-
双氯芬酸钠(diclofenac sodium,DCF)是非甾体抗炎药,其在人体内不能完全代谢,常随排泄物一同进入下水管道,由于具有极性强、难降解的特点,导致其在污水处理厂、土壤、地表水、地下水甚至饮用水中频频检出[1-5]。已有相关研究表明DCF对自然界中的动植物的生存与繁殖产生了一定的不良影响,是一种新型酸性阴离子污染物。JOACHIM等[6]发现,在半自然条件下,暴露在含DCF水体中的植物生物量和鱼类存活量产生显著下降。因此,水中DCF的去除已成为亟待处理的环境问题之一。
目前,膜过滤、高级氧化、生物降解[7]等技术均可以有效去除水中的DCF,但这类方法大多成本高,而且高级氧化法会产生有毒副产物,生物降解耗时较长。吸附法作为一种操作简单、低成本、环境友好型的方法备受重视。吸附剂的经济环保高效性能是影响吸附法广泛应用的关键因素,以生物炭为代表的低价环保高效吸附剂的制备成为吸附法的研究热点。我国是一个农业大国,稻壳作为一种廉价农业废弃物是制备生物炭的优质原料,但原始生物炭往往不能满足对目标污染物的高效去除,常常需要对其改性以提高吸附能力[8]。近年来金属改性生物炭去除水中有机污染物成为研究热点,原始稻壳炭表面带负电,由于静电斥力作用,对作为阴离子的DCF并不能有效吸附,经过Fe、Cu等金属元素改性后,生物炭表面负电荷降低,从而通过配体交换引起表面络合促进对阴离子污染物的吸附性能。另外,改性炭与有机污染物之间的n-π、π-π、氢键、疏水等相互作用也在吸附中起到了重要作用[9-11]。有研究表明,经铜改性后的花椰菜根生物炭显示出比为改性生物炭更高的比表面积,对DCF的去除率可达88.96%[12];铁锰改性炭对DCF的吸附率是商业活性炭的2倍多且具有较优的pH抗干扰能力[13]。此外,一些研究[14]表明,铁氧化物和铜氧化物的水解产物具有与—OH、C=O、—COOH螯合、络合的能力,这对含有羧基的DCF的吸附是有利的。同时,有研究表明,铜的存在可以促进电子转移,通过产生的高还原性氢自由基去除水中的有机物[15-16]。JUAN等[17]在研究Fe/Cu改性城市活性污泥基复合材料对四环素的吸附时发现铜优异的络合能力可以显著影响去除率,在最佳条件下对四环素的最大吸附量可达386.93 mg·g−1,吸附机制主要包括物理吸附、络合作用、氢键、π-π相互作用等。目前使用铁铜改性炭去除水中DCF的研究鲜见报道。因此,本研究采用铜和铁制备改性稻壳炭以研究对水中DCF的去除效果与机理。在吸附剂实际应用中,还需要考虑水质对吸附性能的影响。DCF为酸性阴离子污染物,污水的pH、共存阴离子及有机物均可能对生物炭对DCF的吸附性能造成影响。
本研究以稻壳为原料,通过Fe/Cu盐对其进行改性得到铁铜改性的生物炭,以DCF为目标污染物,考察溶液pH、吸附剂投加量、干扰阴离子及有机物HA对吸附过程的影响,利用吸附动力学模型和吸附等温线模型对实验数据进行拟合,探讨吸附机制,在促进农业废物资源化利用的同时为新型污染物DCF的去除提供基础依据。
-
稻壳来自农田水稻壳,其成分主要为纤维素、半纤维素、木质素和二氧化硅(SiO2)纳米粒子等[18]。将废弃的稻壳用去离子水冲洗干净并于85 ℃的烘箱中干燥,用研磨机研磨成粉末,过筛(60目)备用。实验试剂主要包括氢氧化钠(NaOH)、浓盐酸(HCl)、三氯化铁(FeCl3)、硫酸铜(CuSO4·5H2O)、双氯芬酸钠(DCF)、硫酸钠(Na2SO4)、磷酸钠(Na3PO4·12H2O)、氯化钠(NaCl)、碳酸氢钠(NaHCO3)、腐殖酸(humic acid,HA),以上药品均为分析纯及以上等级。
-
在25 ℃、180 r·min−1的吸附条件下,基于300、400、500、600 ℃下制备的改性稻壳炭分别对0~100 mg·L−1的DCF吸附预实验结果可知,在300 ℃下制备的改性稻壳炭吸附效果最好,故本研究主要讨论300 ℃稻壳炭的吸附特性。原始稻壳炭(BC)的制备是通过将上述过筛的稻壳炭粉末置于管式炉中,在N2气氛下以10 ℃·min−1的升温速率升至300 ℃并保持2 h后取出用超纯水洗涤3~5次,烘箱75 ℃干燥12 h制得,记为BC300。铁铜改性的生物炭的制备过程是在先前研究[19-21]的基础上有所改进,具体做法是将5 g过60目筛后的BC300加至等体积混合的FeCl3(0.8 mol·L−1)和CuSO4·5H2O(0.3 mol·L−1)总计50 mL的混合溶液中,用0.5 mol·L−1的HCl和1.0 mol·L−1NaOH调节pH=5后于85 ℃、180 r·min−1的恒温振荡箱中振荡24 h,然后在75 ℃下干燥12 h。将获得的样品在N2气氛下以10 ℃·min−1的升温速率升至300 ℃并保持2 h。随后,用超纯水洗涤3~5次,然后在75 ℃下干燥12 h,将制备的产物命名为FCBC300。
