-
当前,在我国的重金属污染治理行业中,铅蓄电池行业是重点关注行业之一,也是重金属铅离子(Pb2+)的主要污染来源[1],因其隐蔽性、危害性等特点,在土壤中不断富集,最终对人体产生高毒性和致癌性[2],因此,对于铅污染处理极为紧迫. 吸附、反渗透、化学沉淀、离子交换等是处理重金属污染的典型方法[3],与其他方法相比,吸附法因其技术成熟、成本低、沉淀物生成少等优点在重金属污染治理中被广泛应用[4].
在土壤固相物质中,微生物可将土壤有机质(soil organic matter,SOM)同化为自身组分并在其死亡后以微生物残体的形式逐渐积累在土壤中[5],并与土壤矿物相互作用形成各种矿物-有机复合体、配合物或聚集体[6 − 7],成为稳定土壤有机碳库的重要组成部分,其对土壤重金属的治理起到了关键性的作用. 其中,蒙脱石和赤铁矿常作为典型的土壤矿物对重金属吸附进行研究[8],蒙脱石因其较大的比表面积和较高的阳离子交换能力常被作为吸附剂[9],赤铁矿因其含有铁氧化物且边缘表面带正电[10],使得带负电荷的有机质与其相结合,二者皆可与SOM结合形成有机无机复合体[11]. 目前,土壤对Pb2+的吸附主要受到土壤有机质、黏土、铁和锰氧化物等土壤组分的影响[12],且研究了不同物理化学性质的土壤对Pb2+的吸附,如不同质地、不同有机质浓度和不同阳离子交换容量等[13]. 其中,前人就土壤微生物源有机质对Pb2+的吸附主要研究其整体的有机无机复合体[14],而真菌残体较细菌残体更稳定,且在微生物残体碳中占比更高[15],故本文将微生物分为两类菌群(细菌菌群、真菌菌群),取两种典型的矿物(黏土矿物-蒙脱石、铁氧化物-赤铁矿),研究经培养后的微生物残体及有机无机复合体对Pb2+的吸附作用.
本研究旨在探讨微生物残体(细菌、真菌)及其与黏土矿物(蒙脱石、赤铁矿)形成的有机无机复合体的结合机制,进一步解释矿物与微生物相结合后的有机无机复合体对Pb2+的吸附能力,进而表明微生物残体及其与矿物结合形成有机无机复合体对土壤重金属的吸附不可忽视.
-
本实验土壤采样于云南省昆明市呈贡区平均海拔为2006 m的竹林红壤土. 以该土壤微生物作为源微生物,按照细菌、真菌生长所需配置细菌培养基和真菌培养基[16],进行细菌菌群和真菌菌群的选取、接种及收集.
选取菌群:取200 g土壤样品置于200 mL 水中均匀振荡10 min,之后静置30 min,取上清液约50 mL到离心管中,再次均匀振荡并静置. 取10 mL上清液分别加到已灭菌的细菌培养基和真菌培养基中,放入摇床,设定条件150 r·min−1,温度25 ℃. 通过分光光度法确定细菌和真菌的对数期,并取对数期的细菌菌群和真菌菌群为纯化菌群.
接种菌群:将细菌、真菌培养基分装至250 mL锥形瓶中,分别设两个实验组,一组不添加矿物,另一组向对应锥形瓶分别加入1.0 g蒙脱石和1.0 g赤铁矿. 所有培养基经高压蒸汽灭菌后(121 ℃,30 min),放入紫外操作台中进行接种操作. 取10 mL的纯化菌体对应接种到细菌、真菌培养基及含有矿物的培养基中,放入摇床,设定条件150 r·min−1,温度25 ℃. 在摇床中培养24 h后取出,此时培养基中出现浑浊,随后放入高压蒸汽灭菌锅中进行灭菌,设定温度为120 ℃,时间为30 min.
微生物残体及复合体的收集:将高温灭菌后的微生物残体及复合体置于离心管中,用超纯水多次润洗并离心(
3000 r·min−1,10 min),以去除微生物残体中的溶解性有机碳. 将残体碳及复合体经冷干机干燥,研磨至过200目筛,在-80 ℃下保存备用. 将微生物残体及其复合体分别命名为细菌残体(BN)、细菌-蒙脱石(B-M)、细菌-赤铁矿(B-Fe)、真菌残体(FN)、真菌-蒙脱石(F-M)、真菌-赤铁矿(F-Fe). -
配制0.1 mol·L−1的NaNO3作为背景液,调至pH5备用. 称取Pb(NO3)2用背景液在500 mL烧杯中稀释,稀释后作为母液. Pb2+浓度梯度设置在1—10 mg·L−1,移取母液并用背景液进行稀释. 加入NaN3用以抑制微生物的活动,其浓度为200 mg·L−1. 通过预实验确定BN、FN、B-M、F-M、F-Fe、M的固液比为1:7500,B-Fe、Fe的固液比为1:1000,确保每个吸附剂对Pb2+的吸附率基本在20%—80%之间, 每个浓度设置3个平行. 样品置于25 ℃、120 r·min−1的摇床中避光振荡3 d. 吸附平衡后,样品在
3000 r·min−1下离心10 min,上清液经0.45 μm水相滤膜去除胶体和杂质,收集滤液待测. 根据质量损失加入等体积的背景液进行解吸实验,解吸实验进行3次,解吸平衡后,在3000 r·min−1下离心10 min后经0.45 μm水相滤膜过滤,上清液中Pb2+浓度用火焰原子吸收分光光度计测定. -
样品冷冻干燥后,研磨过200目筛,称取(2±0.1)mg的样品于锡舟中,每个样品设置3次重复,使用元素分析仪(Vario EL II Elemental)进行测定.
-
样品和溴化钾按照1:100比例混合均匀,在玛瑙研钵中充分磨细压片,然后在光谱仪上进行扫描(Varian 640-IR FTIR),扫描次数20,分辨率为8 cm−1,操作范围为400—
4000 cm−1,使用Origin 2018作图. -
有机无机复合体的比表面积、孔径大小是表征吸附剂对污染物吸附物的重要参数指标. 本研究在N2的模式下,采用了BET分析仪(Micromeritics ASAP2020)测定.
-
本研究使用Origin2018软件进行拟合,比较Freundlich模型和Langmuir模型两种吸附等温线的拟合相关系数R2来衡量拟合结果.
Freundlich模型:
式中,Qe为吸附剂的平衡吸附量(mg·g−1);Ce为液相平衡浓度(mg·L−1);KF为Freundlich模型的吸附平衡系数;n为Freundlich模型的非线性系数.
Langmuir模型:
式中,Qe为吸附剂的平衡吸附量(mg·g−1);Qm为最大吸附量(mg·g−1);Ce为液相平衡浓度(mg·L−1);KL为Langmuir模型的吸附平衡系数.
Pb2+在解吸过程中存在解吸滞后现象,该现象通过解吸率的大小(RR)来衡量:
式中,Q0为解吸结束后吸附剂上Pb2+质量(mg·g−1).
-
如表1所示,不同微生物残体及其复合体中的元素组成有所差异,相较于微生物-矿物复合体而言,BN和FN具有较高的含碳量(C%均超过40%),氮(N)、硫(S)含量相差不大,且二者的极性((N+O)/C)和芳香性(H/C)相近. 蒙脱石-微生物复合体的C%均低于对应的赤铁矿-微生物复合体,蒙脱石相比于赤铁矿具有较大的比表面积(蒙脱石的比表面积是赤铁矿的20倍,见表2),但并没有增加微生物残体碳的积累. 有研究指出微生物细胞壁的主要活性基团(羧基和磷酸基)可在细胞表面形成共价键,促进微生物分泌物附着到铁氧化物[17]. 因此,该培养下赤铁矿更有利于微生物残体碳的积累.
此外,各个复合体间的H/C比值各不相同,其表现为B-Fe<FN<F-Fe<BN<F-M<B-M. 一般地,H/C比值越低,芳香性越强,故赤铁矿-微生物复合体的芳香性高于蒙脱石-微生物复合体. 一方面,有研究表明赤铁矿表面富含铁原子配位—OH位点,且高芳香性的有机分子会优先与—OH基团形成配合物[18];另一方面,赤铁矿可通过共沉淀作用与芳香性化合物形成复合体[19]. 这可能是赤铁矿-微生物复合体相比蒙脱石-微生物复合体具有更高的芳香性的重要因素. 综上所述,通过吸附微生物分泌物可能改变矿物的表面性质变化,进一步影响复合体对Pb2+的吸附能力.
如表2所示,BN和FN的孔隙结构相差不大. M的比表面积是Fe的18倍,这是由于M具有较大的内比表面积[20]. 赤铁矿-微生物复合体的比表面积较赤铁矿变化不明显,而蒙脱石-微生物复合体的比表面积较蒙脱石显著降低,但其比表面积是赤铁矿-微生物复合体的四倍以上,蒙脱石-微生物复合体基于自身比表面积对Pb2+可能具有更高的吸附量. 此外,微生物-矿物复合体的平均孔径相较纯矿物均增加,这可能是由于微生物分泌物黏附在矿物表面上,促进了矿物的腐蚀[21],从而增加了其平均孔径.
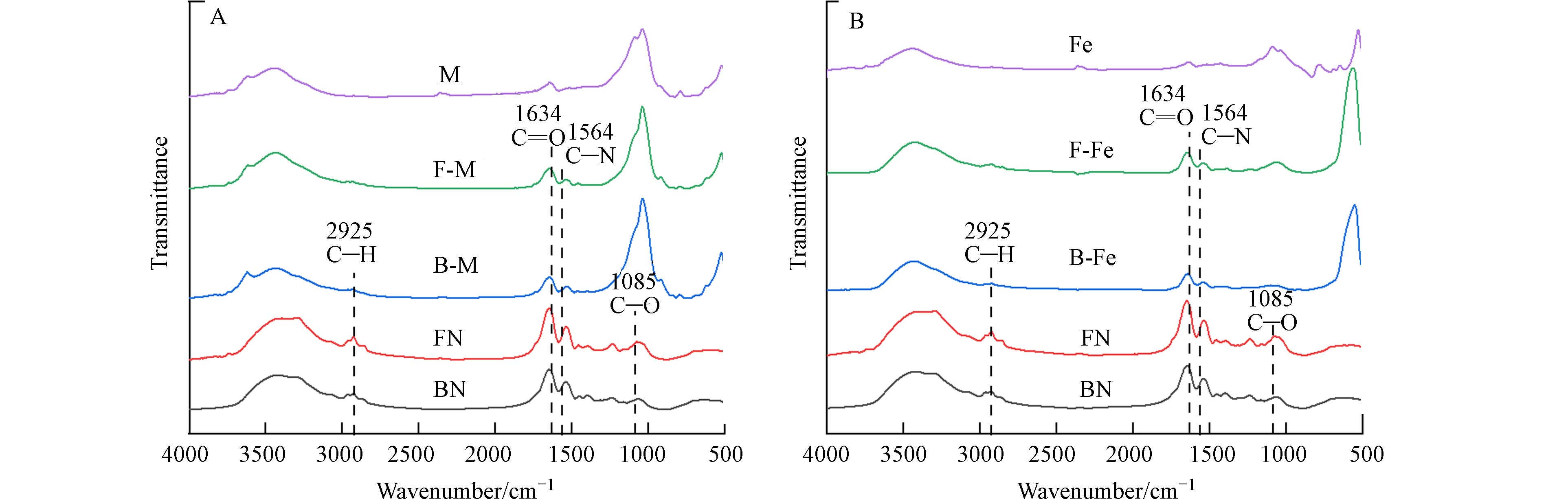
由图1可知,细菌残体和真菌残体在红外光谱图中的特征峰位置类似,说明二者所含官能团种类及相对峰面积基本相同,其中,在
2925 cm−1是脂肪族中—CH2和—CH3的特征峰[22],仅在纯残体组中出现,表明微生物残体相较复合体含有较多的脂肪族化合物. 在1085 cm−1处的吸收峰为C—O—C的伸缩振动,FN在该处的相对峰面积高于BN,表明FN具有更多的多糖类物质. 在1634 cm−1、1564 cm−1处分别表示C=O、C—N的特征峰,各个微生物-矿物复合体在这两处的相对峰面积均高于纯矿物,表明微生物-矿物复合体上结合了含氧、含氮化合物,这可促进复合体与重金属相络合[23],这也可能是复合体对Pb2+表现出较好的络合能力的主要原因. -
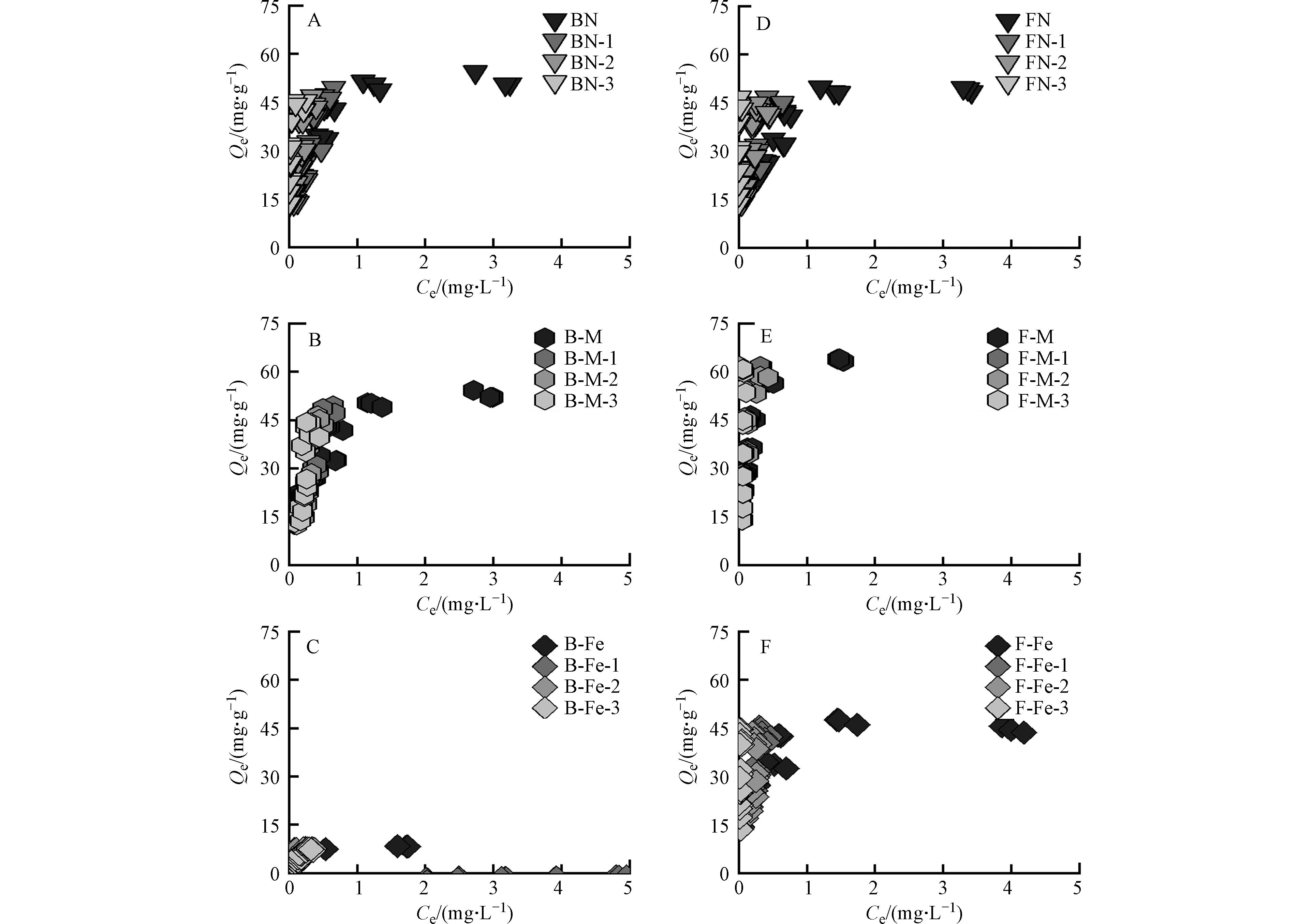
采用Langmuir模型和Freundlich模型对批量吸附实验进行了拟合,如表3所示. 通过比较拟合相关系数R2,Langmuir模型拟合下的R2远低于Freundlich模型拟合,因此,Freundlich模型更适合描述所有吸附剂对Pb2+的吸附作用(R2的范围为0.828—0.957,Fe除外). 在Freundlich模型中,所有吸附剂的n值在0.204—0.626,远低于1,表明微生物残体表面吸附位点分布不均,呈现出较强的吸附异质性.
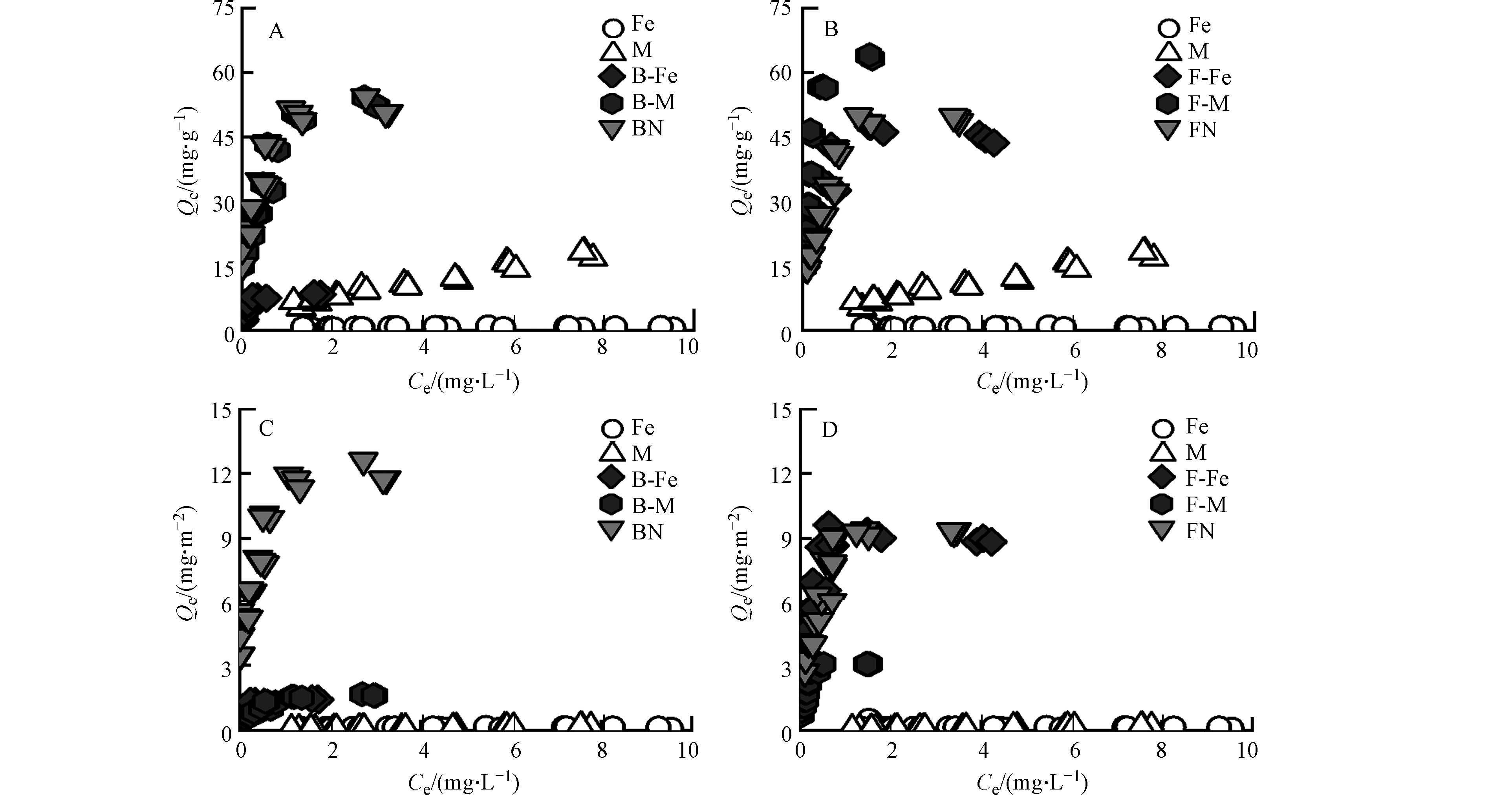
纯矿物、微生物残体及其复合体对Pb2+的吸附等温线如图2所示,各个复合体对Pb2+的吸附容量随着Pb2+的初始浓度增加而增加,随后达到饱和吸附量. 其中,纯矿物对Pb2+的吸附量远低于微生物残体. 由图1可知,微生物残体含有较多的含氧官能团(C—O、C=O),这些官能团可为其表面提供负电荷场所,进一步通过络合作用[24]、静电吸附[25]对Pb2+进行吸附;且细菌残体对Pb2+的吸附量与真菌残体相差不大,因为二者具有相近的元素组成、表面官能团及孔隙结构,因此,细菌残体和真菌残体对Pb2+的吸附量基本一致.
由图2A、图2B可知,矿物-微生物复合体相比于纯矿物而言,对Pb2+均表现出较好的吸附性能. 其中,蒙脱石-微生物复合体对Pb2+的吸附量比赤铁矿-微生物复合体更大. 由图1可知,蒙脱石/赤铁矿-微生物复合体的表面均表现出含氧官能团和含氮官能团,这说明矿物经微生物附着生长后其表面负载了这些活性基团,进而为吸附Pb2+提供更多的吸附位点[26];由表2可知,蒙脱石-微生物复合体的比表面积是赤铁矿-微生物复合体的4倍以上,且蒙脱石-微生物复合体中的矿物基质(蒙脱石)本身具有较好的吸附性能[27]. 因此,蒙脱石-微生物复合体对Pb2+的吸附过程中,除了活性基团提供吸附位点外,还有矿物基质(蒙脱石)提供吸附位点,故蒙脱石-微生物复合体对Pb2+的吸附效果更好.
此外,真菌-矿物复合体对Pb2+的吸附量比细菌-矿物复合体更大,由表1可知,F-M比B-M具有更高的芳香性,可为复合体表面提供更多的酚羟基官能团. 研究表明Pb2+可与酚羟基和羧基官能团相络合[28];又有研究表明复合体中的芳香结构可通过阳离子-π作用与Pb2+相结合[29],故F-M对Pb2+吸附效果更佳. 虽然F-Fe比B-Fe的芳香性低,但研究表明真菌菌丝中会产生大量的活性氧自由基(O2-),这些自由基在赤铁矿存在下能够发生芬顿反应[30],进而产生氢氧根离子(OH-)[31],使F-Fe表面暴露出更多的吸附位点,并通过络合作用与Pb2+相结合[32],增强了其对Pb2+的吸附能力.
由图2C、图2D可知,经比表面积标准化后,蒙脱石-微生物复合体(B-M、F-M)对Pb2+的吸附量Qe值大大降低,这说明二者在单位面积内对Pb2+吸附量较小,蒙脱石复合体吸附Pb2+主要是依赖自身较大的比表面积而非其表面的有机官能团. 而赤铁矿复合体在比表面积标准化前后其Qe值基本不变,赤铁矿复合体对Pb2+的吸附不受比表面积的影响. 在赤铁矿-微生物复合体在形成过程中,其中的铁元素受到微生物分泌物的影响可发生氧化还原反应[33],可促进赤铁矿与微生物分泌物形成配合物[34],该过程主要分为两部分,一是有机质通过特定的化学相互作用(如化学吸附)吸附到赤铁矿表面形成内球配合物[35],并使其表面负载阴离子基团[36];二是可通过非特异性相互作用(如物理吸附、氢键作用和静电吸附)形成外球配合物[37]. 因此,在双层络合机制作用下,在赤铁矿上形成的有机无机复合体中有机官能团较蒙脱石对Pb2+的吸附更强.
-
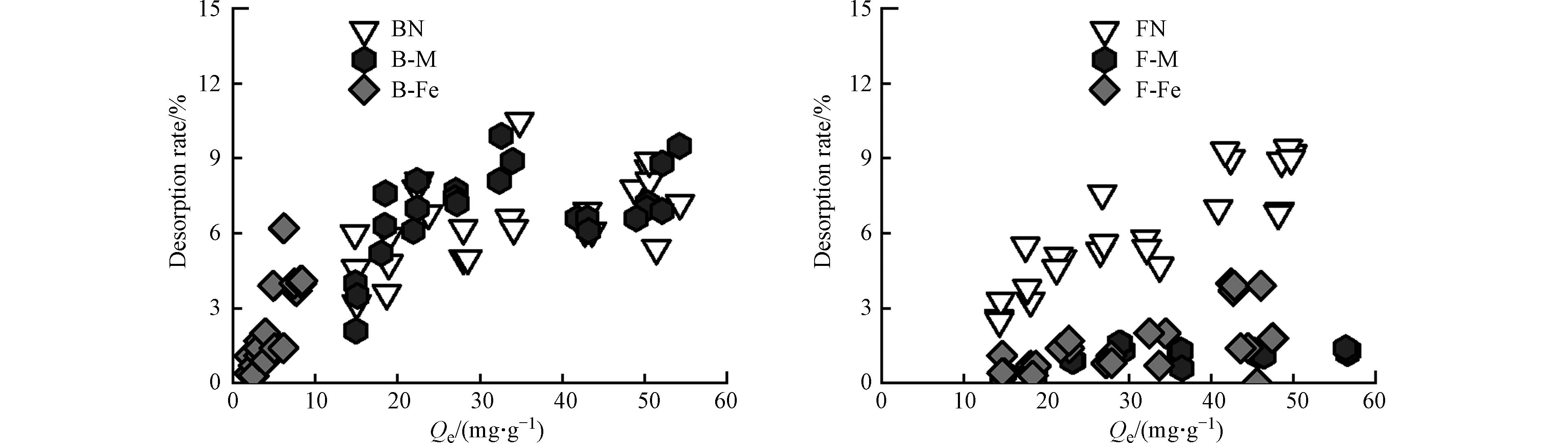
如图3所示,在微生物残体、微生物-矿物复合体对Pb2+的解吸过程中,所有吸附剂在第一次解吸时,Pb2+会有少部分被解吸,而在第二次、第三次解吸时,所有吸附剂基本不解吸,微生物残体及其复合体与Pb2+之间的吸附可能通过物理作用(如静电吸附)和化学作用(如络合作用和阳离子-π作用),且结合后的化学键较难破坏[38],所以吸附在各个吸附剂上的Pb2+难解吸.
如图4所示,微生物残体及其复合体对Pb2+的解吸率(RR)基本在10%以下,表现为BN为8.9%,FN为9.0%,B-M为8.8%,F-M为1.7%,B-Fe为4.1%,F-Fe为1.4%. 各个复合体的解吸率均低于微生物残体,微生物残体(BN、FN)的解吸率RR接近9%,由图2可知,微生物残体对Pb2+的吸附量较大,研究表明微生物残体表面带负电荷[39],故其对Pb2+的吸附机理更多地是静电吸附和络合作用. 其中,静电吸附作用相较其他化学作用并不稳定,因此,微生物残体对Pb2+的吸附量较多,但解吸量也较多. 蒙脱石-微生物复合体较赤铁矿-微生物复合体对Pb2+的吸附量更大,但其解吸率也更大,故基于复合体中的矿物基质(蒙脱石)对Pb2+的吸附量虽多,但并不牢固. 而赤铁矿-微生物复合体的解吸率较蒙脱石-微生物复合体的小,这是由于其表面存在“双层络合”吸附机制[40]. 当Pb2+浓度较高时,可直接与赤铁矿-微生物复合体表面结合,通过配体作用形成较弱的键合[41];在低Pb2+的浓度下,在其表面以羟基为主的内球层发生络合作用[42],故赤铁矿-微生物复合体对Pb2+的吸附更牢固. 综上所述,矿物的存在可以增加有机质对Pb2+的固定作用,蒙脱石-微生物复合体在矿物基质(蒙脱石)的作用下,对Pb2+的吸附量更多;而赤铁矿-微生物复合体在有机官能团的作用下,对Pb2+吸附更牢固.
-
(1)细菌残体和真菌残体的元素含量和性质较为相近,但各个复合体间的组成和性质差异较大. 其中,真菌-矿物复合体相比细菌-矿物复合体具有更高的碳含量且具有更低的极性,赤铁矿-微生物复合体比蒙脱石-微生物复合体具有较高的芳香性.
(2)蒙脱石-微生物复合体对Pb2+的吸附主要受比表面积控制,而赤铁矿-微生物复合体的吸附主要受其表面有机官能团的影响. 经比表面积标准化可知,相较于蒙脱石组,赤铁矿-微生物复合体在单位面积具有更强的吸附能力.
(3)各个复合体相较微生物残体的解吸率(RR)更低,微生物残体通过静电作用对Pb2+的吸附并不牢固. 赤铁矿-微生物复合体较蒙脱石-微生物复合体的解吸率更低,这说明赤铁矿-微生物复合体因较强的络合作用对Pb2+的吸附较为牢固. 同时,真菌-矿物复合体通过更强的阳离子-π作用或络合作用较细菌组对Pb2+的吸附更高且更稳定.
微生物残体及其与矿物形成的复合体对Pb2+的吸附解吸
Adsorption and desorption of Pb2+ by microbial necromass and microbial- mineral complexes
-
摘要: 土壤有机质是控制土壤重金属吸附行为的重要组分之一,最近微生物源有机质被认为是土壤稳态碳的重要组分,然而,微生物源有机质及其与土壤矿物形成的复合体对重金属的吸附解吸行为关注较少. 以微生物(细菌、真菌)为有机质,黏土矿物(蒙脱石、赤铁矿)为矿物基质,制备细菌残体(BN)、真菌残体(FN)、细菌-蒙脱石复合体(B-M)、真菌-蒙脱石复合体(F-M)、细菌-赤铁矿复合体(B-Fe)、真菌-赤铁矿复合体(F-Fe),研究微生物残体及复合体对铅离子(Pb2+)的吸附解吸过程. 结果表明,真菌残体和细菌残体具有相近的元素组成和有机官能团,其对Pb2+的吸附解吸相一致;但相比于微生物-矿物复合体而言,纯残体对Pb2+的吸附量较大,解吸率也较大,吸附并不稳定. 各个复合体中,尽管赤铁矿-微生物复合体对Pb2+的吸附量较蒙脱石-微生物复合体更小,但经比表面积标准化后,蒙脱石-微生物复合体的吸附明显降低,而赤铁矿-微生物复合体的吸附量前后变化不明显且高于蒙脱石组,表明赤铁矿-微生物复合体在单位面积的吸附能力高于蒙脱石-微生物复合体. 真菌-矿物复合体较细菌-矿物复合体具有更高的吸附,经比表面积标准化后,真菌-矿物复合体仍具有更高的吸附. 同时,各个复合体对Pb2+的解吸率表现为B-M>B-Fe>F-M>F-Fe,表明赤铁矿-微生物复合体通过络合作用对Pb2+的吸附更稳定,真菌-矿物复合体通过更强的阳离子-π作用和络合作用对Pb2+的吸附更稳定. 因此,微生物与矿物形成的复合体对Pb2+的吸附能力在土壤重金属修复治理中需要被关注,这为控制土壤重金属的迁移和生物有效性提供了新的思路.Abstract: Soil organic matter (SOM) is one of the important components controlling the adsorption behavior of heavy metals in soil. However, as an important component of stabilized SOM, little attention has been paid to the adsorption/desorption behavior of heavy metals by microbial-derived organic matter and its complexes formed with soil minerals. In this study, the adsorption/desorption of Pb ions (Pb2+) by microbial (bacteria and fungi) and the complexes with clay mineral (montmorillonite and hematite) were studied, specifically, bacterial necromass (BN), fungal necromass (FN), bacteria-montmorillonite complex (B-M), fungus-montmorillonite complex (F-M), bacteria-hematite complex (B-Fe), and fungus-hematite complex (F-Fe). The results showed that the fungal nacromass and bacterial necromass had similar elemental composition and organic functional groups, and their adsorption and desorption for Pb2+ were consistent. However, compared with the microbial-mineral complex, the microbial necromass has a larger adsorption capacity for Pb2+, the desorption rate is also larger, and the adsorption is not stable. In each complex, although the adsorption amount of Pb2+ by hematite-microbial complex was smaller than that of montmorillonite-microbial complex, after the standardization of specific surface area, the adsorption of montmorillonite-microbial complex obviously decreased, while the adsorption of hematite-microbial complex did not change and was higher than that of montmorillonite group. The results showed that the adsorption capacity of hematite-microbial complex was higher than that of montmorillonite-microbial complex. The adsorption of fungus-mineral complex was higher than that of bacteria-mineral complex, and the adsorption of fungus-mineral complex was still higher after the standardization of specific surface area. Meanwhile, the desorption rate of Pb2+ for each complex was in order of B-M>B-Fe>F-M>F-Fe, indicating that the hematite-microbial complex was more stable for Pb2+ adsorption through complexation, and the fungus-mineral complex was more stable for Pb2+ adsorption through cation-π interaction and complexation compared to bacteria-mineral complex. Overall, our study demonstrated the adsorption capacity of different microbial-mineral complex to Pb2+, which will provide a new idea for controlling the migration and bioavailability of heavy metals in soil.
-
Key words:
- microbial necromss /
- clay minerals /
- adsorption and desorption /
- Pb2+.
-

-
表 1 微生物残体及复合体的元素分析
Table 1. Elemental analysis of microbial necromass and complexes
样品
Sample元素质量组成/%
Elemental mass component摩尔原子比
Molar atomic ratioC H O N S H/C O/C (N+O)/C BN 42.1 6.72 31.5 11.1 0.480 1.91 0.561 0.787 B-M 6.72 2.31 11.6 3.24 0.155 4.13 1.29 1.71 B-Fe 9.57 1.03 22.7 2.09 0.239 1.29 1.78 1.97 FN 45.3 6.16 33.6 10.6 0.460 1.63 0.556 0.757 F-M 10.7 1.74 14.6 2.19 0.162 1.96 1.03 1.20 F-Fe 15.6 2.33 24.3 3.92 0.299 1.79 1.17 1.38 注:BN,细菌残体bacterial necromass;FN真菌残体fungal necromass;B-M,细菌残体-蒙脱石bacteria-montmorillonite complex;F-M,真菌残体-蒙脱石fungus -montmorillonite complex;B-Fe,细菌残体-赤铁矿bacteria-hematite complex;F-Fe,真菌残体-赤铁矿fungus -hematite complex. 表 2 微生物残体及复合体的比表面积和孔隙结构
Table 2. Specific surface area and pore structure of microbial necromass and complexes
吸附剂
Adsorbent比表面积/(m2·g−1)
Specific surface area孔容积/(cm3·g−1)
Pore volume平均孔径/nm
Mean pore sizeM 100 0.20 7.76 Fe 5.56 0.03 18.0 BN 4.31 0.01 8.76 B-M 32.6 0.09 10.5 B-Fe 5.83 0.04 25.3 FN 5.32 0.02 11.7 F-M 20.9 0.12 22.6 F-Fe 4.94 0.05 39.4 表 3 微生物残体及复合体的吸附等温线拟合
Table 3. Adsorption isotherm fitting of microbial necromass and complexes
吸附质
Adsorbate吸附剂
AdsorbentLangmuir拟合
Langmuir modelFreundlich拟合
Freundlich modelKL Qm R2 KF n R2 Pb2+ BN 6.21 53.2 0.524 42.7 0.204 0.888 B-M 3.65 56.7 0.765 41.3 0.277 0.887 B-Fe 6.12 0.550 0.725 7.98 0.213 0.828 FN 2.02 63.9 0.902 42.2 0.317 0.935 F-M 7.35 72.2 0.912 83.2 0.306 0.923 Pb2+ F-Fe 7.78 46.5 0.874 42.6 0.216 0.938 M 1.19 10.6 0.445 4.85 0.626 0.957 Fe 1.09 0.823 0.413 0.487 0.210 0.436 -
[1] 鲍雪蓉. 铅蓄电池企业绿化带土壤铅污染特征研究[J]. 资源节约与环保, 2022(7): 116-119. BAO X R. Study on lead pollution characteristics of soil in green belt of lead storage battery enterprises[J]. Resources Economization & Environmental Protection, 2022(7): 116-119 (in Chinese).
[2] ZOU Y D, WANG X X, KHAN A, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review[J]. Environmental Science & Technology, 2016, 50(14): 7290-7304. [3] WAN D, ZHANG N C, CHEN W L, et al. Organic matter facilitates the binding of Pb to iron oxides in a subtropical contaminated soil[J]. Environmental Science and Pollution Research, 2018, 25(32): 32130-32139. [4] ZHAO J, HUANG B W, GAO W, et al. Periodic DFT study on heavy metals Cu(II) and Pb(II) atoms adsorption on Na-montmorillonite (010) edge surface[J]. Solid State Communications, 2023, 366/367: 115171. [5] ZHU X F, JACKSON R D, DeLUCIA E H, et al. The soil microbial carbon pump: From conceptual insights to empirical assessments[J]. Global Change Biology, 2020, 26(11): 6032-6039. doi: 10.1111/gcb.15319 [6] QU C C, CHEN W L, HU X P, et al. Heavy metal behaviour at mineral-organo interfaces: Mechanisms, modelling and influence factors[J]. Environment International, 2019, 131: 104995. [7] BAO Y P, BOLAN N S, LAI J H, et al. Interactions between organic matter and Fe (hydr)oxides and their influences on immobilization and remobilization of metal(loid)s: A review[J]. Critical Reviews in Environmental Science and Technology, 2022, 52(22): 4016-4037. doi: 10.1080/10643389.2021.1974766 [8] 刘洵, 赖潘民旺, 张敏, 等. 微生物-矿物相互作用: 机制与重金属固定效应[J]. 环境化学, 2024, 43(2): 377-392. doi: 10.7524/j.issn.0254-6108.2022080205 LIU X, LAIPAN M W, ZHANG M, et al. Microbe-mineral interactions: Mechanisms and immobilization effect toward heavy metals[J]. Environmental Chemistry, 2024, 43(2): 377-392(in Chinese). doi: 10.7524/j.issn.0254-6108.2022080205
[9] UDDIN M K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade[J]. Chemical Engineering Journal, 2017, 308: 438-462. [10] DIMIRKOU A, IOANNOU A, DOULA M. Preparation, characterization and sorption properties for phosphates of hematite, bentonite and bentonite-hematite systems[J]. Advances in Colloid and Interface Science, 2002, 97(1/2/3): 37-61. [11] MANJAIAH K M, KUMAR S, SACHDEV M S, et al. Study of clay-organic complexes[J]. Current Science, 2010, 98(7): 915-921. [12] VEGA F A, COVELO E F, ANDRADE M L. A versatile parameter for comparing the capacities of soils for sorption and retention of heavy metals dumped individually or together: Results for cadmium, copper and lead in twenty soil horizons[J]. Journal of Colloid and Interface Science, 2008, 327(2): 275-286. doi: 10.1016/j.jcis.2008.08.027 [13] CERQUEIRA B, COVELO E F, ANDRADE L, et al. The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd[J]. Geoderma, 2011, 162(1/2): 20-26. [14] 刘金香, 葛玉杰, 谢水波, 等. 改性微生物吸附剂在重金属废水处理中的应用进展[J]. 微生物学通报, 2020, 47(3): 941-951. LIU J X, GE Y J, XIE S B, et al. Application progress of modified microbial adsorbents for the treatment of heavymetal wastewater[J]. Microbiology China, 2020, 47(3): 941-951 (in Chinese).
[15] WEI J E, ZHANG F F, MA D L, et al. Microbial necromass carbon in estuarine tidal wetlands of China: Influencing factors and environmental implication[J]. Science of the Total Environment, 2023, 876: 162566. [16] BITTAR F, GOURIET F, KHELAIFIA S, et al. FastFung: A novel medium for the culture and isolation of fastidious fungal species from clinical samples[J]. Journal of Microbiological Methods, 2021, 180: 106108. doi: 10.1016/j.mimet.2020.106108 [17] ELZINGA E J, HUANG J H, CHOROVER J, et al. ATR-FTIR spectroscopy study of the influence of pH and contact time on the adhesion of Shewanella putrefaciens bacterial cells to the surface of hematite[J]. Environmental Science & Technology, 2012, 46(23): 12848-12855. [18] LV J T, MIAO Y X, HUANG Z Q, et al. Facet-mediated adsorption and molecular fractionation of humic substances on hematite surfaces[J]. Environmental Science & Technology, 2018, 52(20): 11660-11669. [19] ADHIKARI D, ZHAO Q, DAS K, et al. Dynamics of ferrihydrite-bound organic carbon during microbial Fe reduction[J]. Geochimica et Cosmochimica Acta, 2017, 212: 221-233. doi: 10.1016/j.gca.2017.06.017 [20] CECILIA J A, GARCÍA-SANCHO C, FRANCO F. Montmorillonite based porous clay heterostructures: Influence of Zr in the structure and acidic properties[J]. Microporous and Mesoporous Materials, 2013, 176: 95-102. doi: 10.1016/j.micromeso.2013.03.037 [21] SCHMALENBERGER A, DURAN A L, BRAY A W, et al. Oxalate secretion by ectomycorrhizal Paxillus involutus is mineral-specific and controls calcium weathering from minerals[J]. Scientific Reports, 2015, 5: 12187. doi: 10.1038/srep12187 [22] 常汉达, 王晶, 张凤华. 基于傅里叶红外光谱弃耕地开垦前后土壤有机质结构变化分析[J]. 土壤通报, 2019, 50(2): 333-340. CHANG H D, WANG J, ZHANG F H. Change in soil organic matter structure before and after reclamation for the abandoned farmland based on Fourier transform infrared spectrometer[J]. Chinese Journal of Soil Science, 2019, 50(2): 333-340 (in Chinese).
[23] YE Q Q, LI Q H, LI X. Removal of heavy metals from wastewater using biochars: Adsorption and mechanisms[J]. Environmental Pollutants and Bioavailability, 2022, 34(1): 385-394. doi: 10.1080/26395940.2022.2120542 [24] 郭微, 戴九兰, 王仁卿. 溶解性有机质影响土壤吸附重金属的研究进展[J]. 土壤通报, 2012, 43(3): 761-768. GUO W, DAI J L, WANG R Q. Progress in the effect of dissolved organic matter on adsorption of heavy metals by soil[J]. Chinese Journal of Soil Science, 2012, 43(3): 761-768 (in Chinese).
[25] 姜晶, 邓精灵, 盛光遥. 生物炭老化及其对重金属吸附影响研究进展[J]. 生态环境学报, 2022, 31(10): 2089-2100. JIANG J, DENG J L, SHENG G Y. A review of biochar aging and its impact on the adsorption of heavy metals[J]. Ecology and Environmental Sciences, 2022, 31(10): 2089-2100 (in Chinese).
[26] LU Z F, WANG H M, LI J Y, et al. Adsorption characteristics of bio-adsorbent on chromium(III) in industrial wastewater[J]. Water Science and Technology, 2015, 72(7): 1051-1061. [27] ZHU T T, ZHOU C H, KABWE F B, et al. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites[J]. Applied Clay Science, 2019, 169: 48-66. doi: 10.1016/j.clay.2018.12.006 [28] ZHAO Q J, QIU Y, LAN T, et al. Comparison of lead adsorption characteristics onto soil-derived particulate organic matter versus humic acid[J]. Journal of Soils and Sediments, 2021, 21(7): 2589-2603. doi: 10.1007/s11368-021-02911-4 [29] LU Y, LIANG Y Z, LIU F, et al. Nano scale visualization of enhanced adsorption and distribution of humic acid on hematite: Effect of Pb(II) ions[J]. Chemical Geology, 2020, 541: 119573. doi: 10.1016/j.chemgeo.2020.119573 [30] KRUMINA L, OP de BEECK M, MEKLESH V, et al. Ectomycorrhizal fungal transformation of dissolved organic matter: Consequences for reductive iron oxide dissolution and fenton-based oxidation of mineral-associated organic matter[J]. Frontiers in Earth Science, 2022, 10: 763695. doi: 10.3389/feart.2022.763695 [31] SHAH F, NICOLÁS C, BENTZER J, et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors[J]. The New Phytologist, 2016, 209(4): 1705-1719. [32] LANG M F, YU X Q, LIU J H, et al. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics[J]. Science of the Total Environment, 2020, 722: 137762. doi: 10.1016/j.scitotenv.2020.137762 [33] PORSCH K, DIPPON U, RIJAL M L, et al. In-situ magnetic susceptibility measurements As a tool to follow geomicrobiological transformation of Fe minerals[J]. Environmental Science & Technology, 2010, 44(10): 3846-3852. [34] HOHMANN C, WINKLER E, MORIN G, et al. Anaerobic Fe(II)-oxidizing bacteria show As resistance and immobilize As during Fe(III) mineral precipitation[J]. Environmental Science & Technology, 2010, 44(1): 94-101. [35] JOHNSON S B, YOON T H, BROWN G E. Adsorption of organic matter at mineral/water interfaces: 5. effects of adsorbed natural organic matter analogues on mineral dissolution[J]. Langmuir, 2005, 21(7): 2811-2821. doi: 10.1021/la0481041 [36] OMOIKE A, CHOROVER J, KWON K D, et al. Adhesion of bacterial exopolymers to α-FeOOH: inner-sphere complexation of phosphodiester groups[J]. Langmuir, 2004, 20(25): 11108-11114. doi: 10.1021/la048597+ [37] JOHNSON S B, BROWN G E, HEALY T W, et al. Adsorption of organic matter at mineral/water interfaces. 6. effect of inner-sphere versus outer-sphere adsorption on colloidal stability[J]. Langmuir, 2005, 21(14): 6356-6365. doi: 10.1021/la047030q [38] HINSINGER P, PLASSARD C, TANG C X, et al. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review[J]. Plant and Soil, 2003, 248(1): 43-59. [39] YING-CHIEN C, SU Y P, CHIING-CHANG C, et al. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall[J]. Acta Pharmacologica Sinica, 2004, 25(7): 932-936. [40] RAM R, MORRISROE L, ETSCHMANN B, et al. Lead (Pb) sorption and co-precipitation on natural sulfide, sulfate and oxide minerals under environmental conditions[J]. Minerals Engineering, 2021, 163: 106801. doi: 10.1016/j.mineng.2021.106801 [41] BRADL H B. Adsorption of heavy metal ions on soils and soils constituents[J]. Journal of Colloid and Interface Science, 2004, 277(1): 1-18. doi: 10.1016/j.jcis.2004.04.005 [42] MASON S E, ICEMAN C R, TANWAR K S, et al. Pb(II) adsorption on isostructural hydrated alumina and hematite (0001) surfaces: A DFT study[J]. The Journal of Physical Chemistry C, 2009, 113(6): 2159-2170. -



 下载:
下载: