowhenua2012.11—2013.04TMCs:55.40 μg·L-1;检出Detected:MC-RR、dm-RR、didm-RR、-YR、-LR、dm-LR、didm-LR、-FR、-WR、-LA、-LF-FR、-WR、-LRMicrocystis sp.[41]Sullivans2012.11—2013.04TMCs:3.16 μg·L-1;检出Detected:MC-RR、didm-RR、-YR、-LR、-FR、-WR、-LA、-LY-WR、-LR、-RRDolichospermum lemmermannii[41]阿根廷ArgentinaLos Padres Lake2007.07—08,11—12TMCs:0.16~15.77 μg·L-1;检出Detected:MC-LR、-RR、-YR、-LA-RR,-LA、-LR—[49]南非South AfricaHartbeespoort Dam reservoir2010.12—2011.03TMCs:126.13 μg·g-1(以干质量计 Based on dry mass);检出Detected:MC-RR、-LR、-YR-RRMicrocystis aeruginosa,Microcystis wesenbergii[50]
注:TMCs代表总MCs;N.D代表未检出;—代表数据未提及。
Note: TMCs stands for toal MCs; N.D stands for not detected;— stands for not given.
实际环境中不同的异构体可由不同蓝藻或同种蓝藻的不同菌株产生,这些藻细胞的生物量随生态位的变化而变化。因此,湖泊产毒藻类群落结构的差异可能造成MCs异构体组成不同。在微囊藻或长胞藻为优势属的水域中,MC-LR、MC-RR通常为主要MCs异构体[10, 40, 50]。在红色浮丝藻为优势种的水体中,dmMC-RR浓度通常较高[51-52],意大利Garda湖中检出的dmMC-RR占总MCs的90%以上。Stechlin湖[42]中以卷曲长孢藻和铜绿微囊藻为优势种时,主要MCs异构体为MC-LL,而以水华束丝藻和红色浮丝藻为优势种时主要MCs异构体变为MC-LW。可见,MCs异构体组成与湖泊产毒藻群落结构有着直接关系,而藻类种群丰度又受地理位置、气象条件、水体理化参数等多种因素影响,因此MCs异构体的调控机制涉及多种影响因子,具有一定复杂性,需要开展进一步深入探究。
3 遗传因子对MCs异构体的调控(Regulation of genetics factor to microcystin congeners)
已有研究报道MCs结构的7个肽段均可发生变化,但最常见的是2号和4号位置处的2个可变L-氨基酸(X、Z)的替换以及3号和7号位置甲基化/去甲基化(图1)。不同MCs异构体的出现是由产毒蓝藻中mcy基因编码的差异造成的[53]。
3.1 非特异性非核糖体肽合成酶的影响
微囊藻毒素合成基因mcy中非特异性的非核糖体肽合成酶(NRPSs)可能是造成出现多种MCs异构体的一个重要因素。mcy基因簇中各开放结构域以模块化方式排列,其中每个非核糖体肽合成酶都至少含有一个腺苷酸化域(A域,adenylation domains)、硫酯域(T域,thiolation domain)、氨基酸缩合域(C域,condensation domains)和肽载体蛋白(PCPs,peptidyl carrier protein)。各合成阶段中延长肽段所需的氨基酸底物经过腺苷酸化后被共价键合到磷酸泛肽基辅因子上,与相邻的肽基载体蛋白结构域结合。这些结构域的顺序、数量决定了所得聚酮化合物的结构[54]。遗传序列的改变也可能影响这些结构域的功能,以A域为例,遗传编码影响了其对氨基酸的识别和激活。拟念珠藻(Nostochopsis sp.)152菌株mcyB的第一个模块(mcyB1)中D→Y单个氨基酸变化可能导致2号位L-Arg的引入向L-Hty转移,mcyC中V→I单个氨基酸变化可能导致MCs合成过程4号位L-Arg的引入向L-Har转移[55]。通常情况下NRPS酶的结构保守,但mcy基因簇中NRPS的酶特异性较弱。前文提到非特异性的腺苷酸化域能够激活不同的氨基酸,而非特异性的肽载体蛋白则能将氨基酸以不同顺序的组合[56]。例如,席藻(Phormidium sp.)LP904c菌株mcyB1和mcyC中腺苷酸化域的多特异性,使其在MCs的2号和4号位处选择引入Leu、Arg、Phe、Trp、Tyr、Met、Hph和Hty等多种氨基酸,产生至少16种MCs异构体。但该菌株的mcy基因簇不编码Hph、Hty生物合成的酶,它们的出现可能是由于MCs合成过程中mcyB1和mcyC的腺苷酸化域激活了鸟嘌呤肽生物合成的一部分氨基酸[53]。类似的,微囊藻CAWBG11能够产生至少47种MCs异构体,其中由于控制2号、3号和4号位的mcy底物非特异性产生的MCs异构体有27种,3号位的去甲基异构体的存在表明,mcyB2中A域能同时识别Masp和Asp,因此MCs中大约有2.5%的异构体在第3个位置包含Asp,而不是Masp[57]。
3.2 基因重组的影响
基因重组能使mcy基因簇中模块之间发生片段交换,影响各点位氨基酸的特异性,从而产生不同的异构体。自然环境下,重组是微囊藻菌株中mcy基因变异的一种常见方式,微囊藻属菌株的mcy基因簇中mcyA1、mcyA2、mcyB1和mcyC都易受其影响[54]。Tanabe等[58]确定了1株产自加拿大和10株产自亚洲的产毒微囊藻的mcyA、mcyD、mcyG和mcyJ的基因序列,分析发现mcyA有几处潜在重组区域,但在mcyD、mcyG和mcyJ序列中未检测到重组。Tooming-Klunderud等[59]在mcy的NMT结构域、侧翼dnaN-mcyJ基因的间区中均检测到多个重组事件,同时检测到因重组导致的模块mcyB1和mcyC中A域之间的片段交换。这2个结构域之间的重组可能是微囊藻菌株能够合成MC-RR的关键。Bjørg等[56]对比了11种微囊藻菌株mcyABC基因在序列水平的差异,系统遗传学分析结果表明mcyB1模块的腺苷酸化域有多个系统发育亚组。研究者将能够激活Leu和Arg的mcyB1腺苷酸化域分别命名为mcyB1(B型)(以PCC 7806为例)和mcyB1(C型)(以HUB 5-2-4为例),并依此将实验菌株分为B、C共2类。MCs异构体检测结果表明,B型菌株均可合成MC-LR异构体,而C型菌株则不仅可以合成MC-RR异构型,还能合成出MC-LR和MC-RR混合的MCs。序列分析表明HUB 5-2-4的mcyB1与mcyC间出现明显的基因重组,使mcyB1获得了与mcyC高度相似的腺苷酸化域的序列与底物结合口袋,因此在2号和4号位均能够激活识别Arg,产生MC-RR异构体。部分C型菌株能够同时合成MC-LR可能是由于残基Gly236所致。此后的研究多沿用这种分类[59-60],Meyer等[60]发现能同时合成MC-LR、MC-RR和MC-YR的铜绿微囊藻NIES 843也属于mcyB1(C型)菌株。然而,自然水体中还有产MC-LY、MC-LA等异构体的铜绿微囊藻,其基因型分类尚未建立。因此,异构体合成与基因多态性的研究仍需进行。
值得注意的是,尽管重组增加了mcy基因的多样性,但也可能因此影响mcy基因簇的功能。Fewer等[55]的研究表明重组事件的发生和断点都仅限于腺苷酸化域,mcyB1和mcyC中腺苷酸化域的交换和替换并没有伴随缩合域的转移,不相容的腺苷酸化域和缩合域可能影响多肽组装过程尤其是缩合反应的顺序和时间,甚至导致菌株无法通过NRPS合成所需肽段。
3.3 基因突变的影响
当mcy基因中用于修饰氨基酸的其他结构域(例如差向异构、杂环化、氧化、还原、甲酰化和甲基化等)发生位点突变、缺失和插入导致这些结构域激活或失活时,也会形成不同的MCs异构体[54]。Nishizawa等[61]从铜绿微囊藻K-139的染色体中分离出mcy基因簇,分析发现mcyA1的N-甲基转移酶(NMT)结构域被不含NMT的腺苷酸化结构域取代时,该功能域失活产生了7号位去甲基的MC-LR异构体。
4 环境因子对MCs异构体的调控(Regulation of environmental factor to microcystin congeners)
次生代谢物是有机体在长期进化过程中发展出的,只在生命过程中的某一阶段产生。MCs作为一种次生代谢物,从生物学功能来看,尽管它不是藻类生长所必需的,却是它对生态环境适应的结果,可能与产毒藻抗逆性、信号传导和适应性调节有关。已有的研究表明,光照、温度和营养盐浓度等环境因素都可能影响MCs及其异构体的含量,因此MCs的合成、代谢除了由遗传特性决定外,还受环境条件的影响[62-65]。
4.1 光照的影响
光照是蓝藻进行光合作用的必要条件,光照时间和强度显著影响藻类的生长和代谢[66-67]。Deblois和Juneau[13]通过对比4种产毒和无毒菌株对光照的响应,发现产毒菌株在波动的光照环境中对高光强具有更高的耐受性,低光强/高光强循环诱导使产毒菌株生物量增加259%,无毒菌株减少22%,因此光照时间和强度通过影响藻类的生长、代谢和产毒/无毒菌株竞争,可能间接影响MCs的合成和释放。从分子层面,光照强度通过影响产毒基因mcyA[68-69]、mcyB[70-71]和mcyD[70]的表达直接调控MCs的合成,对其机制的解释主要有以下几种观点:(1)氧化应激。当光强度从7.53 μmol·m-2·s-1增加到45.15 μmol·m-2·s-1时,强光下光捕获复合物的饱和可能导致自由基的产生,mcyB转录物增加,从而促进MCs的合成[71]。(2)mcy基因转录的光依赖性。根据光照条件的不同,微囊藻能够选择性启用不同的mcy转录启动子。铜绿微囊藻PCC7806菌株在31 μmol·m-2·s-1的恒定光下生长时,mcyA和mcyD之间中央启动子区域的2个转录起始位点都能够被检测到;弱光条件(16 μmol·m-2·s-1)下,启动的mcyA和mcyD的转录起始位点分别位于ORF起始密码子上游254 bp和143 bp,产生较长的mcyABC转录物;在强光条件(68 μmol·m-2·s-1)下,启动的mcyA和mcyD的转录起始位点分别位于ORF起始密码子上游206 bp和342 bp,产生较长的mcyD-J转录物[24]。(3)通过光质直接调节或通过另一种调节因子调节。Kaebernick等[70]研究发现,红光下铜绿微囊藻的mcyB和mcyD被上调,首次证明光质会对mcy基因产生影响。另外,他们发现mcy转录物的首次增加发生在弱光条件下,第2次增加发生在31~68 μmol·m-2·s-1之间,与低光强(2~40 μmol·m-2·s-1)时MCs含量的变化一致,因此他们认为影响铜绿微囊藻的光强度<40 μmol·m-2·s-1,同时提出假设:MCs在中低等光强度下合成,但仅在达到某个光照阈值强度时才释放。
迄今为止,关于光照对MCs异构体影响的研究较少,且已有研究的结论各不相同。合成MC-LR与MC-D-(Asp3)-LR铜绿微囊藻PCC7806菌株与合成MC-RR、MC-LR和MC-YR的铜绿微囊藻NIES 843菌株在不同光照条件下各MCs异构体含量占比变化差异不大[60]。但光强能极大影响阿氏浮丝藻产MCs异构体的情况,强光能够上调其mcy的表达,[Asp3]MC-RR的含量随光强增加而减少,而[Asp3]MC-LR的含量则随光强增加而增加[72]。高光强下[Asp3]MC-LR与RR浓度的比值增加了4倍[73]。研究者因此提出了2种假设:(1)光照通过改变结合位点结构直接影响异构体合成。mcyB第一个模块的底物结合位点的因光照不同发生构象变化导致模块的底物特异性的发生变化,引入不同氨基酸进行肽链的延长;(2)通过影响细胞代谢间接影响异构体合成。不同的光照条件引起了细胞内可利用的氨基酸组成的变化,高光照强度下增加光合作用可以提高细胞的C/N比,相对较少的氮可用于合成富氮的精氨酸分子,导致微囊藻毒素合成从[Asp3]MC-RR转移到[Asp3]MC-LR[72]。但也有研究发现,高光强能够促进阿氏浮丝藻毒素的产生,却不能改变异构体的组成[74]。因此,针对光强对MCs异构体的调控机制有待进一步研究。
4.2 温度的影响
自然水体中,MCs的浓度与产毒藻种类与生物量密切相关。温度作为影响蓝藻生长、代谢的重要环境因子,也能影响MCs的产生。多项野外调查结果表明,在中国太湖[75]、巢湖[12]、鄱阳湖[76]和韩国大韩水库[77],水温与产毒微囊藻生物量、细胞内MCs浓度呈正相关。值得注意的是,室内实验研究普遍表明MCs的最高释放温度要高于最佳生长温度。铜绿微囊藻最佳生长温度为25 ℃,但MC-LR释放的最佳温度为28 ℃[78];阿氏浮丝藻的最佳生长温度为19 ℃,但MCs释放的最佳温度在20~25 ℃[79]。研究者们认为这可能是由温度引起的氧化应激所造成,MCs释放对温度响应机制可以防止不利生长条件对细胞的损害。Scherer等[80]发现20~30 ℃范围内,随着温度的升高,铜绿微囊藻上调mcyB和mcyD基因,这说明产毒基因的表达受温度影响,为温度对MCs的调控进一步提供了分子水平上的证据。
然而,温度对MCs异构体的影响至今未有一致的结论。余丽等[12]发现自然水域中水温低于25 ℃时有利于藻类合成MC-LR,高于25 ℃时有利于合成MC-RR,但Xie等[81]在美国密歇根州中放置的浮游植物透析袋实验表明水温不是造成MCs异构体组成差异的原因。室内实验中,铜绿微囊藻在20 ℃时更多合成MC-RR(占83.75%),在28 ℃时更多合成MC-LR(占95.27%)[82];当温度从28 ℃升高至36 ℃时,MC-LR与MC-LA浓度比值增加[83];但Peng等[84]研究发现,当温度从18 ℃升高至30 ℃时,MC-LR的占比下降,MC-RR和MC-YR的占比增加,但亮氨酸与精氨酸浓度的比值,酪氨酸与精氨酸浓度的比值均未改变;鱼害微囊藻(Microcystis ichthyoblabe)随温度升高,其产生的MC-LR和MC-RR的浓度比值增加,且比值>1,表明高温可能导致毒性较强的MCs异构体(如MC-LR)占主导地位[85]。以上研究结果的差异可能是由不同物种和菌株对温度的特异性响应造成的,也可能是由产毒藻类群落结构差异造成,但细胞因温度升高而上调藻细胞MCs合成以及MCs异构体占比变化的原因目前尚不清楚。
4.3 营养元素的影响
磷常被认为是大部分水体藻类生长的限制性因素,其与MCs产生之间的关系也被广泛研究。大量研究表明磷浓度是影响野外环境中微囊藻生物量和产毒基因型丰度的主要因素[12, 81, 86-87]。Oh等[87]提出,磷限制下MC-LR与-RR含量的比值变大。类似的,余丽等[12]在巢湖的研究也发现到磷盐浓度与胞内MC-RR占比显著正相关,与胞内MC-LR占比呈显著负相关。史红星等[88]的室内培养实验表明磷的不同形态对MCs合成也有影响,![]() 比β甘油磷酸钠更易于MCs的产生。因此,尽管MCs的元素构成中不含磷,但磷浓度和磷形态仍可能通过影响藻类生长、代谢、群落结构,进而影响细胞内外MCs异构体组成。在分子层面,低磷浓度下微囊藻的磷酸结合蛋白(pstS和sphX)和碱性磷酸酶(phoX)基因的表达被上调了50倍~400倍,这表明MCs合成可能是对磷胁迫的应激反应,并通过pstS、sphX和phoX这3种基因来有效地转运磷酸盐,利用磷的有机来源,在缺磷情况下形成藻华[89]。而Ludwig和Bryant[90]发现磷胁迫下聚球藻中差异表达明显的基因并不多,这可能是由于不同的藻类对磷的反应不同[91],从而造成基因表达上存在差异。
比β甘油磷酸钠更易于MCs的产生。因此,尽管MCs的元素构成中不含磷,但磷浓度和磷形态仍可能通过影响藻类生长、代谢、群落结构,进而影响细胞内外MCs异构体组成。在分子层面,低磷浓度下微囊藻的磷酸结合蛋白(pstS和sphX)和碱性磷酸酶(phoX)基因的表达被上调了50倍~400倍,这表明MCs合成可能是对磷胁迫的应激反应,并通过pstS、sphX和phoX这3种基因来有效地转运磷酸盐,利用磷的有机来源,在缺磷情况下形成藻华[89]。而Ludwig和Bryant[90]发现磷胁迫下聚球藻中差异表达明显的基因并不多,这可能是由于不同的藻类对磷的反应不同[91],从而造成基因表达上存在差异。
氮是藻类生长的必需元素,在藻细胞物质能量代谢过程中起重要作用[86]。氮浓度1 mg·L-1时,铜绿微囊藻生长受到抑制,随着氮浓度增加,铜绿微囊藻生物量和MC-LR产生量也显著增加[92]。一方面,产毒藻生物量的增加意味着藻类群体具备更大产毒潜力;另一方面,合成MCs所用到的氨基酸均是由不同形式的氮通过一些转化同化而成,氮浓度变化可能直接调控MCs的合成过程[93]。一些研究通过基因组学、转录组学的方法发现氮浓度影响mcy基因的表达,氮限制条件下铜绿微囊藻中参与MCs合成的酶基因被下调,而涉及剪裁和转运的基因被上调[94-96],但在氮胁迫下,所有涉及MCs合成的基因均被上调[97],为氮对MCs的调控提供了直接证据。值得注意的是,mcy基因簇中的启动子能与响应氮代谢环境和氧化还原变化的关键基因全局氮调控子(ntcA)结合,藻细胞通过调节氮同化基因和mcy基因的转录来响应氮浓度变化[94, 98-99](图3)。当细胞处于低氮水平时,pipX与磷酸化的PII蛋白分离并与ntcA-2-OG相互作用,从而影响氮同化相关基因的表达,伴随细胞内2-OG含量升高,进一步增加ntcA和PmcyA/D启动子的亲和力[100]。已有研究显示,MCs异构体组成受氮源可利用性差异的影响较大。由于不同氨基酸所含氮原子数目不同(如亮氨酸含有1个氮原子而精氨酸含有4个氮原子),藻细胞可能通过调节氮代谢过程,改变细胞内氨基酸组成和含量,进而影响MCs异构体的相对丰度。Qian等[101]通过同位素标记方法研究发现藻细胞对水中不同氮源利用的顺序是不同的,在氮的胁迫下MC-LY会显著增加。在培养基中添加亮氨酸时,阿氏浮丝藻产生的[Asp3]MC-LR/RR比率明显增加;添加精氨酸时,2种异构体的比率减少[73];而在长期缺氮的条件下,突然的氮富集会使阿氏浮丝藻细胞中的氮碳比、天冬氨酸和精氨酸迅速增加,[Asp3]MC-RR在氮脉冲后强烈增加,而[Asp3]MC-LR增加较少[102]。随着培养基中硝酸盐的消耗,含精氨酸的MCs异构体的相对丰度会降低[103]。
锌、铁是藻细胞生长过程中所必需的微量元素[104]。已有研究表明,水体中锌、铁的浓度不仅影响藻类的生长也可能影响其产毒[105-106]。过高浓度的锌会影响藻类的光合作用、渗透平衡,甚至造成藻细胞的裂解死亡,这可能导致产毒藻胞内MCs随细胞破裂而释放到水环境中[106]。铁离子可通过Fur与mcy启动子区域结合,在mcy基因表达层面直接调节MC-LR的合成[105],伴随铁离子浓度下降,铜绿微囊藻中mcyD基因显著上调[107]。当铁浓度<2.5 μmol·L-1时,藻类生长受到抑制,但MCs增加了20%~40%[108]。
参与细胞功能(例如膜运输、pH调节、酶活性和基因表达等)的其他营养盐对藻细胞生长和产毒的研究相对较少。Sandrini等[109]发现微囊藻对低浓度的钾离子比对钠、锂等其他碱金属阳离子更加敏感。12 mmol·L-1的钾离子对铜绿微囊藻PCC 7005和NIES-843的生长速率影响极大,但PCC 7806及其无毒突变PCC 7806均不受其影响。由于该研究未分析它们产生的MCs含量和mcy基因表达的相关情况,因此无法判断钾离子对藻细胞产毒能力的影响。
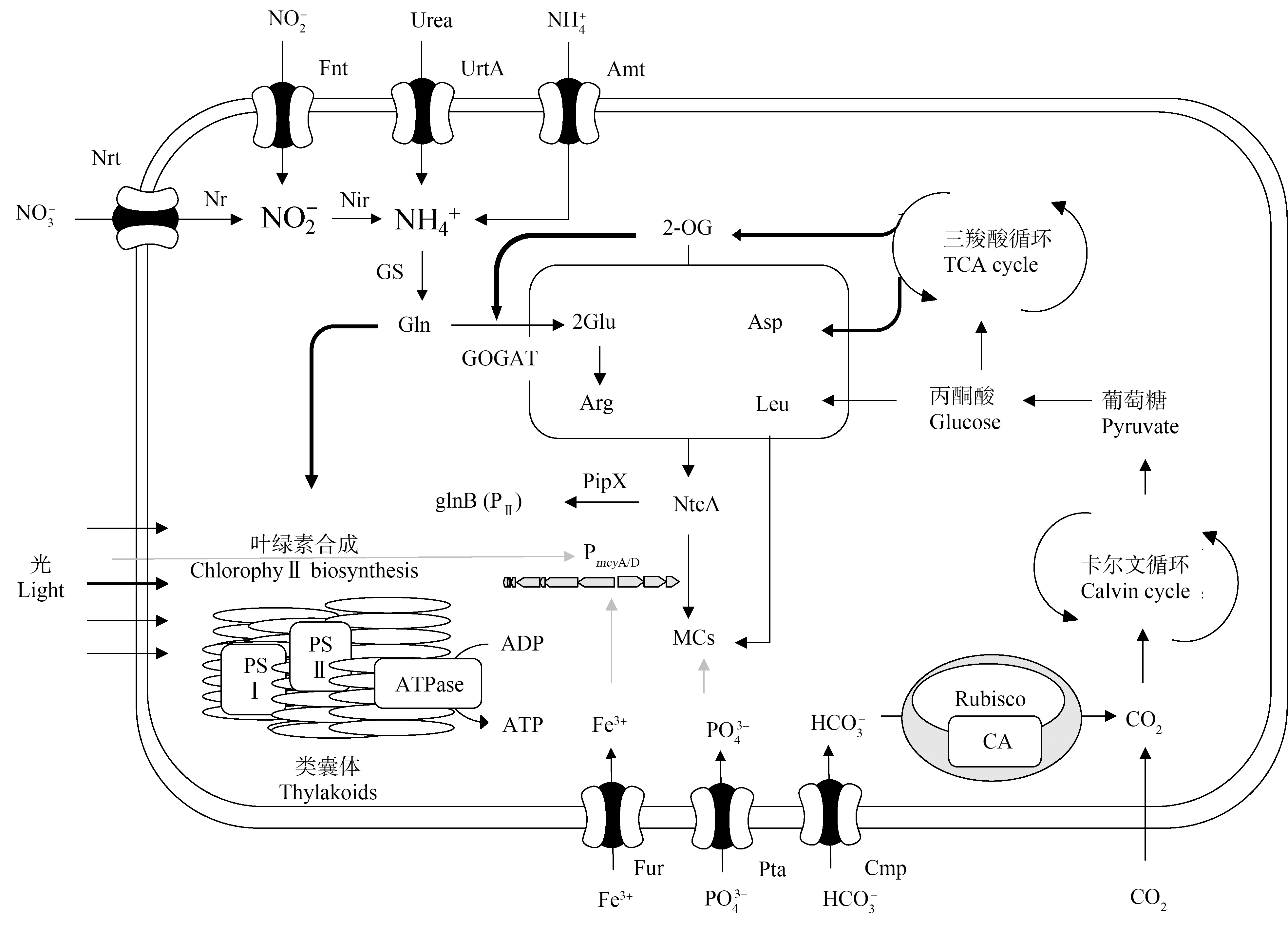
图3 产毒藻细胞中可能涉及的对MCs合成的调控机制(改自文献[97, 103])
Fig. 3 Schematic diagram of potential pathways involved in the regulation of MCs biosynthesis in a microcystin-producing cyanobacterium (Modified from literature [97, 103])
5 展望(Prospects)
经过多年的发展,MCs的相关研究已经获得了很多重要成果,但大多是原位调查数据的统计分析结果,深入研究MCs异构体调控机理的较少。室内实验由于选择的产毒菌株不同,MCs异构体对环境因子的影响机制也存在较大争议。未来应加强以下几个方面的研究。
(1)进一步加强对微囊藻产毒驱动机制的研究。目前环境因子与MCs总量和异构体组成相关性研究存在结论不一致的现象,微囊藻对特定环境条件的分子反应尤其是特定温度的反应机制在很大程度上是未知的,有待继续深入研究。
(2)结合MCs的生物学功能研究其调控机制。目前不同异构体在藻细胞信号传导和适应性调节的过程中起到的功能是否有所区别尚不清楚,MCs异构体含量变化是否会引起产毒藻内结合蛋白质靶标的构象变化和结合力变化,以促进或抑制蛋白质降解来应对环境变化还需要进一步验证。
(3)加强组学技术的应用。环境胁迫下产毒藻细胞可能出现养分获取、光合作用和MCs合成释放等的交叉调节反应,传统技术往往难以捕捉其关键变化,而组学和测序技术能够在分子层面更加整体和精确地认识环境变化引起的藻细胞内各种表达水平。
(4)建立并完善以MCs异构体为区分标准的微囊藻分类。目前尚未有区分并量化产不同MCs异构型的微囊藻丰度的方法,存在一定的技术壁垒。因此,有必要加快识别不同亚型的微囊藻的分子生物学方法(如重测序、单细胞测序等技术)的建立和完善,为揭示产毒菌株细胞个体、群体差异,探究产毒细胞个体和群落产毒的规律提供技术手段。
(5)加强产生MCs藻类的系统性研究。鱼害微囊藻、阿氏浮丝藻等其他藻种以及野外混合藻的产毒影响与单藻源微囊藻的结果相差迥异,且缺少对这些藻种系统性的研究,对于一些特定环境下的产毒规律值得进一步挖掘。
(6)加强对MCs构效关系的研究。MCs具有结构多样性,异构体之间的结构差异使其具有不同的活性(亲疏水性、毒性效应等)。由于MCs不同位点的取代复杂性,目前异构体构效关系的研究并不完善,亟需进一步加强,这对全面了解异构体环境归趋、体内代谢、毒性效应及其降解特性等方面均有重要意义。
[1] Díez-Quijada L, Puerto M, Gutiérrez-Praena D, et al. Microcystin-RR: Occurrence, content in water and food and toxicological studies. A review [J]. Environmental Research, 2019, 168: 467-489
[2] Hinojosa M G, Gutiérrez-Praena D, Prieto A I, et al. Neurotoxicity induced by microcystins and cylindrospermopsin: A review [J]. Science of the Total Environment, 2019, 668: 547-565
[3] 国晓春, 卢少勇, 谢平, 等. 微囊藻毒素的环境暴露、毒性和毒性作用机制研究进展[J]. 生态毒理学报, 2016, 11(3): 61-71
Guo X C, Lu S Y, Xie P, et al. Environmental exposure, toxicity and toxic mechanism of microcystins: A review [J]. Asian Journal of Ecotoxicology, 2016, 11(3): 61-71 (in Chinese)
[4] Tillett D, Dittmann E, Erhard M, et al. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: An integrated peptide-polyketide synthetase system [J]. Chemistry & Biology, 2000, 7(10): 753-764
[5] Mello F D, Braidy N, Marçal H, et al. Mechanisms and effects posed by neurotoxic products of cyanobacteria/microbial eukaryotes/dinoflagellates in algae blooms: A review [J]. Neurotoxicity Research, 2018, 33(1): 153-167
[6] Su X M, Steinman A D, Xue Q J, et al. Evaluating the contamination of microcystins in Lake Taihu, China: The application of equivalent total MC-LR concentration [J]. Ecological Indicators, 2018, 89: 445-454
[7] Meriluoto J, Spoof L, Codd G A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis [M]. John Wliley & Sons, Ltd., 2018: 1405-1406
[8] Cerasino L, Salmaso N. Diversity and distribution of cyanobacterial toxins in the Italian subalpine lacustrine district [J]. Oceanological and Hydrobiological Studies, 2012, 41(3): 54-63
[9] Harke M J, Steffen M M, Gobler C J, et al. A review of the global ecology, genomics, and biogeography of the toxic Cyanobacterium, Microcystis spp. [J]. Harmful Algae, 2016, 54: 4-20
[10] Zhang D W, Liao Q G, Zhang L, et al. Occurrence and spatial distributions of microcystins in Poyang Lake, the largest freshwater lake in China [J]. Ecotoxicology, 2015, 24(1): 19-28
[11] Su X M, Xue Q J, Steinman A D, et al. Spatiotemporal dynamics of microcystin variants and relationships with environmental parameters in Lake Taihu, China [J]. Toxins, 2015, 7(8): 3224-3244
[12] 余丽, 朱广伟, 孔繁翔, 等. 巢湖微囊藻毒素异构体组成的时空分布特征及影响因子[J]. 湖泊科学, 2019, 31(3): 700-713
Yu L, Zhu G W, Kong F X, et al. Spatiotemporal characteristics of microcystin variants composition and associations with environmental parameters in Lake Chaohu, China [J]. Journal of Lake Sciences, 2019,31(3): 700-713 (in Chinese)
[13] Deblois C P, Juneau P. Comparison of resistance to light stress in toxic and non-toxic strains of Microcystis aeruginosa (cyanophyta)(1) [J]. Journal of Phycology, 2012, 48(4): 1002-1011
[14] Taranu Z E, Pick F R, Creed I F, et al. Meteorological and nutrient conditions influence microcystin congeners in freshwaters [J]. Toxins, 2019, 11(11): 620
[15] He Q, Kang L, Sun X F, et al. Spatiotemporal distribution and potential risk assessment of microcystins in the Yulin River, a tributary of the Three Gorges Reservoir, China [J]. Journal of Hazardous Materials, 2018, 347: 184-195
[16] Campos A, Vasconcelos V. Molecular mechanisms of microcystin toxicity in animal cells [J]. International Journal of Molecular Sciences, 2010, 11(1): 268-287
[17] Chen L, Chen J, Zhang X Z, et al. A review of reproductive toxicity of microcystins [J]. Journal of Hazardous Materials, 2016, 301: 381-399
[18] Li J M, Li R H, Li J. Current research scenario for microcystins biodegradation - A review on fundamental knowledge, application prospects and challenges [J]. Science of the Total Environment, 2017, 595: 615-632
[19] 何君, 张欣然, 杨欣. 化学氧化法去除微囊藻毒素的研究进展[J]. 化学通报, 2018, 81(11): 981-985, 991
He J, Zhang X R, Yang X. Progress in the removal of microcystins by chemical oxidation [J]. Chemistry, 2018, 81(11): 981-985, 991 (in Chinese)
[20] 王逸飞, 倪利晓, 岳菲菲, 等. 环境水体微囊藻毒素的光催化降解途径与机制研究[J]. 环境科技, 2020, 33(1): 7-12
Wang Y F, Ni L X, Yue F F, et al. Photocatalytic degradation pathway and mechanism of microcystins in environmental water [J]. Environmental Science and Technology, 2020, 33(1): 7-12 (in Chinese)
[21] Pivokonsky M, Kloucek O, Pivokonska L. Evaluation of the production, composition and aluminum and iron complexation of algogenic organic matter [J]. Water Research, 2006, 40(16): 3045-3052
[22] Dittmann E, Neilan B A, Erhard M, et al. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806 [J]. Molecular Microbiology, 1997, 26(4): 779-787
[23] Christiansen G, Kurmayer R, Liu Q, et al. Transposons inactivate biosynthesis of the nonribosomal peptide microcystin in naturally occurring Planktothrix spp. [J]. Applied and Environmental Microbiology, 2006, 72(1): 117-123
[24] Kaebernick M, Dittmann E, Börner T, et al. Multiple alternate transcripts direct the biosynthesis of microcystin, a cyanobacterial nonribosomal peptide [J]. Applied and Environmental Microbiology, 2002, 68(2): 449-455
[25] Cullen A, Pearson L A, Mazmouz R, et al. Heterologous expression and biochemical characterisation of cyanotoxin biosynthesis pathways [J]. Natural Product Reports, 2019, 36(8): 1117-1136
[26] Xie L Q, Park H D. Determination of microcystins in fish tissues using HPLC with a rapid and efficient solid phase extraction [J]. Aquaculture, 2007, 271(1-4): 530-536
[27] Zhang X Y, Cai X X, Zhang X Y, et al. Simultaneous rapid determination of 12 microcystins and one nodularin in water by direct injection-ultra performance liquid chromatography-triple quadrupole mass spectrometry [J]. Chinese Journal of Chromatography, 2017, 35(12): 1286-1293
[28] Oehrle S A, Southwell B, Westrick J. Detection of various freshwater cyanobacterial toxins using ultra-performance liquid chromatography tandem mass spectrometry [J]. Toxicon, 2010, 55(5): 965-972
[29] Kim M S, Kim H H, Lee K M, et al. Oxidation of microcystin-LR by ferrous-tetrapolyphosphate in the presence of oxygen and hydrogen peroxide [J]. Water Research, 2017, 114: 277-285
[30] Nummer S A, Weeden A J, Shaw C, et al. Updating the ELISA standard curve fitting process to reduce uncertainty in estimated microcystin concentrations [J]. MethodsX, 2018, 5: 304-311
[31] Heussner A H, Winter I, Altaner S, et al. Comparison of two ELISA-based methods for the detection of microcystins in blood serum [J]. Chemico-Biological Interactions, 2014, 223: 10-17
[32] He X X, Stanford B D, Adams C, et al. Varied influence of microcystin structural difference on ELISA cross-reactivity and chlorination efficiency of congener mixtures [J]. Water Research, 2017, 126: 515-523
[33] Metcalf J S, Codd G A. Analysis of cyanobacterial toxins by immunological methods [J]. Chemical Research in Toxicology, 2003, 16(2): 103-112
[34] 吴丹青. 太湖水中微囊藻毒素的检测技术及其方法比对分析[D]. 杭州: 浙江工业大学, 2017: 10-13
Wu D Q. Detection methods of microcystins in water of Taihu Lake and their comparison analysis [D]. Hangzhou: Zhejiang University of Technology, 2017: 10-13 (in Chinese)
[35] Via-Ordorika L, Fastner J, Kurmayer R, et al. Distribution of microcystin-producing and non-microcystin-producing Microcystis sp. in European freshwater bodies: Detection of microcystins and microcystin genes in individual colonies [J]. Systematic and Applied Microbiology, 2004, 27(5): 592-602
[36] Otten T G, Xu H, Qin B, et al. Spatiotemporal patterns and ecophysiology of toxigenic microcystis blooms in Lake Taihu, China: Implications for water quality management [J]. Environmental Science & Technology, 2012, 46(6): 3480-3488
[37] Griffiths D J, Saker M L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin [J]. Environmental Toxicology, 2003, 18(2): 78-93
[38] Park H D, Iwami C, Watanabe M F, et al. Temporal variabilities of the concentrations of intra- and extracellular microcystin and toxic Microcystis species in a hypertrophic lake, Lake Suwa, Japan (1991-1994) [J]. Environmental Toxicology, 1998, 13(1): 61-72
[39] Bormans M, Amzil Z, Mineaud E, et al. Demonstrated transfer of cyanobacteria and cyanotoxins along a freshwater-marine continuum in France [J]. Harmful Algae, 2019, 87: 101639
[40] Vareli K, Touka A, Theurillat X, et al. Microcystins in two low nutrient lakes in the Epirus region of north-west Greece [J]. CLEAN - Soil Air Water, 2015, 43(9): 1267-1356
[41] Wood S A, Maier M Y, Puddick J, et al. Trophic state and geographic gradients influence planktonic cyanobacterial diversity and distribution in New Zealand Lakes [J]. FEMS Microbiology Ecology, 2016, 93(2): fiw234
[42] Dadheech P K, Selmeczy G B, Vasas G, et al. Presence of potential toxin-producing cyanobacteria in an oligo-mesotrophic lake in Baltic Lake District, Germany: An ecological, genetic and toxicological survey [J]. Toxins, 2014, 6(10): 2912-2931
[43] 葛思敏. 重点湖泊微囊藻毒素时空分布特征及综合健康风险评估[D]. 北京: 中国环境科学研究院, 2021: 26-30
Ge S M. Spatiotemporal distribution characteristics of microcystins in key lakes and comprehensive health risk assessment [D]. Beijing: Chinese Research Academy of Environmental Sciences, 2021: 26-30 (in Chinese)
[44] Greer B, McNamee S E, Boots B, et al. A validated UPLC-MS/MS method for the surveillance of ten aquatic biotoxins in European brackish and freshwater systems [J]. Harmful Algae, 2016, 55: 31-40
[45] Farkas O, Gyémant G, Hajdú G, et al. Variability of microcystins and its synthetase gene cluster in Microcystis and Planktothrix waterblooms in shallow lakes of Hungary [J]. Acta Biologica Hungarica, 2014, 65(2): 227-239
[46] Cerasino L, Shams S, Boscaini A, et al. Multiannual trend of microcystin production in the toxic cyanobacterium Planktothrix rubescens in Lake Garda (Italy) [J]. Chemistry and Ecology, 2016, 32(5): 492-506
![]() W, Piontek M,
W, Piontek M,  uszczyńska K. The occurrence of potential harmful cyanobacteria and cyanotoxins in the Obrzyca River (Poland), a source of drinking water [J]. Toxins, 2020, 12(5): 284
uszczyńska K. The occurrence of potential harmful cyanobacteria and cyanotoxins in the Obrzyca River (Poland), a source of drinking water [J]. Toxins, 2020, 12(5): 284
[48] Oehrle S, Rodriguez-Matos M, Cartamil M, et al. Toxin composition of the 2016 Microcystis aeruginosa bloom in the St. Lucie Estuary, Florida [J]. Toxicon, 2017, 138: 169-172
[49] Amé M V, Galanti L N, Menone M L, et al. Microcystin-LR, -RR, -YR and-LA in water samples and fishes from a shallow lake in Argentina [J]. Harmful Algae, 2010, 9(1): 66-73
[50] Mbukwa E A, Msagati T A M, Mamba B B. Quantitative variations of intracellular microcystin-LR, -RR and-YR in samples collected from four locations in Hartbeespoort Dam in North West Province (South Africa) during the 2010/2011 summer season [J]. International Journal of Environmental Research and Public Health, 2012, 9(10): 3484-3505
[51] Briand J F, Jacquet S, Flinois C, et al. Variations in the microcystin production of Planktothrix rubescens (cyanobacteria) assessed from a four-year survey of lac du Bourget (France) and from laboratory experiments [J]. Microbial Ecology, 2005, 50(3): 418-428
[52] Rohrlack T, Edvardsen B, Skulberg R, et al. Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form sub-populations with dissimilar ecological traits [J]. Limnology and Oceanography, 2008, 53(4): 1279-1293
[53] Shishido T K, Jokela J, Humisto A, et al. The biosynthesis of rare homo-amino acid containing variants of microcystin by a benthic cyanobacterium [J]. Marine Drugs, 2019, 17(5): 271
[54] Christiansen G, Yoshida W Y, Blom J F, et al. Isolation and structure determination of two microcystins and sequence comparison of the McyABC adenylation domains in Planktothrix species [J]. Journal of Natural Products, 2008, 71(11): 1881-1886
[55] Fewer D P, Rouhiainen L, Jokela J, et al. Recurrent adenylation domain replacement in the microcystin synthetase gene cluster [J]. BMC Evolutionary Biology, 2007, 7: 183
[56] Bjørg M, Gudrun B, Skulberg O M, et al. Natural variation in the microcystin synthetase operon mcyABC and impact on microcystin production in Microcystis strains [J]. Journal of Bacteriology, 2003, 185(9): 2774-2785
[57] Puddick J, Prinsep M R, Wood S A, et al. High levels of structural diversity observed in microcystins from Microcystis CAWBG11 and characterization of six new microcystin congeners [J]. Marine Drugs, 2014, 12(11): 5372-5395
[58] Tanabe Y, Kaya K, Watanabe M M. Evidence for recombination in the microcystin synthetase (mcy) genes of toxic cyanobacteria Microcystis spp. [J]. Journal of Molecular Evolution, 2004, 58(6): 633-641
[59] Tooming-Klunderud A, Mikalsen B, Kristensen T, et al. The mosaic structure of the mcyABC operon in Microcystis [J]. Microbiology, 2008, 154(Pt 7): 1886-1899
[60] Meyer S, Kehr J C, Mainz A, et al. Biochemical dissection of the natural diversification of microcystin provides lessons for synthetic biology of NRPS [J]. Cell Chemical Biology, 2016, 23(4): 462-471
[61] Nishizawa T, Asayama M, Fujii K, et al. Genetic analysis of the peptide synthetase genes for a cyclic heptapeptide microcystin in Microcystis spp. [J]. The Journal of Biochemistry, 1999, 126(3): 520-529
[62] Lürling M, van Oosterhout F, Faassen E. Eutrophication and warming boost cyanobacterial biomass and microcystins [J]. Toxins, 2017, 9(2): 64
[63] Pearson L A, Dittmann E, Mazmouz R, et al. The genetics, biosynthesis and regulation of toxic specialized metabolites of cyanobacteria [J]. Harmful Algae, 2016, 54: 98-111
[64] Peng G T, Lin S J, Fan Z Q, et al. Transcriptional and physiological responses to nutrient loading on toxin formation and photosynthesis in Microcystis aeruginosa FACHB-905 [J]. Toxins, 2017, 9(5): 168
[65] Wang Z C, Zhang Y, Huang S, et al. Nitrogen limitation significantly reduces the competitive advantage of toxic Microcystis at high light conditions [J]. Chemosphere, 2019, 237: 124508
[66] 殷燕, 张运林, 王明珠, 等. 光照强度对铜绿微囊藻(Microcystis aeruginosa)和斜生栅藻(Scenedesmus obliqnus)生长及吸收特性的影响[J]. 湖泊科学, 2012, 24(5): 755-764
Yin Y, Zhang Y L, Wang M Z, et al. Effects of different irradiation intensity on the growth and absorption properties of Microcystis aeruginosa and Scenedesmus obliqnus [J]. Journal of Lake Sciences, 2012, 24(5): 755-764 (in Chinese)
[67] 孙昕, 何飞飞, 李鹏飞, 等. 光照周期性波动对铜绿微囊藻(Microcystis aeruginosa)和斜生栅藻(Scenedesmus obliquus)生长的抑制[J]. 湖泊科学, 2018, 30(5): 1309-1318
Sun X, He F F, Li P F, et al. The growth inhibition of Microcystis aeruginosa and Scenedesmus obliquus by periodic light fluctuation [J]. Journal of Lake Sciences, 2018, 30(5): 1309-1318 (in Chinese)
[68] Salvador D, Churro C, Valério E. Evaluating the influence of light intensity in mcyA gene expression and microcystin production in toxic strains of Planktothrix agardhii and Microcystis aeruginosa [J]. Journal of Microbiological Methods, 2016, 123: 4-12
[69] Pineda-Mendoza R M, Zú iga G, Martínez-Jerónimo F. Microcystin production in Microcystis aeruginosa: Effect of type of strain, environmental factors, nutrient concentrations, and N: P ratio on mcyA gene expression [J]. Aquatic Ecology, 2016, 50(1): 103-119
iga G, Martínez-Jerónimo F. Microcystin production in Microcystis aeruginosa: Effect of type of strain, environmental factors, nutrient concentrations, and N: P ratio on mcyA gene expression [J]. Aquatic Ecology, 2016, 50(1): 103-119
[70] Kaebernick M, Neilan B A, Börner T, et al. Light and the transcriptional response of the microcystin biosynthesis gene cluster [J]. Applied and Environmental Microbiology, 2000, 66(8): 3387-3392
[71] Kim H R, Kim C K, Ahn T S, et al. Effects of temperature and light on microcystin synthetase gene transcription in Microcystis aeruginosa [J]. Key Engineering Materials, 2005, 277-279: 606-611
[72] Tonk L, Visser P M, Christiansen G, et al. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity [J]. Applied and Environmental Microbiology, 2005, 71(9): 5177-5181
[73] Tonk L, van de Waal D B, Slot P, et al. Amino acid availability determines the ratio of microcystin variants in the cyanobacterium Planktothrix agardhii [J]. FEMS Microbiology Ecology, 2008, 65(3): 383-390
[74] Xie L, Rediske R R, Hong Y, et al. The role of environmental parameters in the structure of phytoplankton assemblages and cyanobacteria toxins in two hypereutrophic lakes [J]. Hydrobiologia, 2012, 691(1): 255-268
[75] Li D M, Zheng H Y, Pan J L, et al. Seasonal dynamics of photosynthetic activity, Microcystis genotypes and microcystin production in Lake Taihu, China [J]. Journal of Great Lakes Research, 2017, 43(4): 710-716
[76] 袁丽娟, 廖且根, 张莉, 等. 鄱阳湖微囊藻毒素时空分布格局及其与理化和生物因子的关系[J]. 环境科学, 2018, 39(1): 450-459
Yuan L J, Liao Q G, Zhang L, et al. Seasonal and spatial variations of microcystins and their relationships with physiochemical and biological factors in Poyang Lake [J]. Environmental Science, 2018, 39(1): 450-459 (in Chinese)
[77] Joung S H, Oh H M, Ko S R, et al. Correlations between environmental factors and toxic and non-toxic Microcystis dynamics during bloom in Daechung Reservoir, Korea [J]. Harmful Algae, 2011, 10(2): 188-193
[78] 吴溶, 崔莉凤, 卢珊, 等. 温度光照对铜绿微囊藻生长及藻毒素释放的影响[J]. 环境科学与技术, 2010, 33(S1): 33-36, 51
Wu R, Cui L F, Lu S, et al. The influence of temperature and illumination on the Microcystis production [J]. Environmental Science & Technology, 2010, 33(S1): 33-36, 51 (in Chinese)
[79] Walls J T, Wyatt K H, Doll J C, et al. Hot and toxic: Temperature regulates microcystin release from cyanobacteria [J]. Science of the Total Environment, 2018, 610-611: 786-795
[80] Scherer P I, Raeder U, Geist J, et al. Influence of temperature, mixing, and addition of microcystin-LR on microcystin gene expression in Microcystis aeruginosa [J]. MicrobiologyOpen, 2017, 6(1): e00393
[81] Xie L Q, Rediske R R, Gillett N D, et al. The impact of environmental parameters on microcystin production in dialysis bag experiments [J]. Scientific Reports, 2016, 6: 38722
[82] Amé M V, Wunderlin D A. Effects of iron, ammonium and temperature on microcystin content by a natural concentrated Microcystis aeruginosa population [J]. Water, Air, and Soil Pollution, 2005, 168(1): 235-248
[83] Van der Westhuizen A, Eloff J. Effect of temperature and light (fluence rate) on the composition of the toxin of the cyanobacterium Microcystis aeruginosa (UV-006) [J]. Archiv Für Hydrobiologie, 1986, 108(2): 145-154
[84] Peng G T, Martin R M, Dearth S P, et al. Seasonally relevant cool temperatures interact with N chemistry to increase microcystins produced in lab cultures of Microcystis aeruginosa NIES-843 [J]. Environmental Science & Technology, 2018, 52(7): 4127-4136
[85] Mowe M A D, Porojan C, Abbas F, et al. Rising temperatures may increase growth rates and microcystin production in tropical Microcystis species [J]. Harmful Algae, 2015, 50: 88-98
[86] Xu Y, Wang G X, Yang W B, et al. Dynamics of the water bloom-forming Microcystis and its relationship with physicochemical factors in Lake Xuanwu (China) [J]. Environmental Science and Pollution Research, 2010, 17(9): 1581-1590
[87] Oh H M, Lee S J, Jang M H, et al. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat [J]. Applied and Environmental Microbiology, 2000, 66(1): 176-179
[88] 史红星, 王庚, 王晨宇, 等. 微囊藻毒素产生过程中磷素行为与作用研究[J]. 环境科学, 2011, 32(10): 2916-2919
Shi H X, Wang G, Wang C Y, et al. Effect ofphosphorus on the production of microcystin [J]. Environmental Science, 2011, 32(10): 2916-2919 (in Chinese)
[89] Harke M J, Berry D L, Ammerman J W, et al. Molecular response of the bloom-forming cyanobacterium, Microcystis aeruginosa, to phosphorus limitation [J]. Microbial Ecology, 2012, 63(1): 188-198
[90] Ludwig M, Bryant D A. Acclimation of the global transcriptome of the cyanobacterium Synechococcus sp. strain PCC 7002 to nutrient limitations and different nitrogen sources [J]. Frontiers in Microbiology, 2012, 3: 145
[91] Vézie C, Rapala J, Vaitomaa J, et al. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations [J]. Microbial Ecology, 2002, 43(4): 443-454
[92] 付保荣, 鲁男, 苗斌, 等. 环境因子对铜绿微囊藻生长和产毒的影响[J]. 辽宁大学学报: 自然科学版, 2015, 42(1): 85-90
Fu B R, Lu N, Miao B, et al. Effects of environmental factors on growth and toxin production of Microcystis aeraginosa [J]. Journal of Liaoning University: Natural Sciences Edition, 2015, 42(1): 85-90 (in Chinese)
[93] Davis T W, Bullerjahn G S, Tuttle T, et al. Effects of increasing nitrogen and phosphorus concentrations on phytoplankton community growth and toxicity during Planktothrix blooms in Sandusky Bay, Lake Erie [J]. Environmental Science & Technology, 2015, 49(12): 7197-7207
[94] Harke M J, Gobler C J. Daily transcriptome changes reveal the role of nitrogen in controlling microcystin synthesis and nutrient transport in the toxic cyanobacterium, Microcystis aeruginosa [J]. BMC Genomics, 2015, 16: 1068
[95] Zhou Y P, Zhang X F, Li X, et al. Evaluation of changes in Microcystis aeruginosa growth and microcystin production by urea via transcriptomic surveys [J]. Science of the Total Environment, 2019, 655: 181-187
[96] Tang X M, Krausfeldt L E, Shao K Q, et al. Seasonal gene expression and the ecophysiological implications of toxic Microcystis aeruginosa blooms in Lake Taihu [J]. Environmental Science & Technology, 2018, 52(19): 11049-11059
[97] Zhou Y P, Li X, Xia Q Q, et al. Transcriptomic survey on the microcystins production and growth of Microcystis aeruginosa under nitrogen starvation [J]. Science of the Total Environment, 2020, 700: 134501
[98] Neilan B A, Pearson L A, Muenchhoff J, et al. Environmental conditions that influence toxin biosynthesis in cyanobacteria [J]. Environmental Microbiology, 2013, 15(5): 1239-1253
[99] Zilliges Y, Kehr J C, Meissner S, et al. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of microcystis under oxidative stress conditions [J]. PLoS One, 2011, 6(3): e17615
[100] Kuniyoshi T M, Gonzalez A, Lopez-Gomollon S, et al. 2-oxoglutarate enhances NtcA binding activity to promoter regions of the microcystin synthesis gene cluster [J]. FEBS Letters, 2011, 585(24): 3921-3926
[101] Qian Z Y, Chen X, Zhu H T, et al. Study on the cyanobacterial toxin metabolism of Microcystis aeruginosa in nitrogen-starved conditions by a stable isotope labelling method [J]. Journal of Hazardous Materials, 2019, 373: 558-564
[102] van de Waal D B, Ferreruela G, Tonk L, et al. Pulsed nitrogen supply induces dynamic changes in the amino acid composition and microcystin production of the harmful cyanobacterium Planktothrix agardhii [J]. FEMS Microbiology Ecology, 2010, 74(2): 430-438
[103] Puddick J, Prinsep M R, Wood S A, et al. Modulation of microcystin congener abundance following nitrogen depletion of a Microcystis batch culture [J]. Aquatic Ecology, 2016, 50(2): 235-246
[104] 王举, 李婧, 陈荣, 等. 锌在不同磷源条件下对铜绿微囊藻生长与产毒的影响[J]. 生态毒理学报, 2018, 13(5): 226-234
Wang J, Li J, Chen R, et al. Effect of zinc on growth and toxin production of Microcystis aeruginosa under different phosphorus sources [J]. Asian Journal of Ecotoxicology, 2018, 13(5): 226-234 (in Chinese)
[105] Sevilla E, Martin-Luna B, Vela L, et al. Iron availability affects mcyD expression and microcystin-LR synthesis in Microcystis aeruginosa PCC7806 [J]. Environmental Microbiology, 2008, 10(10): 2476-2483
[106] Pereira D A, Pimentel J, Bird D F, et al. Changes in oligopeptide production by toxic cyanobacterial strains under iron deficiency [J]. Aquatic Microbial Ecology, 2015, 74(3): 205-214
[107] Alexova R, Fujii M, Birch D, et al. Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation [J]. Environmental Microbiology, 2011, 13(4): 1064-1077
![]() M, Aegerter R. Influence of trace metals on growth and toxin production of Microcystis aeruginosa [J]. Toxicon, 1993, 31(3): 293-305
M, Aegerter R. Influence of trace metals on growth and toxin production of Microcystis aeruginosa [J]. Toxicon, 1993, 31(3): 293-305
[109] Sandrini G, Huisman J, Matthijs H C P. Potassium sensitivity differs among strains of the harmful cyanobacterium Microcystis and correlates with the presence of salt tolerance genes [J]. FEMS Microbiology Letters, 2015, 362(16): fnv121