-
采用元素分析仪(EA)测定生物炭的元素组成(艾力蒙塔 UNICUBE);采用ICP-OES/MS测定生物炭的金属元素组成(美国 Agilent 720ES OES);通过场发射扫描电子显微镜(SEM)分析生物炭表面形貌特征(蔡司Sigma 300,Oxford Xplore 50);通过傅里叶变换红外光谱仪(FTIR)对其表面官能团进行表征(赛默飞 Thermo Scientific Nicolet iS20);通过X-射线衍射光谱(XRD)测定其组成成分(日本理学 rigaku Ultima IV);通过pH漂移法[22]测定生物炭的零电位点(梅特勒-托利多 SevenCompact S220)。
-
吸附实验在恒温振荡箱中进行24 h,温度保持在25 ℃,转速为180 r·min−1,实验结束吸取上清液经0.45 μm滤膜过滤后,分析滤液中DCF质量浓度。DCF质量浓度利用紫外分光光度法(λ=273 nm)进行测定[23]。影响因素实验、吸附动力学及吸附等温线实验具体设置如下。
1)溶液初始pH对吸附的影响。分别取0.05 g的生物炭到100 mL离心管中,加入50 mL初始质量浓度为10 mg·L−1的DCF溶液,利用0.1~2 mol·L−1的HCl和NaOH调节初始pH分别为3、5、7、9、11,每组设置3个平行样、1个空白。
2)投加量对吸附的影响。分别取0.01、0.02、0.03、0.04、0.05、0.06、0.07g的生物炭到100mL离心管中,加入50 mL质量浓度为10 mg·L−1的初始DCF溶液,调节初始pH为7,每组设置3个平行样、1个空白。
3)共存阴离子及有机物HA对吸附的影响。分别制备含有10 mg·L−1的DCF和5 mmol·L−1的阴离子(SO42−、PO43−、Cl−、HCO3−)混合溶液,10 mg·L−1的DCF和10 mg·L−1HA的混合溶液,调节pH为7,取50 mL于离心管中,分别加入0.05 g的FCBC300进行吸附实验,每组设置3个平行样、1个空白。
4)吸附动力学实验。制备质量浓度为10 mg·L−1的DCF溶液,调节pH=7,取50 mL于离心管中,分别加入0.05 g的FCBC300,分别在振荡一定时间后快速取样,经0.45 μm滤膜过滤后测定清液中DCF质量浓度。
5)吸附等温线实验。制备50~100 mg·L−1的DCF溶液,取50 mL于离心管中,分别加入0.05 g的FCBC300,控制反应温度为25 ℃、pH=7、于180 r·min−1恒温振荡箱中振荡24 h,经0.45 μm滤膜过滤后测定。
-
1)稻壳炭对DCF的吸附量和去除率根据式(1)和式(2)计算。
式中:
$ {Q}_{\mathrm{e}} $ 是吸附平衡时的吸附量,mg·g−1;$ {C}_{\mathrm{e}} $ 是吸附平衡时DCF的质量浓度,mg·L−1;$ {C}_{\mathrm{o}} $ 是初始DCF的质量浓度,mg·L−1;V是DCF溶液的体积,L;m是吸附剂的添加量,g;R为去除率,%。2)吸附动力学模型。为研究FCBC对DCF的吸附机理,分别利用准一级(式(3))、准二级模型(式(4))对其进行拟合。
式中:
$ {Q}_{\mathrm{e}} $ 是吸附平衡时的吸附量,mg·g−1;$ {Q}_{t} $ 是t时刻对DCF的吸附量,mg·g−1;t是吸附时间,min;$ {K}_{1} $ 、$ {K}_{2} $ 分别是是一级、二级反应速率常数,min−1、g·(mg·min)−1;3)吸附等温线模型。为研究FCBC对DCF的吸附特性,分别利用Langmuir(式(5))和Freundlich等温吸附模型(式(6))对其进行拟合。
式中:
$ {Q}_{\mathrm{e}} $ 是吸附平衡时的吸附量,mg·g−1;$ {Q}_{\mathrm{m}} $ 是理论最大吸附量,mg·g−1;$ {C}_{\mathrm{e}} $ 是吸附平衡时DCF的质量浓度,mg·L−1;$ {K}_{\mathrm{l}} $ 是Langmuir等温吸附常数,L·mg−1;$ {K}_{\mathrm{f}} $ 是Freundlich等温吸附常数,mg(1-1/n)·L1/n·g−1;n是Freundlich模型常数。 -
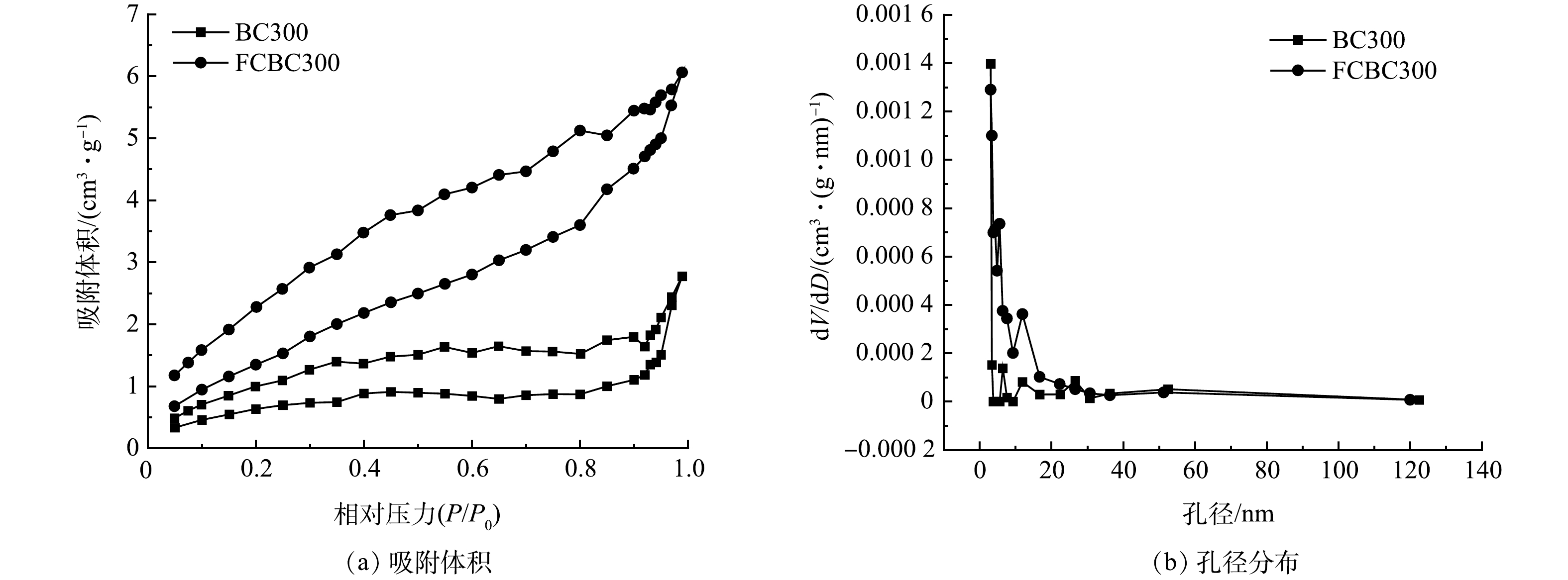
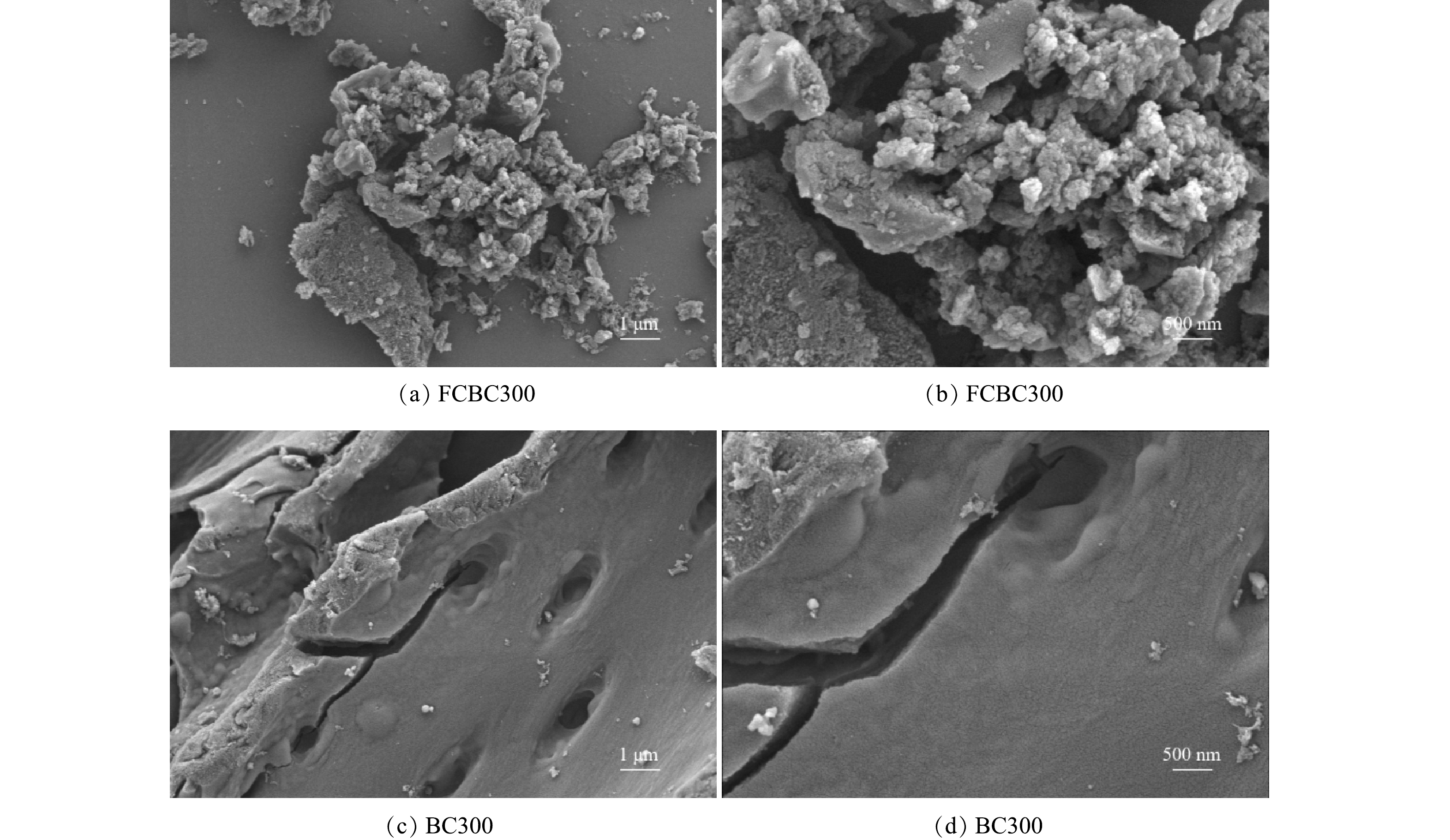
1) SEM、BET分析。经扫描电子显微镜比较改性前后稻壳炭的结构变化,结果如图1所示,BC300表面孔隙较少且呈现为光滑的管束结构。而FCBC300表面出现了大量近似球形的颗粒物质,并以链状聚集在一起,这种团聚效应可能归因于纳米颗粒之间的磁力和范德华力,表面变得凹凸粗糙且排列杂序无章,这与Fe-Cu/聚乙烯吡咯烷酮改性的生物炭SEM表征结果一致[24-25]。以上结果表明金属氧化物颗粒可能成功负载到了稻壳炭的表面上,具体负载情况需通过XRD进行进一步地分析印证。由图2可以看出,2种生物炭的孔径大多分布在0~40 nm,氮气吸脱附等温线均为具有滞后环的Ⅳ型等温线,表明吸附剂含有大量介孔,且孔径分布不均匀[26]。由表1可知,改性前后生物炭的平均孔径略微下降,但比表面积由2.717 m2·g−1增加至6.139 m2·g−1,说明改性增加了生物炭的比表面积,在静电引力下这可能有利于与更多的DCF接触,同时改性生物炭表面负载的铁与铜可能通过与阴离子DCF的络合作用而加快反应速率[27]。
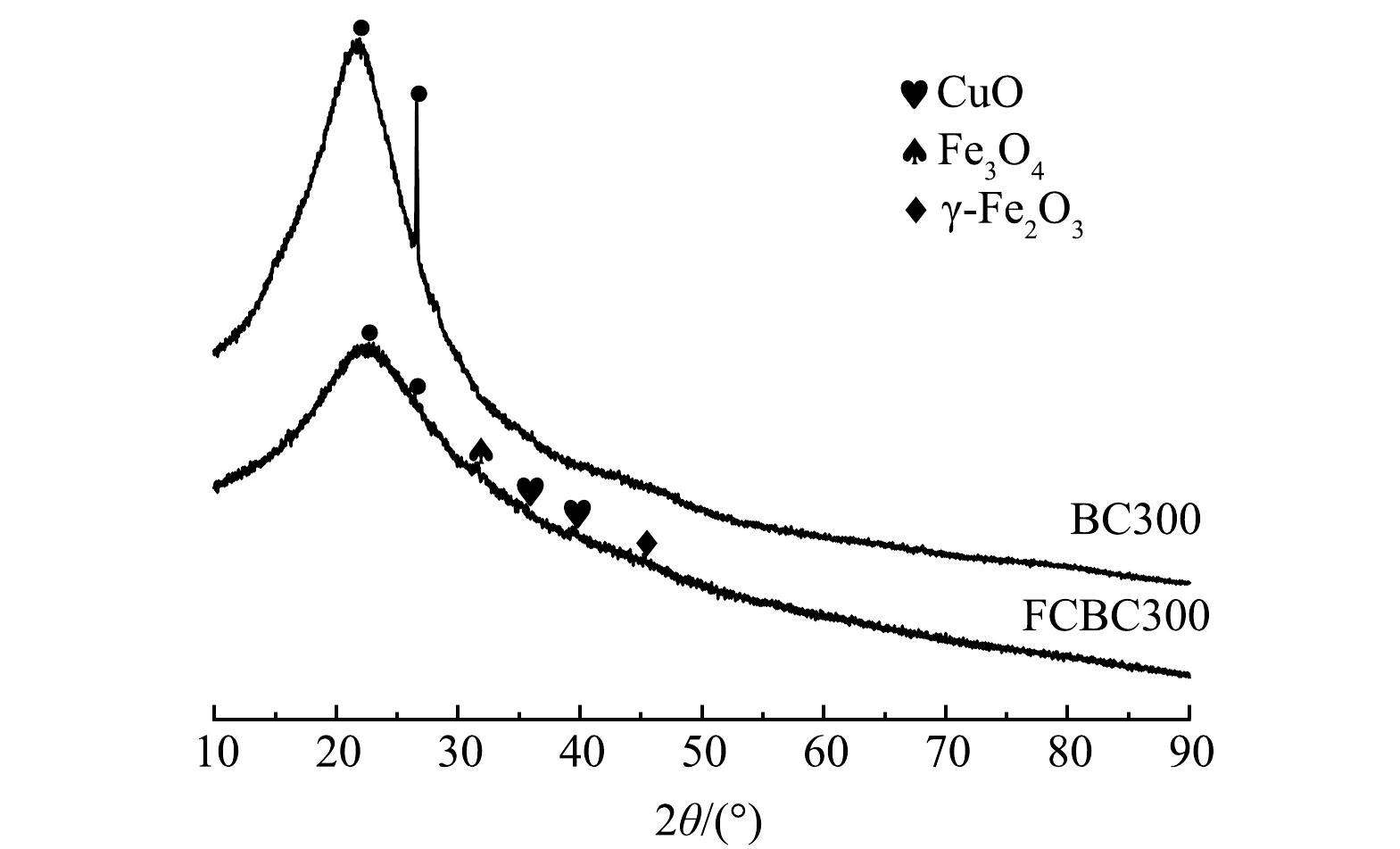
2) XRD分析。XRD常用来定性表征物体的结晶度。FCBC300和BC300的XRD图谱如图3所示,在2θ=20°~24°处均存在一个较宽的衍射峰,说明该结构具有长程有序性,这个重叠的衍射峰主要为无定型碳和SiO2晶面。另外,由图3中可以看出,原始稻壳炭(BC300)在2θ=26.68°处有一个尖锐的衍射峰,该峰代表SiO2的存在[28]。改性后的炭在2θ=20°~24°的衍射峰变宽大且平缓、2θ=26.68°的衍射峰明显减弱,可能一方面是由于SiO2与氢氧化钠反应使得SiO2的含量减少,另一方面是由于改性稻壳炭乱层石墨结构微晶增多[29]。另外,观察发现FCBC300在2θ=31.41°、45.23°出现了新的衍射峰,分别对应Fe3O4、γ-Fe2O3[30-31],在2θ=35.77°、39.40°出现的衍射峰对应CuO[32-33]。这进一步表明金属氧化物颗粒成功负载到了稻壳炭的表面上。但这些衍射峰并未发生明显变化,表明了无定形Fe/Cu的形成,这可能是主要活性吸附组分。
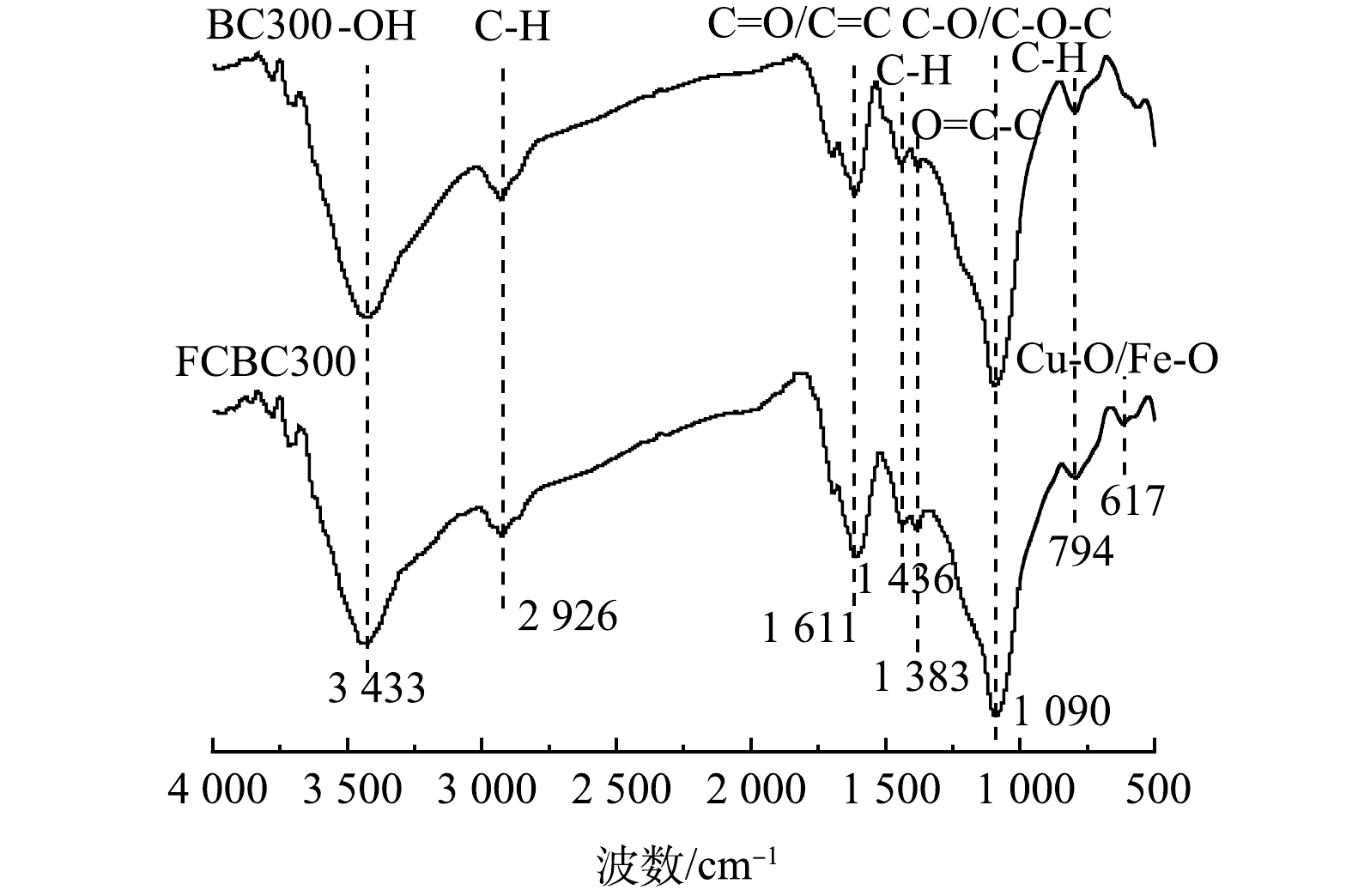
3) FTIR分析。不同稻壳炭的FTIR分析如图4所示。可见,BC300和FCBC300吸收峰的位置大致相同。在3 433 cm−1附近出现的衍射峰证明了—OH的存在,2 926 cm−1附近的衍射峰为脂肪族化合物C—H的对称伸缩振动和非对称伸缩振动。在1 611 cm−1附近出现了芳环C=O/C=C的伸缩振动;在1 436 cm−1附近出现了C—H面内弯曲振动峰;在794 cm−1附近出现的吸收峰,则是由芳环C—H面外弯曲振动峰引起的。1 090 cm−1附近可能为醇的C—O伸缩振动或者脂肪性C—O—C的伸缩振动;在1 383 cm−1附近出现的吸收峰可能是—COOH的O=C—C拉伸引起的,且此处改性稻壳炭的吸收峰峰值略微增强,说明含氧官能团数量有所增加;改性稻壳炭在617 cm−1处出现了一个新的吸收峰为Cu—O/Fe—O的振动吸收峰[34],这与XRD衍射光谱相对应。改性后不一定会引起稻壳炭表面官能团的显著变化,主要含氧官能团均为—OH、C=O、—COOH,这一研究结果与前人一致,任洁青等[35]通过NaOH和FeCl3溶液制备的改性稻壳炭,在改性前后仅峰面积与吸收强度发生了变化,但吸收峰的位置基本一致。
4)元素组成。生物炭的组成元素主要是C、H、O、N 4种元素。H/C的摩尔比反映了生物炭的芳香性,H/C与芳香性负相关,芳香性越高,物质的稳定性越好,越不易分解;O/C的摩尔比反映了生物炭的亲水性,O/C与亲水性正相关;(O+N)/C的摩尔比反映了生物炭的极性,(O+N)/C与极性正相关。由表2可知,BC300主要包含C、H和O,FCBC300主要包含C、H、O、Fe和Cu,表明Fe、Cu的成功负载。改性后,C、H元素的含量分别从48.48%、3.71%升至56.18%、3.75%,其中H的升幅较小;O、N元素的含量分别从21.29%、0.55%降至18.45%、0.37%,其中N降幅最大,这可能与热解过程中稻壳中的含N物质转化为NOx、NH3、HCN、HNCO等气体逸出和分配至焦油中有关[36]。因而,H/C、O/C、(O+N)/C的值均变小,分别下降了0.12、1.33、0.09,这表明改性后的稻壳热解过程中发生了脱氢聚合、脱羧和脱水反应[37],即改性后的生物炭芳香性增强,物质稳定性好,而含氧官能团含量、极性和亲水性降低,说明FCBC300对DCF的去除可能与改性稻壳炭表面官能团与DCF之间的π-π相互作用有关[9]。
-
考虑到天然水体的pH,本研究主要探索pH在3~11范围内对DCF吸附的影响。由图5(b)可知,原始稻壳炭BC300在pH=3时的去除率较高,在pH=5~11内吸附性极弱,几乎没有吸附。这能是因为强酸性条件下原始稻壳炭表面带正电荷,DCF通过静电引力吸附到原始稻壳炭表面。而pH≥5时,原始稻壳炭表面带负电荷,DCF主要以阴离子形式存在,强大的静电斥力可能会阻碍DCF的吸附[38]。经改性后的稻壳炭FCBC300吸附性有所提升,在pH=3时去除效果最好,随着pH升高去除率降低,当pH=11时,去除率下降至24.83%,吸附量下降至3.10 mg·g−1,这表明吸附剂对DCF的吸附是一个依赖pH的过程。另外,在pH=3~9内吸附量保持在10.76~11.08 mg·g−1,表明其具有较宽的pH适用范围。研究富含铁的微生物制备生物炭对DCF的吸附时也有类似发现[39-41],认为铁改性生物炭的pH耐受性是因为除了静电作用外,氢键作用、π-π相互作用等可能也发挥着重要作用。这在实际污水处理时具有更好降低成本的优势。而在pH=11时去除率显著降低除了受OH−与带负电的DCF竞争吸附位点外,还与FCBC300的表面零电荷点有关(pHpzc=3.74)。如图5(a)所示,当pH>pHpzc时,FCBC300表面带负电,而DCF的pKa=4.10[42],在碱性条件下DCF阴离子占主导地位,所以随着pH升高静电斥力逐渐增大,从而减弱了DCF的吸附。值得注意的是,在pH=11时,FCBC300对DCF的吸附能力显著降低,但FCBC300的吸附容量仍远高于BC300,约是其8倍,说明此时络合反应在其中也起着重要贡献,类似于针铁矿对DCF的吸附,在pH>pHpzc时的吸附是由于针铁矿与DCF表面羧基的络合[43]。
-
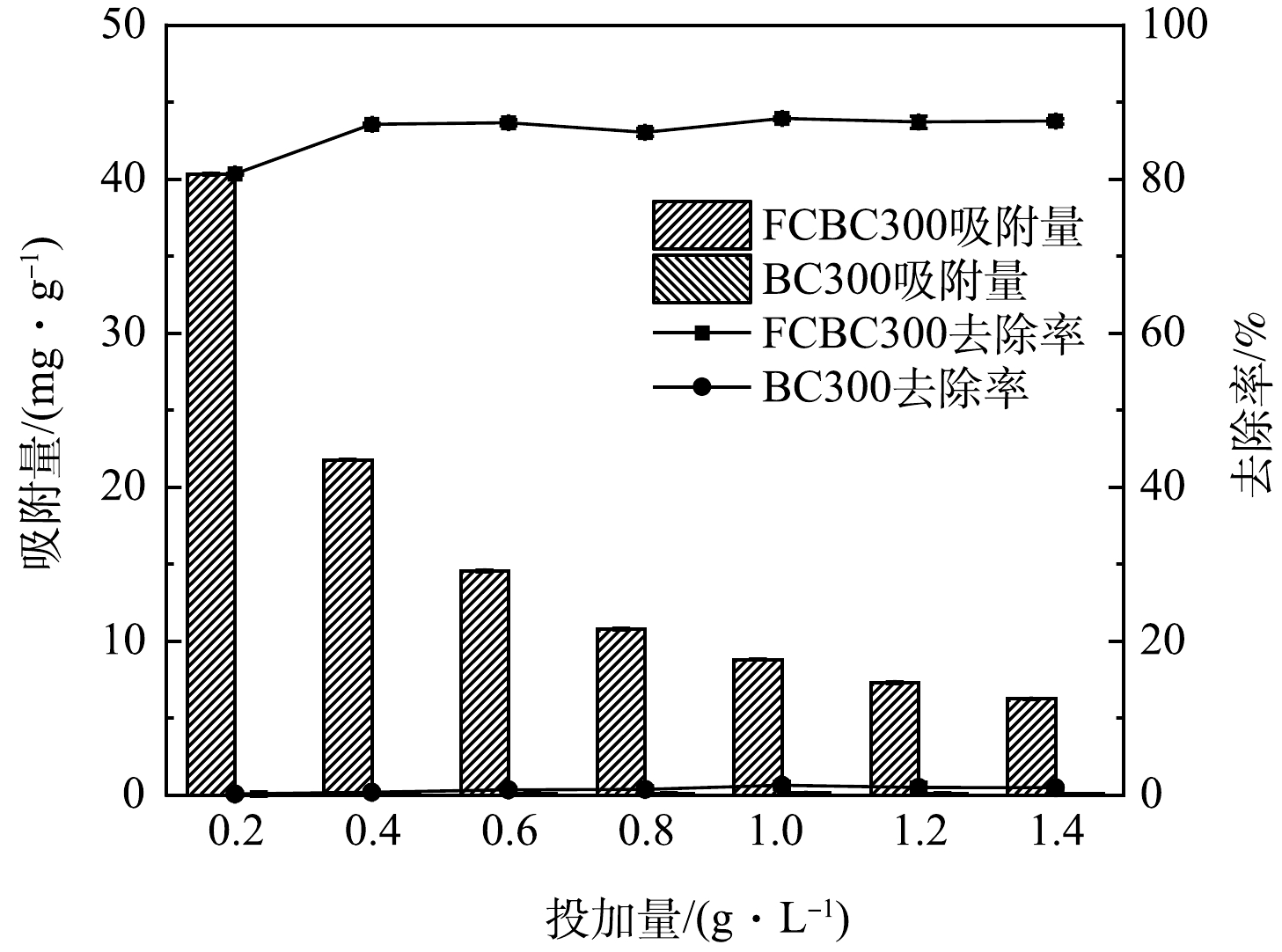
如图6所示,BC300在中性条件下的吸附效果甚微,而FCBC300的吸附效果明显提升。随着投加量的增加,FCBC300对DCF的单位吸附量逐渐下降,去除率上升后逐渐趋向平稳。投加量为0.2 g·L−1时去除率为80.66%,当投加量增加至1 g·L−1时去除率达87.87%,随后逐渐趋于平稳。在吸附的过程中,刚开始去除率随着投加量的增加而增加,是因为一开始FCBC300的活性位点与投加量成正相关,投加量越多对应的活性位点数越多,因而去除率快速增长[44];而DCF与FCBC300的质量比逐渐降低,导致单位质量生物炭对DCF的利用率降低,从而吸附量呈现下降趋势,这可能与改性稻壳炭在较高质量浓度下发生聚集降低表面活性位点有关[45]。当达到一定程度时,去除率趋于平缓,这可能是因为大量FCBC300的加入改变了吸附剂/液体悬浮液的黏度,阻止了DCF在FCBC300表面的扩散[46]。
-
生物炭在实际应用中往往会受到水体中阴离子和有机物的影响,不同阴离子(SO42−、PO43−、Cl−、HCO3−)及有机物HA对改性稻壳炭吸附过程中的影响如图7所示。FCBC300的抗干扰能力较好,在SO42−、PO43−、Cl−和HCO3−共存的溶液中,DCF的去除率最低,仍可保持在约83.76%,其中PO43−的存在不影响改性稻壳炭对DCF的吸附作用,可能是由于在pH=7的条件下,改性稻壳炭表面带负电,而PO43−所带的负电荷最多,与改性稻壳炭的静电斥力最大,因而不影响改性稻壳炭对DCF的吸附;SO42−、Cl−、HCO3−对DCF的吸附存在抑制作用,吸附量从8.80 mg·g−1分别下降至8.54、8.46、8.38 mg·g−1,这可能与该3种离子所带的负电荷相对较少,静电斥力减弱有关。此外,共存阴离子会降低DCF的溶解度,从而降低了边界层的传质推力[47]。此外,在不同阴离子存在下,FCBC300对DCF的去除率下降均不足5%,说明其具有较好的抗阴离子干扰性。HA对DCF的吸附存在明显抑制作用,吸附量下降至0.61 mg·g−1,这是因为相比DCF的羧基,HA丰富的羧基、羟基、酚基等官能团可以通过氢键、络合作用、离子交换等方式与吸附剂及吸附剂表面的金属离子发生作用,从而与DCF竞争FCBC300表面的吸附位点。在研究HA对二甲基胂酸在磁铁矿上吸附过程的影响时也有同样的结果,HA与磁铁矿吸附剂之间的氢键、阳离子键桥、表面配位交换等作用抑制了磁铁矿对二甲基胂酸的去除[48-49]。
-
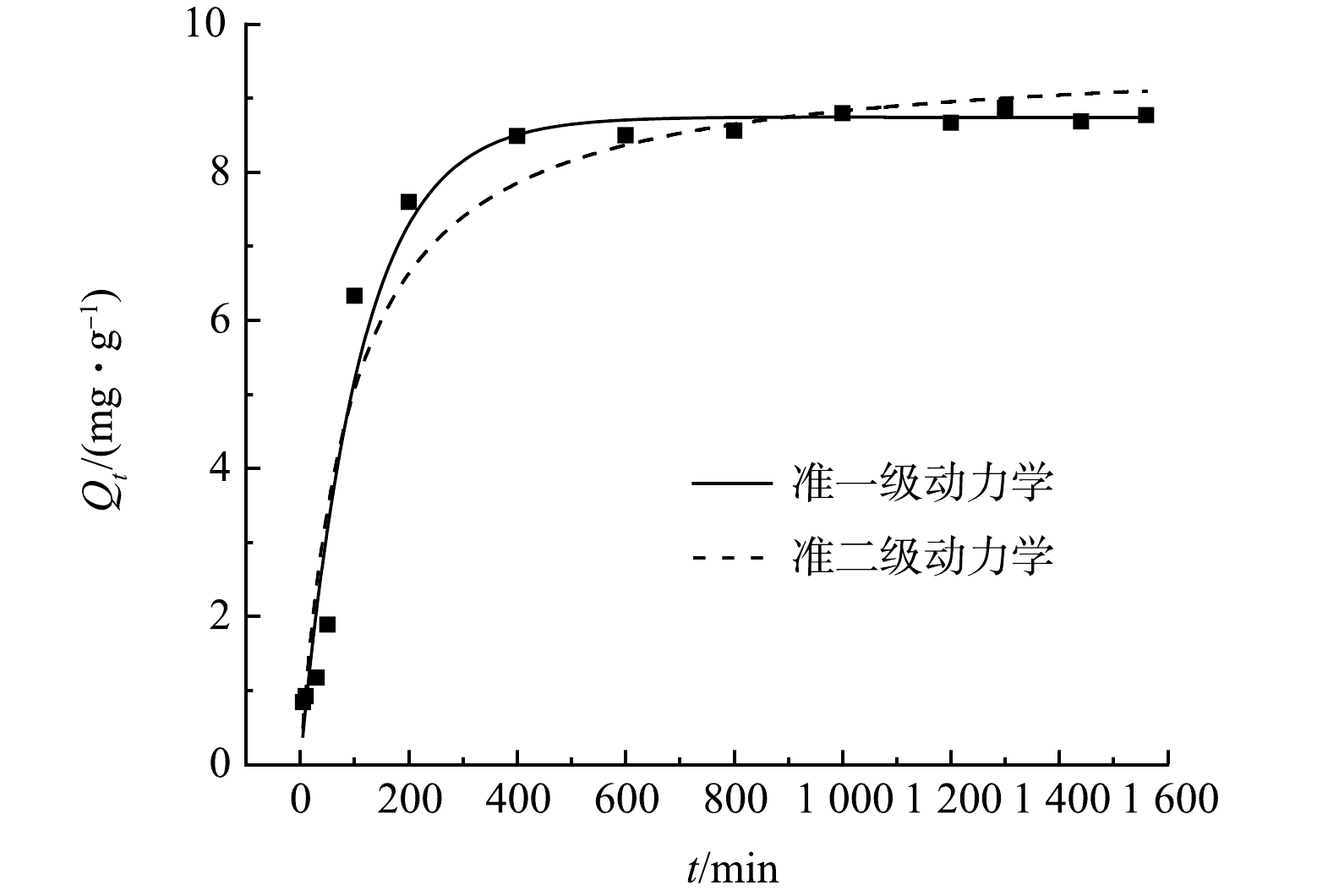
为更好地了解FCBC300对DCF的吸附行为,本实验采用准一级动力学模型、准二级动力学模型对其吸附过程进行模拟分析,结果见图8和表3所示。由表3可见,准一级动力学和准二级动力学相关系数拟合度均较好(R2>0.95),但准一级动力学模型的拟合度更优(R2=0.972 4),且计算所得的理论吸附量(8.744 1 mg·g−1)与实际吸附量(8.79 mg·g−1)更加接近,说明FCBC300对DCF的吸附行为由2种方式共同作用,以物理作用为主、化学作用为辅[50]。
-
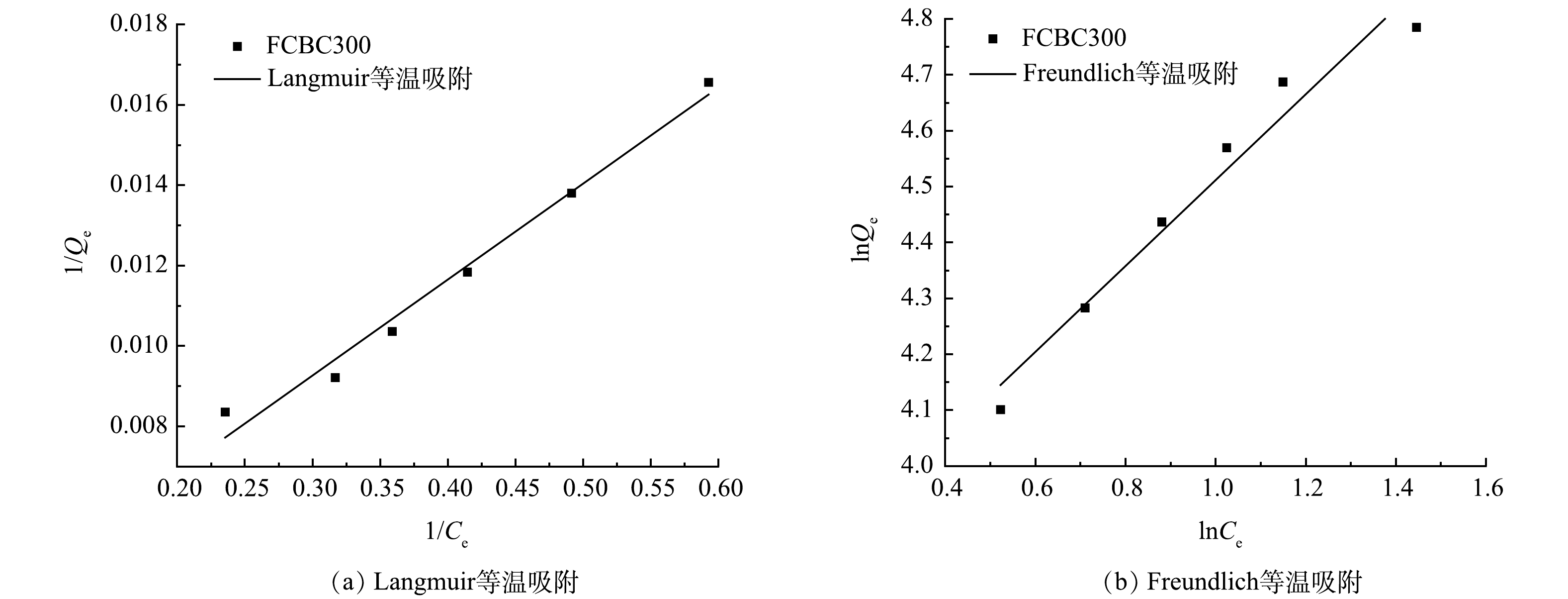
分别用Langmuir等温吸附方程和Freundlich等温吸附方程对吸附数据进行拟合,拟合曲线和参数分别如图9和表4所示,平衡时的吸附量与DCF初始质量浓度呈正相关,这可能是由于高质量浓度可以增加边界层的传质推理,从而增加FCBC300与DCF之间的接触[51];Langmuir和Freundlich等温吸附方程与实验数据均具有较好的相关性(R2>0.96),但Langmuir的拟合程度更好(R2=0.978 3),表明单层吸附是有利的,这一结果与高铁酸钾活化多孔石墨生物炭高效去除水中DCF的结果一致[52]。Langmuir方程拟合计算出的最大吸附量理论值为476.190 5 mg·g−1;另外n>1,说明FCBC300对DCF的吸附过程易于进行。
综上所述,本研究旨在通过废弃物资源化利用,以稻壳为原料制备铁铜改性稻壳炭探究其对DCF的去除效果和影响因素。但由于实验条件和研究时间的有限性,本研究尚存在一定的不足之处,如本研究制备的材料采用二次热解的方法存在一定的经济损失,在后续的研究中可进一步探讨一次热解和二次热解对改性稻壳炭理化性质及吸附性能的影响;在影响DCF吸附的影响因素方面,除了本实验探究的部分阴离子和有机物HA的影响外,在实际水体中仍存在诸如Na+、K+、NO3−、药物等其他阴离子、阳离子和有机物等物质,这些因素均会影响吸附效果,在后续的研究中有待进一步的探究与完善。
-
1)改性后的稻壳炭表面粗糙,比表面积增大,孔隙结构以介孔为主;FCBC300含有Cu—O/Fe—O的振动峰且芳香性增强、亲水性降低;表面的负载物包含Fe3O4、γ-Fe2O3和CuO,但衍射峰强度较弱,表明了无定形Fe/Cu的形成。
2) FCBC300处理DCF时,FCBC300在初始pH=3~9的溶液中均具有较高的吸附能力,在pH=5~9的范围内,对DCF的吸附量约是BC300的20倍以上;PO43−不影响改性稻壳炭对DCF的吸附作用,SO42−、Cl−、HCO3−和HA则存在抑制作用。DCF的去除机制以静电作用为主。
3) FCBC300对DCF的吸附过程符合准一级动力学、Langmuir等温吸附方程,这表明FCBC300对DCF的吸附是单层吸附,吸附过程由两种方式共同作用,以物理作用为主、化学作用为辅。
可见,铁铜改性稻壳炭对DCF的吸附能力明显提升,且具有较好的抗阴离子干扰性和pH缓冲性,本研究为废水处理中的DCF去除,提供了节本增效的新思路和基础数据。
铁铜改性稻壳炭对双氯芬酸钠吸附性能
Performance of Fe/Cu modified rice husk biochar on diclofenac sodium adsorption
-
摘要: 以稻壳为原料制备铁铜改性生物炭(FCBC300),采用扫描电子显微镜、元素组成分析仪、X-射线衍射光谱仪、傅里叶红外光谱仪对其进行了系列基础理化性质表征,通过批量吸附实验研究了FCBC300在不同pH和干扰离子及有机物腐殖酸(HA)等条件下对双氯芬酸钠(DCF)的去除效果和吸附机制。结果表明,改性后稻壳炭表面负载Fe3O4、γ-Fe2O3和CuO,芳香性增强、亲水性降低,对DCF的吸附性能大幅度提升。pH=5~9条件下,改性稻壳炭对DCF的吸附量约是未改性稻壳炭的20倍,吸附机制以静电作用为主;PO43−的存在对吸附几乎无影响,SO42−、Cl−和HCO3−对DCF的去除虽有轻微抑制作用,但去除率下降幅度均低于5%,HA存在则明显抑制吸附能力;FCBC300对DCF的吸附过程,更符合准一级动力学模型和Langmuir等温吸附模型,预测最大吸附量为476.190 5 mg·g−1。综上所述,FCBC300体现出良好的pH缓冲性、抗离子干扰性和高吸附性能,在废水处理中有较好的应用前景。Abstract: Rice husk was used to prepare iron-copper modified biochar (FCBC300), and its physicochemical properties were characterized by using Fourier transform infrared spectroscopy, X-ray diffraction spectroscopy, elemental composition analysis, and scanning electron microscopy. The effect and mechanism of diclofenac sodium (DCF) removal by FCBC300 were investigated through adsorption batch tests at different pH, interfering ions and organic humic acid (HA). The results showed that Fe3O4, γ-Fe2O3, and CuO loading onto the surface of FCBC300 could increase its aromaticity and decrease its hydrophilicity, as well as largely increase the adsorption performance on DCF. The adsorption capacity of DCF on FCBC was about 20 times that of the unmodified rice husk charcoal at pHs of 5~9. Electrostatic interactions dominated the adsorption mechanism. The coexisting PO43− had hardly effect on DCF removal, while coexisting SO42−, Cl−, and HCO3− slightly inhibited DCF removal with less than 5% reduction rate. HA had an obviously inhibitory effect on DCF adsorption on FCBC300. The fitting results with adsorption kinetic and isothermal modes revealed that the DCF adsorption process on FCBC300 was more consistent with the quasi-first-order kinetic model and the Langmuir model. The maximum adsorption capacity was estimated to be 476.190 5 mg·g−1. FCBC300 is a promising material for wastewater treatment because of its strong adsorption capabilities, anti-ion interference, and pH buffering capabilities.
-
Key words:
- rice husk biochar /
- modification /
- diclofenac sodium /
- adsorption
-

-
表 1 BC300和FCBC300的比表面积、介孔孔径及孔容
Table 1. Specific surface area, mesopore size and volume of BC300 and FCBC300
生物炭 比表面积/
(m2·g−1)平均孔径/
nm总孔容/
(cm3·g−1)BC300 2.717 6.315 0.004 FCBC300 6.139 6.110 0.008 表 2 BC300和FCBC300的元素组成
Table 2. Elements content analysis of BC300 and FCBC300
吸附剂 元素组成 H/C O/C (O+N)/C C/% H/% O/% N/% Fe/% Cu/% BC300 48.48 3.71 21.29 0.55 1.50×10−2 2.00×10−4 0.92 5.27 0.34 FCBC300 56.18 3.75 18.45 0.37 3.91 0.95 0.80 3.94 0.25 注:C、H、O、N为质量百分比,H/C、O/C、(O+N)/C为摩尔比。 表 3 FCBC300的吸附动力学拟合参数
Table 3. Adsorption kinetics fitting parameters of FCBC300
生物炭
类型准一级动力学方程 准二级动力学方程 Qe/(mg·g−1) K1/min−1 R2 Qe/(mg·g−1) K2/(g·mg−1·min−1) R2 FCBC300 8.744 1 8.96×10−3 0.972 4 9.626 7 1.15×10−3 0.951 6 表 4 FCBC300的吸附等温线拟合参数
Table 4. Adsorption isotherm parameter of FCBC300
生物炭
类型Langmuir方程 Freundlich方程 Qm/(mg·g−1) Kl/(L·mg−1) R2 n Kf/(mg(1-1/n)·L1/n·g−1) R2 FCBC300 476.190 5 0.087 9 0.978 3 1.301 7 42.222 8 0.962 5 -
[1] 王龙, 朱丹, 曹云霄, 等. 北京市污水处理厂出水中药物和个人护理品的季节变化及其生态风险评价[J]. 环境科学学报, 2021, 41(7): 2922-2932. [2] MIRIAM B, CARMEN C, PABLO A L. Monitoring the occurrence of pharmaceuticals in soils irrigated with reclaimed wastewater[J]. Environmental Pollution, 2018, 235: 312-321. doi: 10.1016/j.envpol.2017.12.085 [3] 王丽, 陈凡, 易皓, 等. 东江下游药物和个人护理品污染特征及风险评价[J]. 河南师范大学学报(自然科学版), 2014, 42(6): 79-85. [4] LI Z P, YU X P, YU F R, et al. Occurrence, sources and fate of pharmaceuticals and personal care products and artificial sweeteners in groundwater[J]. Environmental science and pollution research international, 2021, 28(17): 20903-20920. doi: 10.1007/s11356-021-12721-3 [5] CARMONA E, ANDREU V, PICÓ Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water[J]. Science of the Total Environment, 2014, 484: 53-63. doi: 10.1016/j.scitotenv.2014.02.085 [6] JOACHIM S, BEAUDOUIN R, DANIELE G, et al. Effects of diclofenac on sentinel species and aquatic communities in semi-natural conditions[J]. Ecotoxicology and Environmental Safety, 2021, 211: 111812. doi: 10.1016/j.ecoenv.2020.111812 [7] TAOUFIK N, BOUMYA W, JANANI F, et al. Removal of emerging pharmaceutical pollutants: A systematic mapping study review[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104251. doi: 10.1016/j.jece.2020.104251 [8] 郑姗, 郑刘根, 张燕海, 等. 改性芦苇生物炭对水中硫酸盐的吸附性能及机理[J]. 环境工程学报, 2022, 16(12): 3916-3925. [9] LONAPPAN L, ROUISSI T, BRAR K S, et al. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics[J]. Bioresource Technology, 2018, 249: 386-394. doi: 10.1016/j.biortech.2017.10.039 [10] LIU S, XU W, LIU Y, et al. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water[J]. Science of the Total Environment, 2017, 592. [11] LIU SB, LI MF, LIU G, et al. Removal of 17 β-estradiol from aqueous solution bygraphene oxide supported activated magnetic biochar: adsorptionbehavior and mechanism[J]. Taiwan Institute Chemical Engineers, 2019, 102: 330-339. doi: 10.1016/j.jtice.2019.05.002 [12] LIANG G W, HU Z Z, WANG Z W, et al. Effective removal of carbamazepine and diclofenac by CuO/Cu2O/Cu-biochar composite with different adsorption mechanisms[J]. Environmental science and pollution research international, 2020, 27(36): 45435-45446. doi: 10.1007/s11356-020-10284-3 [13] ZHANG B, MEI M, LI K W, et al. One-pot synthesis of MnFe2O4 functionalized magnetic biochar by the sol-gel pyrolysis method for diclofenac sodium removal[J]. Journal of Cleaner Production, 2022, 381(P1). [14] YANG X, XU G, YU H, et al. Preparation of ferric-activated sludge-based adsorbent from biological sludge for tetracycline removal[J]. Bioresource Technology, 2016, 211: 566-573. doi: 10.1016/j.biortech.2016.03.140 [15] WU Y, YUE Q, GAO Y, et al. Performance of bimetallic nanoscale zero-valent iron particles for removal of oxytetracycline[J]. Journal of Environmental Sciences, 2018, 69(7): 173-182. [16] DUAN J, ZHU H, XU F, et al. A new approach to 4-chlorophenol dechlorination on monometallic copper compared to its Cu/Fe bimetallic system[J]. Chemical Engineering Journal, 2016, 304: 282-288. doi: 10.1016/j.cej.2016.06.089 [17] MA J, ZHOU B, ZHANG H, et al. Activated municipal wasted sludge biochar supported by nanoscale Fe/Cu composites for tetracycline removal from water[J]. Chemical Engineering Research and Design, 2019, 149: 209-219. doi: 10.1016/j.cherd.2019.07.013 [18] 丰祎. 生物质稻壳制备硅基(SiO2、SiOx和Si)/碳复合材料及其储锂性能的研究[D]. 长春: 吉林大学, 2021, 209-219. [19] 张彦彬, 杨会国, 马丽萍, 等. 生物质热解制备多孔吸附材料的研究进展[J]. 应用化工, 2023, 52(11): 3101-3106. [20] SEPÚLVEDA P, RUBIO A M, BALTAZAR E S, et al. As(V) removal capacity of FeCu bimetallic nanoparticles in aqueous solutions: The influence of Cu content and morphologic changes in bimetallic nanoparticles[J]. Journal of Colloid And Interface Science, 2018, 524: 177-187. doi: 10.1016/j.jcis.2018.03.113 [21] ELYOUNSSI K, HALIM M. An investigation on the texture and microstructure of carbonized charcoals produced by two-step pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2014, 109: 258-265. doi: 10.1016/j.jaap.2014.06.003 [22] 谷娟. 餐厨垃圾水热炭的制备及对六价铬和布洛芬的吸附研究[D]. 重庆: 重庆大学, 2022. [23] NAVARRO C, MILENA Y , PIRAJÁN M, et al. Processing of fique bagasse waste into modified biochars for adsorption of caffeine and sodium diclofenac[J]. Brazilian Journal of Chemical Engineering, 2021, 39(4): 1-16. [24] HOU W H, WANG S, LI Y, et al. Influence of modified biochar supported Fe–Cu/polyvinylpyrrolidone on nitrate removal and high selectivity towards nitrogen in constructed wetlands[J]. Environmental Pollution, 2021, 289: 117812-117812. doi: 10.1016/j.envpol.2021.117812 [25] 朱建龙, 郭硕铖, 徐伟杰, 等. 纳米铁改性生物炭制备及其对含铬废水的吸附性能[J]. 环境工程学报, 2023, 17(9): 2891-2898. [26] 吴嘉煦, 李凯, 孙鑫, 等. 载镧酒糟污泥生物炭对磷的吸附性能及机理[J]. 环境工程学报, 2022, 16(12): 3884-3894. [27] 梁金浩, 许兵, 张钰卿, 等. 金属盐改性活性氧化镁对饮用水中氟离子的去除性能[J]. 环境工程学报, 2023, 17(7): 2181-2191. [28] MENG F Y, SOMG M, CHEN Y Y, et al. Promoting adsorption of organic pollutants via tailoring surface physicochemical properties of biomass-derived carbon-attapulgite[J]. Environmental Science and Pollution Research, 2020, 28(9): 11106-11118. [29] 吕松磊. 稻壳基活性炭的制备、改性以及对苯酚的吸附机理研究[D]. 北京: 北京化工大学, 2020. [30] 陈涛. 稻壳基磁性活性炭的制备及其在水处理中的应用研究[D]. 南京: 东南大学, 2018. [31] 王江南, 孙晓雪, 杨玲辉等. 壳聚糖、铁锰改性稻壳生物炭的表征及其Cd2+吸附性能研究[J]. 农业环境科学学报, 2023, 42(9): 1964-1973. [32] WEI X Q, WANG X, GAO B, et al. Facile Ball-Milling Synthesis of CuO/Biochar Nanocomposites for Efficient Removal of Reactive Red 120[J]. ACS omega, 2020, 5(11): 5748-5755. doi: 10.1021/acsomega.9b03787 [33] 刘佳乐. 生物炭负载铁铜双金属复合物活化氧气吸附和降解四环素[D]. 长沙: 湖南大学, 2022. [34] ANDRESSA C F, EDSON L F, GUILHERME L D. Preparation and characterization of NiFe2O4/activated carbon composite as potential magnetic adsorbent for removal of ibuprofen and ketoprofen pharmaceuticals from aqueous solutions[J]. Journal of Cleaner Production, 2019, 229: 828-837. doi: 10.1016/j.jclepro.2019.05.037 [35] 任洁青, 王朝旭, 张峰, 等. 改性稻壳生物炭对水中Cd2+的吸附性能研究[J]. 生态与农村环境学报, 2021, 37(1): 73-79. [36] 董智伟, 左宁, 王彦, 等. 热解污泥生物炭化学组成及环境效应研究进展[J]. 环境污染与防治, 2019, 41(4): 479-484. [37] LEENA A P, KURIAN J. Hydrothermal carbonization of oily sludge for solid fuel recovery investigation of chemical characteristics and combustion behaviour[J]. Journal of Analytical and Applied Pyrolysis, 2021, 157: 105235. doi: 10.1016/j.jaap.2021.105235 [38] SURIYANON N, PUNYAPALAKUL P, NGAMCHARUSSRIVICHAI C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials[J]. Chemical Engineering Journal, 2013, 214: 208-218. doi: 10.1016/j.cej.2012.10.052 [39] LUO H, ZHANG Y, XIE Y, et al. Iron-rich microorganism-enabled synthesis of magnetic biocarbon for efficient adsorption of diclofenac from aqueous solution[J]. Bioresource Technology, 2019, 282: 310-317. doi: 10.1016/j.biortech.2019.03.028 [40] BHADRA N B, SEO W P, JHUNG H S. Adsorption of diclofenac sodium from water using oxidized activated carbon[J]. Chemical Engineering Journal, 2016, 301: 27-34. doi: 10.1016/j.cej.2016.04.143 [41] FENG Z, ODELIUS K, RAJARAO K G, et al. Microwave carbonized cellulose for trace pharmaceutical adsorption[J]. Chemical Engineering Journal, 2018, 346: 557-566. doi: 10.1016/j.cej.2018.04.014 [42] DAI C, GEISSEN S, ZHANG Y, et al. Selective removal of diclofenac from contaminated water using molecularly imprinted polymer microspheres[J]. Environmental Pollution, 2011, 159(6): 1660-1666. doi: 10.1016/j.envpol.2011.02.041 [43] ZHAO Y, LIU F, QIN X. Adsorption of diclofenac onto goethite: Adsorption kinetics and effects of pH[J]. Chemosphere, 2017, 180: 373-378. doi: 10.1016/j.chemosphere.2017.04.007 [44] 李乃玮, 陈月琴, 罗维, 等. 壳聚糖/生物炭复合材料对Cr离子的吸附性能[J]. 应用化工, 2022, 51(2): 406-410+425. doi: 10.3969/j.issn.1671-3206.2022.02.021 [45] LONAPPAN L, ROUISSI T, BRAR K S, et al. Adsorption of diclofenac onto different biochar microparticles: Dataset–Characterization and dosage of biochar[J]. Data in Brief, 2018, 16: 460-465. doi: 10.1016/j.dib.2017.10.041 [46] SAJAB S M, CHIA H C, ZAKARIA S, et al. Citric acid modified kenaf core fibres for removal of methylene blue from aqueous solution[J]. Bioresource Technology, 2011, 102(15): 7237-7243. doi: 10.1016/j.biortech.2011.05.011 [47] ZAHRA S, HOCHEOL S, AMIT B. Efficient removal of diclofenac and cephalexin from aqueous solution using Anthriscus sylvestris-derived activated biochar[J]. Science of the Total Environment, 2020, 745: 140789. doi: 10.1016/j.scitotenv.2020.140789 [48] 才金玲, 谢玉洁, 刘兰军, 等. 腐殖酸的性质及应用进展[J/OL][J]. 应用化工, 2023, 52(12): 3418-3422+3427. [49] 谢青青, 马晓燕, 艾迪娅·阿不来提, 等. 腐殖酸对二甲基胂酸在磁铁矿上吸附过程的影响[J]. 环境化学, 2023, 42(2): 658-670. [50] 高琦, 黄海龙, 韩卢, 等. PEI改性磁性壳聚糖微球的制备及其对布洛芬的吸附性能[J]. 功能高分子学报, 2020, 33(2): 165-171. [51] GAO T, SHI WS, ZHAO MX, et al. Preparation of spiramycin fermentation residue derived biochar for effective adsorption of spiramycin from wastewater[J]. Chemosphere, 2022, 296: 133902-133902. doi: 10.1016/j.chemosphere.2022.133902 [52] TAM M T N, LIU Y, BASHIR H, et al. Efficient removal of diclofenac from aqueous solution by potassium ferrate-activated porous graphitic biochar: ambient condition influences and adsorption mechanism[J]. International Journal of Environmental Research and Public Health, 2019, 17(1): 291-291. doi: 10.3390/ijerph17010291 -



 下载:
下载: