离子液体(ionic liquids, ILs)是由有机阳离子和无机或有机阴离子组成在低于100 ℃的温度下以熔融状态存在的有机盐。由于其具有低蒸发、低易燃性和高热稳定性等特点,被作为传统挥发性有机化学品的替代品广泛应用于液液分离、萃取和医药等领域[1]。鉴于ILs不断扩大的应用及其残留的持久性,其生态毒性效应受到关注,关于ILs对大型蚤、斑马鱼和藻类的毒性效应已经证实了其潜在的生态毒性,毒性作用取决于其结构和受试生物体[2-5]。研究表明,ILs对土壤污染也不容忽视,且会对蚯蚓、土壤微生物群落等产生影响[2, 6],目前关于ILs对植物毒性的研究包括对浮萍、水稻、萝卜和玉米等种子萌发、植株生长、氧化胁迫和光合色素含量变化的影响等[7-8],但对于不同植物类型的毒性差异少有涉及,而拟南芥和小麦是分别是理想的双子叶和单子叶模式生物。
本文研究3种ILs对拟南芥和小麦生长抑制(表型、叶质量)和光合作用(叶绿素含量、荧光成像和叶绿素荧光参数)的影响,解析不同碳链长度ILs的毒性差异以及不同植物的响应效应,研究结果可为合理开发和使用环境友好型ILs提供理论支持,为ILs的环境安全性评价提供理论依据。
1 材料与方法(Materials and methods)
1.1 植物幼苗培养及处理
拟南芥(哥伦比亚野生型,Arabidopsis thaliana, Col)和小麦(Triticum aestivum L.)种子消毒春化后于培养液中光照培养箱培育,7 d后分别选取长势良好的幼苗,加入不同浓度ILs进行培养(25.0 ℃,光暗比为16 h∶8 h,光照40~70 μmol·m-2·s-1)。
1.2 ILs对植物幼苗的生长抑制
植物生长抑制实验根据OECD化学品测试指南,测定不同处理下7 d后的植物茎叶质量,每个处理组设置3个平行。
1.3 植物叶绿素含量的测定
使用SPAD-502叶绿素含量测定仪,根据植物叶片在2种波长(650 nm和940 nm)下的光学浓度差,测定植物叶片相对叶绿素含量。
1.4 植物叶绿素荧光参数测定
植物叶片暗处理20 min后,参考Lefebvre等[9]的研究,在光化光的光强度分别为0、1、36、81、144、256、361、484、625和841 μmol·m-2·s-1时,用叶绿素荧光仪测量最小荧光(F0)、最大荧光产量(Fm)、最大光化学量子产量(Fv/Fm)、实际光化学量子产量(Y(Ⅱ))和PSⅡ调节性能量耗散的量子产量(Y(NPQ)),并绘制快速光响应曲线。
1.5 数据统计与分析
实验数据用Microsoft Excel 2019和Origin 2019进行处理,用SPSS 26进行单因素方差分析(ANOVA),用Tukey法进行显著性检验,数据结果均采用means±SD的形式表示。
2 结果与讨论(Results and discussion)
2.1 ILs对植物幼苗的生长抑制
在3种不同碳链长度ILs暴露处理7 d后,拟南芥叶片颜色和叶片大小均发生变化,与对照组叶片相比,ILs处理组的叶片均明显变小(图1)。在[C6mim]NO3和[C8mim]NO3处理组,拟南芥主叶脉白化,叶片出现褪绿黄化(图1(a)和1(b));在0.5 mg·L-1 [C12mim]NO3处理组叶片出现褐色,高浓度下叶片部分坏死(图1(c))。选择毒性胁迫较大的[C12mim]NO3处理小麦,根长和叶片大小随ILs浓度升高而缩短或缩小(图1(d))。
ILs对植物茎叶质量有明显抑制作用(表1),表现为明显的剂量-效应关系。暴露于[C6mim]NO3、[C8mim]NO3和[C12mim]NO3相同处理浓度(2 mg·L-1)下,拟南芥的茎叶质量分别为对照组的63.64%、35.82%和9.09%,说明ILs对拟南芥毒性作用为[C12mim]NO3>[C8mim]NO3>[C6mim]NO3,其毒性随碳链长度增加而增大。[C12mim]NO3处理组对拟南芥和小麦的EC50分别为0.167 mg·L-1和1.13 mg·L-1。小麦受到的胁迫比拟南芥轻,说明小麦和拟南芥对ILs的敏感性存在差异。
2.2 ILs对植物光合作用的影响
2.2.1 ILs对植物叶绿素含量的影响
ILs处理后,拟南芥叶绿素含量随ILs浓度增加明显减少(表1)。2 mg·L-1 [C6mim]NO3、[C8mim]NO3和[C12mim]NO3处理组的叶绿素含量分别为对照组的81.25%、53.13%和15.63%,说明碳链越长其毒性越强。以往的研究也发现拟南芥和藻类的叶绿素含量随ILs碳链长度增加而降低[10]。
[C6mim]NO3和[C8mim]NO3处理下拟南芥幼苗的新叶与老叶的叶绿素含量均明显降低;在[C12mim]NO3处理组,老叶叶绿素含量下降,而其新叶叶绿素含量却呈升高趋势,0.5 mg·L-1 [C12mim]NO3处理组的新生叶片叶绿素含量为对照组的140%,而老叶只有对照组的48.20%。即[C12mim]NO3的新生叶片的生长状态比老叶更好,这可能是植物在暴露于过强的毒性胁迫时的一种自我防御。面对环境胁迫时植物优先将营养集中调往新生叶片,舍弃老旧枯萎叶片而保证植株新生部分存活[11]。小麦叶片叶绿素含量随ILs浓度增加而降低(表1),2 mg·L-1浓度处理下小麦叶片叶绿素含量为对照组的91.43%,拟南芥叶片仅为15.26%,这说明小麦的叶绿素减少量更小,这与茎叶质量变化规律一致。

图1 3种离子液体(ILs)处理后植物幼苗形态学的变化
注:(a)~(c)拟南芥;(d)小麦。
Fig. 1 The morphological changes of plant seedlings after three ionic liquids (ILs) treatments
Note: (a)~(c) Arabidopsis thaliana; (b) Triticum aestivum L..
表1 ILs对植物生长及叶绿素含量的影响
Table 1 The effect of ILs on plant growth and chlorophyll content
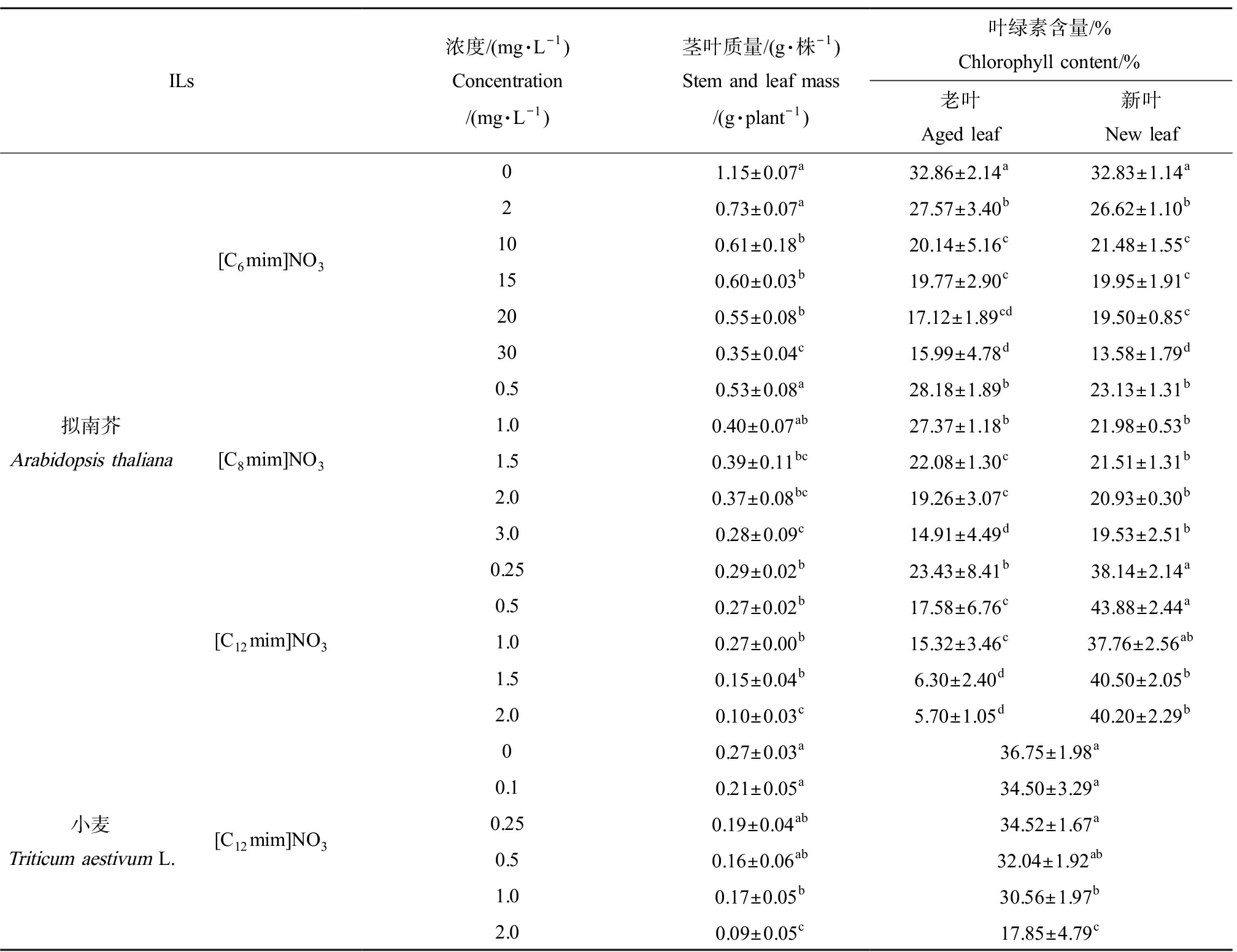
ILs浓度/(mg·L-1)Concentration/(mg·L-1)茎叶质量/(g·株-1)Stem and leaf mass/(g·plant-1)叶绿素含量/%Chlorophyll content/%老叶Aged leaf新叶New leaf拟南芥Arabidopsis thaliana[C6mim]NO3[C8mim]NO3[C12mim]NO301.15±0.07a32.86±2.14a32.83±1.14a20.73±0.07a27.57±3.40b26.62±1.10b100.61±0.18b20.14±5.16c21.48±1.55c150.60±0.03b19.77±2.90c19.95±1.91c200.55±0.08b17.12±1.89cd19.50±0.85c300.35±0.04c15.99±4.78d13.58±1.79d0.50.53±0.08a28.18±1.89b23.13±1.31b1.00.40±0.07ab27.37±1.18b21.98±0.53b1.50.39±0.11bc22.08±1.30c21.51±1.31b2.00.37±0.08bc19.26±3.07c20.93±0.30b3.00.28±0.09c14.91±4.49d19.53±2.51b0.250.29±0.02b23.43±8.41b38.14±2.14a0.50.27±0.02b17.58±6.76c43.88±2.44a1.00.27±0.00b15.32±3.46c37.76±2.56ab1.50.15±0.04b6.30±2.40d40.50±2.05b2.00.10±0.03c5.70±1.05d40.20±2.29b小麦Triticum aestivum L.[C12mim]NO300.27±0.03a36.75±1.98a0.10.21±0.05a34.50±3.29a0.250.19±0.04ab34.52±1.67a0.50.16±0.06ab32.04±1.92ab1.00.17±0.05b30.56±1.97b2.00.09±0.05c17.85±4.79c
注:同列不同字母表示组间差异显著(P<0.05)。
Note: Different letters in the same column indicate significant differences between groups (P<0.05).
2.2.2 ILs对植物叶绿素荧光参数的影响
测定叶绿素荧光可以得到光合作用的过程信息[12],不同浓度ILs处理下植物幼苗叶绿素荧光参数如表2所示,包括最小荧光(F0,与叶片叶绿素浓度有关)、最大荧光产量(Fm,反映经过PSⅡ的电子传递情况)和最大光化学量子产量(Fv/Fm,反映PSⅡ反应中心的光能转换效率,不受物种生长阶段影响,非胁迫条件下变化极小,胁迫环境下表现为下降)[13]。
在2 mg·L-1 [C6mim]NO3、[C8mim]NO3和[C12mim]NO3处理组,老叶的F0值分别为对照组的1.07倍、1.23倍和2.23倍,说明碳链长度越长,对叶片光能效率影响越大。[C6mim]NO3和[C8mim]NO3处理组,新叶和老叶的F0均随ILs浓度增加而升高,这可能是由于植物的电子传递通路被切断,植物受到的氧化损伤增加,使得PSⅡ发生光失活,导致其反应中心流失,光合系统受到抑制而使F0上升[14];Fm均随ILs浓度增加而降低,表明光系统Ⅱ受到胁迫,导致其类囊体膜受损或类囊体失去活性,影响植物吸收光能的效率[15];新叶和老叶Fv/Fm随ILs浓度增加而下降,表明叶片中的光抑制以及开放PSⅡ中心活性下调。而[C12mim]NO3处理下拟南芥叶片的荧光参数变化不同,老叶F0随ILs浓度增加而增加,而新叶F0随ILs浓度增加而下降,新叶中Fv/Fm变化较小,而在老叶中显著下降,仅为对照的11.89%,说明老叶受到较强胁迫,但新叶叶片吸收光能效率反而增加,这与叶绿素含量的结果一致。
表2 3种ILs处理下植物叶片的荧光参数
Table 2 Fluorescence parameters of plant leaves under three ILs treatments

ILs种类浓度/(mg·L-1)Concentration/(mg·L-1)F0FmFv/Fm新叶New leaf老叶Aged leaf新叶New leaf老叶Aged leaf新叶New leaf老叶Aged leaf拟南芥Arabidopsis thaliana[C6mim]NO3[C8mim]NO3[C12mim]NO300.0900.0860.2930.3130.6930.72520.0910.0920.2880.3130.6840.706100.1100.1030.2720.3060.5960.663150.1550.1090.2660.3040.4170.641200.1720.1160.2620.2830.3440.590300.1950.1270.2590.2530.2470.49800.0830.0890.3500.3020.7630.7050.50.0980.0900.2800.3110.6500.7111.00.1000.0910.2690.3030.6280.7001.50.1530.1020.2550.2820.4000.6382.00.1590.1090.2450.2810.3510.6123.00.2050.1330.2420.2730.1530.51300.1060.0890.3100.2950.6580.6980.250.1030.0930.2830.2650.6360.6490.50.0900.1140.2950.2340.6950.5131.00.0900.1140.2580.2290.6510.5021.50.0780.1160.2470.2190.6840.4702.00.0740.1980.2350.2160.6850.083小麦Triticum aestivum L.[C12mim]NO300.1160.3160.5880.10.1220.2930.5810.250.1310.2870.5470.50.1350.2850.5311.00.1420.2790.5312.00.1450.2620.417
注:F0表示最小荧光;Fm表示最大荧光产量;Fv/Fm表示最大量子产量。
Note: F0 means minimum fluorescence; Fm means maximum fluorescence; Fv/Fm means maximal quantum efficiency.
随ILs浓度增加小麦叶片F0升高、Fm降低,Fv/Fm降低,说明小麦的光合作用过程受到胁迫。在相同浓度处理下(2 mg·L-1),小麦叶片和拟南芥叶片的F0值分别为对照组的125.00%和222.47%,Fm值分别为对照组的82.91%和73.22%,说明ILs对小麦光合作用的影响小于拟南芥。
相关性分析表明,光合作用参数与生长抑制率具有较好的相关性,[C6mim]NO3处理下拟南芥幼苗的新叶和老叶叶绿素含量与抑制率的相关系数(r2)分别为0.9496和0.8906,[C8mim]NO3和[C12mim]NO3处理下新叶与老叶叶绿素含量与抑制率的r2分别为0.9965和0.6365、0.6476和0.7418;[C12mim]NO3处理下小麦叶片叶绿素含量和Fv/Fm与抑制率的r2分别为0.8117和0.8643,说明ILs可能通过抑制植物光合作用而影响植物生长[16]。
2.3 ILs对植物荧光外观的影响
光合图谱颜色代表了Fv/Fm的值,图片下方的色带从左到右(从橙色到蓝色)为Fv/Fm值增大。而Fv/Fm的值越小表示植物受到的胁迫越大(图2)。
在[C6mim]NO3处理组中,随ILs浓度升高(10 mg·L-1以上),拟南芥叶片的荧光图颜色从纯蓝色转变为叶片中心出现绿色,ILs对拟南芥造成光合胁迫;在[C8mim]NO3处理组中,随ILs浓度升高(1 mg·L-1以上),荧光成像图颜色由纯蓝色转变为叶片大范围出现绿色,拟南芥受到光合胁迫,且[C8mim]NO3对拟南芥的光合胁迫比[C6mim]NO3更强;[C12mim]NO3处理组中,随ILs浓度升高(1 mg·L-1以上),叶片图像甚至开始出现橙色,少数叶片出现枯萎破损。在同一浓度处理下(2 mg·L-1),[C12mim]NO3造成的光合胁迫(橙黄色)比[C8mim]NO3(绿色)和[C6mim]NO3(蓝色)更强,因此进一步说明3种供试ILs的毒性大小为:[C12mim]NO3>[C8mim]NO3>[C6mim]NO3(图2(a))。
在0.5 mg·L-1和1 mg·L-1 [C12mim]NO3浓度下,小麦叶尖呈现少量绿色,2 mg·L-1处理叶尖有少量橙色出现(图2(b)),而在同一浓度下的拟南芥叶片已完全转为橙绿色。这说明ILs对小麦存在光合胁迫,但胁迫的程度小于拟南芥。
2.4 ILs对植物Y(Ⅱ)和Y(NPQ)的影响
为了进一步明晰ILs胁迫下PSⅡ系统变化,测定了植物叶片Y(Ⅱ)和Y(NPQ)[17]。Y(Ⅱ)反映的是叶片的实际光能转化效率,表示PSⅡ的实际光合效率[18],Y(NPQ)是指非光化学猝灭量子产率,指PSⅡ调节性能量耗散(如将过量光能耗散为热)的量子产率[19]。
随ILs浓度增高,拟南芥叶片Y(Ⅱ)下降(图3(a)),说明拟南芥的实际光合效率下降,这可能与PSⅡ的光捕获复合体破坏有关。光捕获复合体是光系统Ⅰ和光系统Ⅱ之间的结构,其作用是维持2个光系统的能量平衡,而有毒物质会破坏这种平衡,从而影响光合作用[20]。在同等条件(36 μmol·m-2·s-1,2 mg·L-1)下,[C6mim]NO3和[C8mim]NO3处理组的Y(Ⅱ)值分别为对照组的98.96%和25.13%,说明[C8mim]NO3对拟南芥的胁迫更强。[C12mim]NO3处理组的Y(Ⅱ)值差别不大,说明该组植物样品受到胁迫较小,这可能由于植物受高毒性污染物胁迫时,会产生某种自我防御[21]。
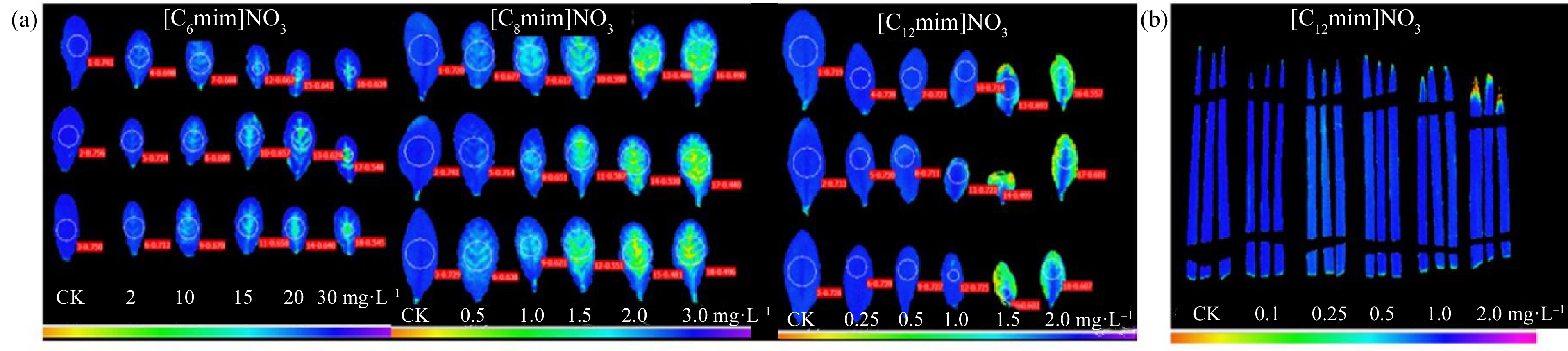
图2 ILs处理后植物叶片叶绿素荧光图
注:(a)拟南芥;(b)小麦。
Fig. 2 Chlorophyll fluorescence of plant leaves treated with three ILs
Note: (a) Arabidopsis thaliana; (b) Triticum aestivum L..
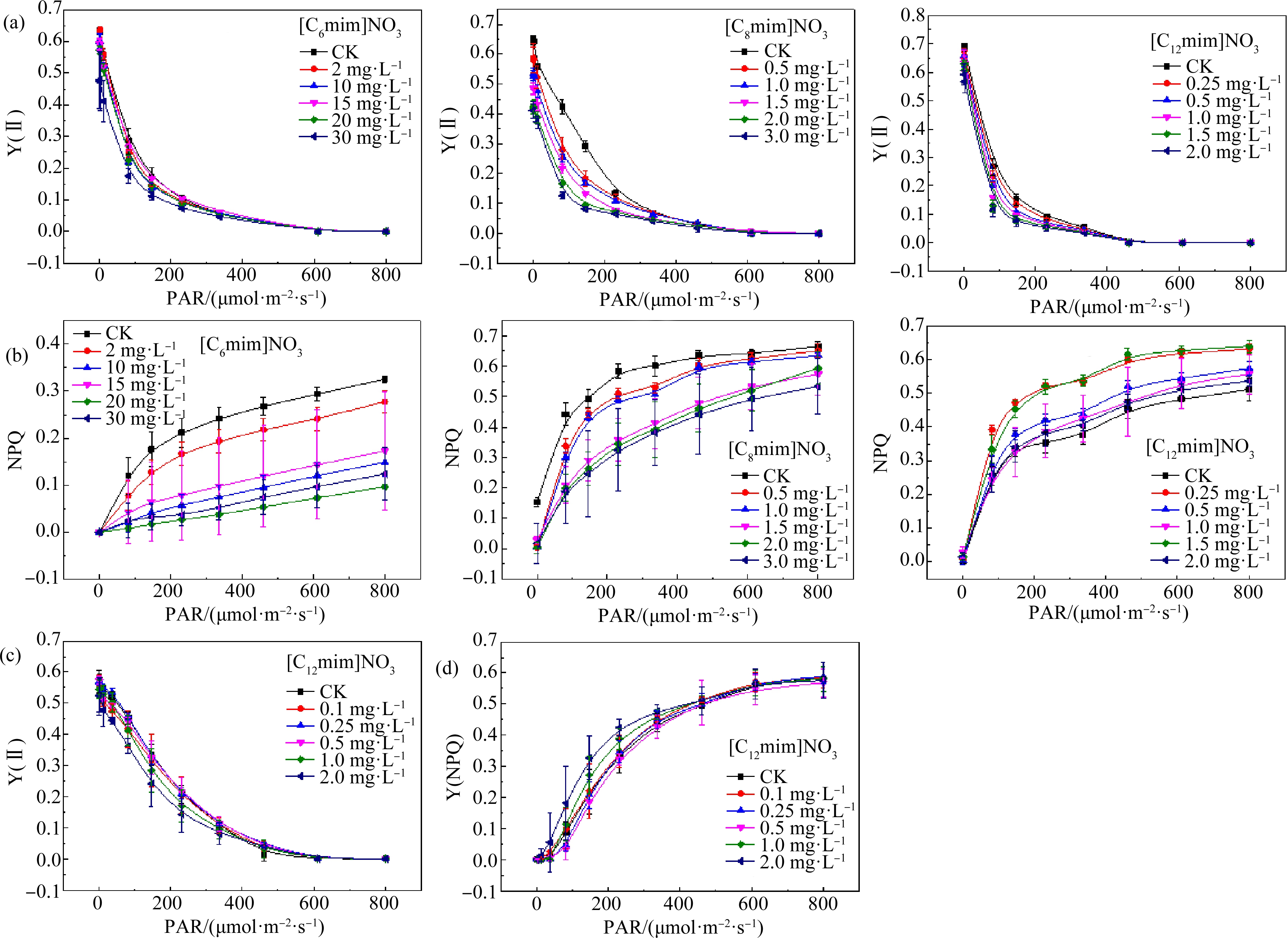
图3 ILs对植物Y(Ⅱ)和Y(NPQ)的影响
注:(a)和(b)拟南芥;(c)和(d)小麦;PAR表示光合有效辐射,Y(Ⅱ)表示实际光合效率,Y(NPQ)表示非光化学猝灭量子产率。
Fig. 3 The effect of ILs on plant Y (Ⅱ) and Y(NPQ)
Note: (a) and (b) Arabidopsis thaliana; (c) and (d) Triticum aestivum L.; PAR means photosynthetically active radiation; Y(Ⅱ) means effective photosynthetic efficiency; Y(NPQ) means non photochemical quenching quantum yield.
拟南芥的Y(NPQ)值随[C6mim]NO3和[C8mim]NO3浓度升高而降低(图3(b)),Y(NPQ)下降说明叶绿素a对电子的传导率降低,影响PSⅡ正常运作,从而影响植物的正常光合作用[22]。在36 μmol·m-2·s-1光强和2 mg·L-1浓度下,[C6mim]NO3、[C8mim]NO3和[C12mim]NO3处理组的Y(NPQ)分别是对照组的95.37%、81.91%和116.30%。[C8mim]NO3处理组的Y(NPQ)值比[C6mim]NO3低,说明[C8mim]NO3毒性更强;而[C12mim]NO3处理组的Y(NPQ)却比对照组高,说明拟南芥受到的胁迫较小,可能是植物在面对高毒性污染物时产生的某种自我防御。
在[C12mim]NO3处理下,小麦叶片的Y(Ⅱ)值和Y(NPQ)与对照没有显著差异(图3(c)和3(d)),说明[C12mim]NO3对小麦叶片实际光合效率和电子的传导率影响不大。
本文研究了3种不同碳链长度咪唑硝酸盐ILs对拟南芥和小麦的生长抑制作用,其毒性大小为[C12mim]NO3>[C8mim]NO3>[C6mim]NO3。3种供试ILs对拟南芥和小麦叶片的叶绿素含量均有明显影响,叶绿素合成受到严重抑制,且ILs的碳链长度越长,拟南芥幼苗受到的光合胁迫越强。[C6mim]NO3和[C8mim]NO3处理组及[C12mim]NO3处理组老叶的拟南芥叶片荧光参数F0随ILs浓度增加而升高,Fm、Fv/Fm、Y(Ⅱ)和Y(NPQ)随ILs浓度增加而下降,说明ILs可以通过破坏PSⅡ的光捕获复合体和降低光合作用电子传导率来影响光能转化效率和PSⅡ的正常运作,进而影响植物正常光合作用。而[C12mim]NO3处理下拟南芥新叶叶绿素含量上升,F0下降,Fm、Fv/Fm、Y(Ⅱ)和Y(NPQ)升高,说明植物受到高毒性污染物胁迫时,会产生某种自我防御。[C12mim]NO3对小麦的光合作用影响要小于拟南芥,因此研究ILs毒性时应考虑不同植物类型的毒性效应。
[1] Petkovic M, Seddon K R, Rebelo L P, et al. Ionic liquids: A pathway to environmental acceptability [J]. Chemical Society Reviews, 2011, 40(3): 1383-1403
[2] Amde M, Liu J F, Pang L. Environmental application, fate, effects, and concerns of ionic liquids: A review [J]. Environmental Science & Technology, 2015, 49(21): 12611-12627
[3] Thuy Pham T P, Cho C W, Yun Y S. Environmental fate and toxicity of ionic liquids: A review [J]. Water Research, 2010, 44(2): 352-372
[4] Bubalo M C, ![]() I R, et al. A brief overview of the potential environmental hazards of ionic liquids [J]. Ecotoxicology and Environmental Safety, 2014, 99: 1-12
I R, et al. A brief overview of the potential environmental hazards of ionic liquids [J]. Ecotoxicology and Environmental Safety, 2014, 99: 1-12
[5] Cho C W, Pham T P T, Zhao Y F, et al. Review of the toxic effects of ionic liquids [J]. The Science of the Total Environment, 2021, 786: 147309
[6] Mrozik W, Jungnickel C, Paszkiewicz M, et al. Interaction of novel ionic liquids with soils [J]. Water, Air, and Soil Pollution, 2013, 224: 1759
[7] Li Y J, Yang M, Liu L, et al. Effects of 1-butyl-3-methylimidazolium chloride on the photosynthetic system and metabolism of maize (Zea mays L.) seedlings [J]. Ecotoxicology and Environmental Safety, 2018, 161: 648-654
[8] Li M, Xue Y L, Liu Z J, et al. Toxic effect and mechanism of four ionic liquids on seedling taproots of Arabidopsis thaliana [J]. Environmental Science and Pollution Research International, 2018, 25(15): 14703-14712
[9] Lefebvre S, Mouget J L, Lavaud J. Duration of rapid light curves for determining the photosynthetic activity of microphytobenthos biofilm in situ [J]. Aquatic Botany, 2011, 95(1): 1-8
[10] Pham T P, Cho C W, Min J, et al. Alkyl-chain length effects of imidazolium and pyridinium ionic liquids on photosynthetic response of Pseudokirchneriella subcapitata [J]. Journal of Bioscience and Bioengineering, 2008, 105(4): 425-428
[11] Himelblau E, Amasino R M. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence [J]. Journal of Plant Physiology, 2001, 158(10): 1317-1323
[12] Wang H, Jin M K, Xu L L, et al. Effects of ketoprofen on rice seedlings: Insights from photosynthesis, antioxidative stress, gene expression patterns, and integrated biomarker response analysis [J]. Environmental Pollution, 2020, 263(Pt A): 114533
[13] Liu J H, Hou H, Zhao L, et al. Protective effect of foliar application of sulfur on photosynthesis and antioxidative defense system of rice under the stress of Cd [J]. The Science of the Total Environment, 2020, 710: 136230
[14] Aro E M, Virgin I, Andersson B. Photoinhibition of photosystem Ⅱ. Inactivation, protein damage and turnover [J]. Biochimica et Biophysica Acta, 1993, 1143(2): 113-134
[15] Liu H J, Zhang S X, Zhang X Q, et al. Growth inhibition and effect on photosystem by three imidazolium chloride ionic liquids in rice seedlings [J]. Journal of Hazardous Materials, 2015, 286: 440-448
[16] Liu H J, Xia Y L, Cai W D, et al. Enantioselective oxidative stress and oxidative damage caused by Rac- and S-metolachlor to Scenedesmus obliquus [J]. Chemosphere, 2017, 173: 22-30
[17] Tan S L, Liu T, Zhang S B, et al. Balancing light use efficiency and photoprotection in tobacco leaves grown at different light regimes [J]. Environmental and Experimental Botany, 2020, 175: 104046
[18] Kramer D M, Johnson G, Kiirats O, et al. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes [J]. Photosynthesis Research, 2004, 79(2): 209
[19] Krause G, Jahns P. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: Characterization and function [M]// Chlorophyll a Fluorescence. Springer, 2004: 463-495
[20] Basso S, Simionato D, Gerotto C, et al. Characterization of the photosynthetic apparatus of the Eustigmatophycean Nannochloropsis gaditana: Evidence of convergent evolution in the supramolecular organization of photosystem Ⅰ [J]. Biochimica et Biophysica Acta, 2014, 1837(2): 306-314
[21] 侯秀富, 郭沛涌, 张华想, 等. 水体悬浮颗粒物对斜生栅藻生理生化及光合活性的影响[J]. 环境科学学报, 2013, 33(5): 1446-1457
Hou X F, Guo P Y, Zhang H X, et al. Effects of water suspended particulate matter on the physiological and photosynthetic activity of Scenedesmus obliquus [J]. Acta Scientiae Circumstantiae, 2013, 33(5): 1446-1457 (in Chinese)
[22] Shahzadi A K, Bano H, Ogbaga C C, et al. Coordinated impact of ion exclusion, antioxidants and photosynthetic potential on salt tolerance of ridge gourd [Luffa acutangula (L.) Roxb. [J]. Plant Physiology and Biochemistry: PPB, 2021, 167: 517-528