-
氯消毒被广泛用于世界各地的饮用水输配系统[1]。氯消毒可杀死各种病原微生物,防止水致疾病的传播,是保障人群身体健康的一种有效手段。然而,氯是一种化学氧化剂,在氯消毒过程中,它会与水中的天然有机物发生反应形成消毒副产物,其中最常见的是三卤甲烷和卤代乙酸,消毒副产物可能导致膀胱癌等疾病[2]。
在饮用水输配过程中,管网铁腐蚀和腐蚀产物的释放与饮用水水质直接相关。尽管全球多地已开始将老旧无内衬铸铁管更换淘汰,但无内衬铸铁管在给水管网中仍然广泛使用。有研究发现,调节进水余氯浓度对无内衬铸铁管的铁释放具有一定调控作用,但不同腐蚀产物类型会影响余氯的调控效应[3]。与此同时,管道中的腐蚀产物为细菌生长提供了庇护所,给水管网中的生物膜是导致水质恶化病原菌的重要来源[4]。水质的变化可能导致生物膜不稳定,使得细菌、金属离子和其它有害物质释放水中[5-6]。因此,余氯的变化也可能影响水的化学和微生物稳定性。而三磷酸腺苷 (ATP) 分析被认为是在氯化和非氯化饮用水环境中测定细菌活力的高效和前瞻性工具[7]。
铁颗粒物引发变色 (如“黄水”) 是全世界自来水投诉中最常见的问题[8]。由于铁颗粒物、余氯和微生物之间的相互作用,在铁颗粒物存在情况下余氯对水质的影响非常复杂。有研究表明,铁质管道中消毒剂衰减速率明显高于塑料管材。这是由于,消毒剂会持续对腐蚀产物进行再氧化,促使体系中的消毒剂加快衰减;与此同时,消毒剂的强氧化作用可剥离锈垢加剧水质变色恶化[9]。值得注意的是,铁颗粒物由于其形貌特征和自由基路径能够损伤细胞,导致潜在健康风险[10];此外,铁颗粒物还能够通过影响消毒副产物的形成过程加剧水质风险[11-12]。因此,了解余氯对无内衬铸铁管中的水质影响对于保障饮用水安全非常重要。
本研究拟通过浸泡实验和烧杯实验,研究了实际老旧无内衬铸铁管中初始氯对浓度水质的影响。一方面,通过实际管段浸泡实验,在低氯条件下,对比不同初始氯浓度下管道中浊度、总铁浓度、以及三磷酸腺苷等指标的变化,探究余氯通过影响铁释放引发的微生物风险;另一方面,预先收集管段中的颗粒物,在不同氯条件下,对比初始氯浓度对颗粒物浊度、表面电位、粒径等性质的影响,探究余氯通过影响颗粒物性质引发的化学风险。本研究结果可有助于明确给水管网中余氯与铁释放共存时引发的水质风险。
-
1) 采样方法。对中国北方某城市频繁发生“黄水”的小区进行了实地调研,截取了长度为1 m的入楼管管道,管材为无内衬铸铁管,管径为100 mm,管龄约为 (20±5) a。利用软毛刷刷取管道表层疏松沉积物,将获得的疏松沉积物冷冻干燥,再通过90目 (160 μm) 筛网进行筛分。将截取的管道进一步截为20 cm的小管段共3段以制造小型管式反应器。
2) 管段浸泡实验方法。实验水样均为自来水,浊度为 (0.3±0.1) NTU、余氯为 (0.5±0.1) mg·L−1 (Cl2) 、溶解氧为 (9.2±0.4) mg·L−1。将自来水静置30 min至余氯完全被消耗。对于初始氯为0.5和1 mg·L−1的样品,向溶液中加入游离氯,以将初始氯浓度调整为目标值 (以Cl2形式,由NaClO制备) 。浸泡实验中,用搅拌器以100 r∙min−1搅拌反应器以使水质均匀,然后用无菌注射器在0~12 h从水面以下10 cm处取样进行相关测试。
3) 颗粒物烧杯实验方法。将预先获得的疏松沉积物配置成颗粒物浓度100 mg·L−1的分散液,对于初始氯为1和10 mg·L−1的样品,向溶液中加入游离氯,以将初始氯浓度调整为目标值 (以Cl2形式,由NaClO制备) 。摇床中振荡180 r∙min−1,0~48 h后进行相关测试。
-
浊度计 (2100Q,哈希公司) ;便携式多参数仪 (HQ 40d,哈希公司) ;表面电位和粒径使用zeta电位分析仪 (Malvern,Zetasizer2000) 测试; 电感耦合等离子体质谱仪 (ICP-MS,NexlON 300X,美国 PerkinElmer公司) ;使用游离氯试剂盒 (DR300,哈希公司) 测定氯浓度。为了测定消毒副产物三卤甲烷 (THMs) 和卤乙酸 (HAAs) ,使用甲基叔丁基醚作为萃取剂提取水样,并通过配备电子捕获检测器 (GC/ECD,7890B,美国安捷伦公司) 和HP-5熔融石英毛细管柱 (30 m×0.25 mm,0.25 μm,美国安捷伦公司) 的气相色谱仪进行分析。傅里叶变换红外光谱法 (FTIR,Nicolet iN10MX,美国Thermo Fisher Scientific公司) 用于分析胞外聚合物 (EPS) 的官能团。使用BacTiter GloTM (G8231;Promega Corporation公司) 和GloMax20/20光度计 (美国Turner BioSystems公司) 测量三磷酸腺苷 (ATP) 物质的量浓度。
-
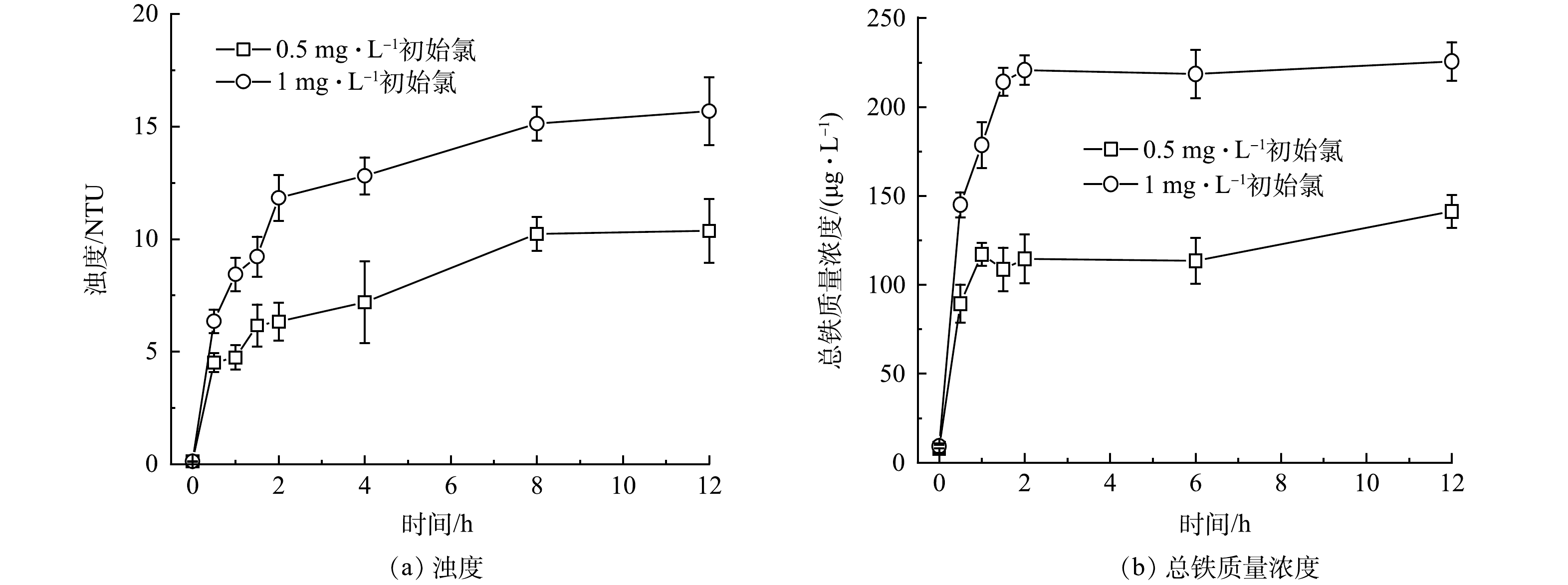
给水管网中铁颗粒的形成将导致浊度增加。因此,研究了无内衬铸铁管中初始氯质量浓度对浊度的变化的影响 (图1(a)) 。在12 h内,管道中水的浊度随着时间增长而增大、随初始氯质量浓度增加而增大。与此同时,总铁质量浓度也随着初始氯的增加而增加 (图1(b)) 。原因可能是氯会将腐蚀结垢破坏为疏松多孔结构,降低结垢对管道的保护作用[13],促进管道中铁释放。在该体系内,余氯在2 h内被消耗完全,因此氯对管壁的破坏主要发生在2 h以内,在2~12 h被破坏的管壁向水中进行铁释放。释放的腐蚀产物不仅会引起水质恶化,同时也削弱了腐蚀垢对内部金属的保护作用,促进了进一步腐蚀的发生,余氯还可将二价铁氧化为三价铁,使阳极反应生成的二价铁减少,间接促进了阳极反应的进行[14]。
-
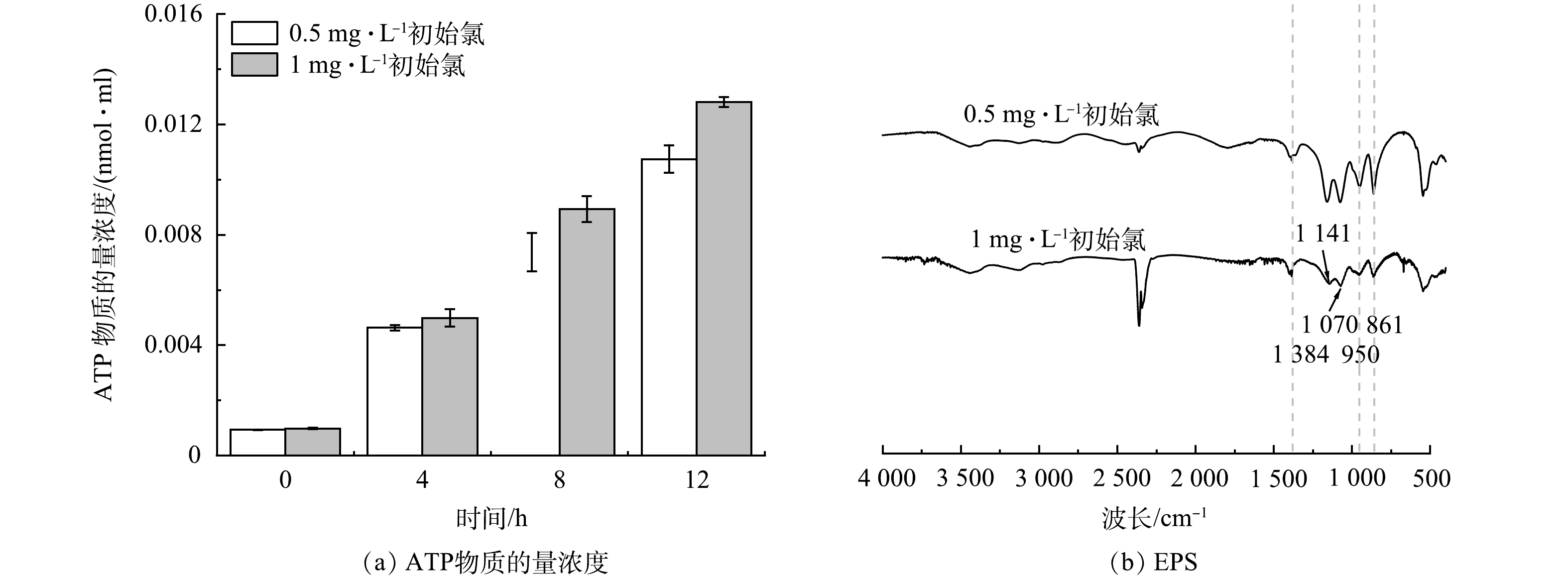
为了研究初始氯质量浓度对无内衬铸铁管中微生物稳定性的影响,对比了不同初始氯质量浓度下的ATP物质的量浓度变化 (图2(a)) 。钟丹等[15]发现,随着投氯量增大,聚乙烯和不锈钢管内生物量均逐渐降低,但是铸铁管内生物量反而升高,这一现象表明,余氯能够影响管壁生物膜的稳定性,但该过程中细菌活性的变化尚不明确。ATP分子是细胞中许多代谢过程的主要能量载体,是反映细菌生存能力的有效指标,NESCERECKA等[7]的研究表明,ATP的增长比生物量的增殖更快。本研究结果发现,随着初始氯质量浓度的增加,ATP明显增加,这表明在高初始氯下无衬里铸铁管道中的活性生物质浓度更高,也说明了初始氯质量浓度能够显著影响管壁生物膜的稳定性和微生物的活性。这可能是因为,在高初始氯质量浓度下,水中受损细菌释放的ATP将促进从生物膜释放的细菌再生到水中,并且ATP可在细菌增殖期间用作磷源,导致ATP物质的量浓度增加[7]。此外,微生物可以从管道中的生物膜释放到水中[16]。因此,含氯量较高的水可以提供更多的ATP作为磷源用于从管道转移到水中的生物量的生长。此外,高残留氯会将水中的有机物降解为小分子的生物可同化有机碳,这将进一步促进细菌的生长[13]。多糖的存在促进了EPS的粘附强度,而蛋白质的粘附效果较小。在初始氯为0.5和1 mg·L−1时,多糖/蛋白质分别为1.28和1.71。更大的EPS产生和增强的细胞粘附与生物膜生长有关[17]。在FTIR (图2(b)) 中,1 384 cm−1的峰值为羧酸盐的对称拉伸振动[18]。800~1 200 cm−1处的峰为糖类的特征峰,861 cm−1的峰主要与b-半乳糖残基中异聚碳的C-H弯曲有关[19]。950 cm−1带是由于3,6-脱水-D-半乳糖的出现。1 070 cm−1和1 141 cm−1带由几种振动模式贡献,即C—O和C—C拉伸以及C—C—O与C—C/H变形[18, 20]。EPS对细菌的自我保护和粘附能力具有重要影响,EPS的峰值在1 mg·L−1初始氯质量浓度下比0.5 mg·L−1更弱,这表明微生物1 mg·L−1初始氯质量浓度下自我保护能力较弱。由此可见,在铁释放严重的无衬里铸铁管道中,消毒剂的增加可能会加剧水质恶化并产生微生物风险。
-
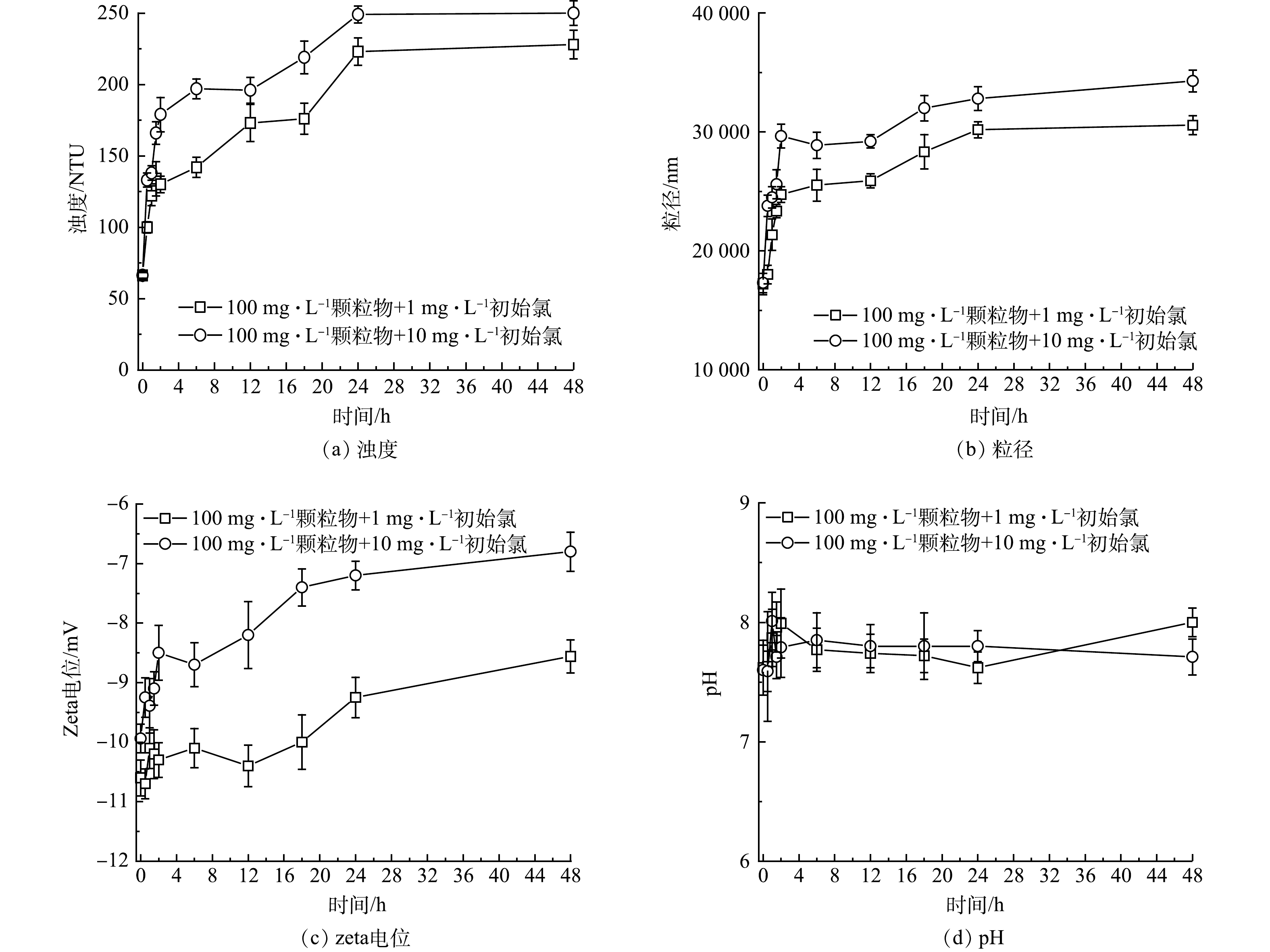
铁颗粒物变色是一个普遍引起公众关注的水质恶化问题。为了研究加氯对颗粒物引发变色的影响,将管网颗粒物配置成100 mg·L−1的溶液研究氯对管网颗粒物影响。加氯后,样品颜色明显变深、浊度明显变大 (图3(a)) ,这表明加氯加剧了颗粒物的致色能力,并进一步证实氯化会增加变色风险。颗粒物的粒径随时间逐渐变大,且在高初始氯质量浓度下粒径更大 (图3(b)) 。所有颗粒物的初始表面电荷均为负电荷,反应过程中,颗粒物的表面电荷随时间增长而减少 (图3(c)) ,可见氯通过降低颗粒物表面电荷从而降低静电排斥,加剧了颗粒物的团聚,导致粒径变大。在反应过程中,溶液pH随时间增长略有稍高,但整体上初始氯质量浓度的改变对溶液的pH影响较小 (图3(d)) 。
图4为初始氯对管网颗粒物分散液中2种典型的消毒副产物三卤甲烷 (图4(a)) 和卤乙酸 (图4(b)) 的影响。水中初始的三卤甲烷和卤乙酸浓度分别是38.2和35.5 μg·L−1,三卤甲烷随时间先升高后降低,卤乙酸随时间变化稍有升高后保持稳定。在1.5 h之前,三卤甲烷浓度明显提升而卤乙酸浓度仅小幅增加,这可能是该颗粒物对三卤甲烷的生成具有一定的催化作用。已有文献报道,铁氧化物可以通过在二价和三价态之间进行氧化还原循环来催化特定化合物的氧化[12, 21],碳阳离子形成和脱芳构化被认为是铁氧化物对消毒副产物生成的一个重要催化机制[22]。在1.5 h之后,三卤甲烷质量浓度大幅降低,但卤乙酸质量浓度基本不变,这可能是由于该颗粒物对于三卤甲烷比对卤乙酸具有更强的吸附性能。CHEN等[23]发现,虽然预成型MnOx对有机污染物的累积可以忽略不计,但锰颗粒物的形成过程可能会累积有机污染物。本研究的结果表明,预成型铁氧化物对于消毒副产物可能具有一定的富集累积作用。此外,管壁生物膜中的微生物也可能会降解水中天然有机物,导致 DBPs 的迁移转化[24]。
-
1) 氯能够促进管壁铁释放引发浊度增大和总铁升高,加剧水质变色风险。
2) 高初始氯质量浓度下三磷酸腺苷升高,这表明在铁释放严重的无衬里铸铁管道中,增大氯的投加量可能引发管壁生物膜释放导致微生物风险。
3) 氯能够通过降低颗粒物表面电荷从而降低静电排斥、加剧颗粒物的团聚,使得粒径变大、浊度升高,提升了颗粒物致色能力,同时颗粒物对消毒副产物可能通过富集累积作用引发水质化学风险。
老旧无内衬铸铁管中氯对水质的影响
Effect of free chlorine on drinking water quality in old unlined cast iron pipes
-
摘要: 给水管网铁释放对于饮用水水质具有重要影响。通过浸泡实验和烧杯实验探究实际老旧无内衬铸铁管中氯对铁腐蚀的影响。一方面,通过实际管段浸泡实验,对比了不同初始氯浓度下管道中水质变化,发现在高初始氯下浊度和三磷酸腺苷增大,这表明在铁释放严重的无衬里铸铁管道中,消毒剂的增加可能引发水质微生物风险;另一方面,通过烧杯实验进一步确认了氯对管网颗粒物结构的影响,发现氯能够通过降低颗粒物表面电荷导致静电排斥降低,从而加剧了颗粒物的团聚,导致粒径变大、浊度升高,且颗粒物对消毒副产物三卤甲烷具有一定的富集累积作用,引发水质化学风险。本研究结果可为了解给水管网中氯引发的黄水风险提供参考。Abstract: Iron release in drinking water distribution system has an important impact on drinking water quality. The influence of chlorine on iron release in the actual old unlined cast iron pipe was studied through soaking experiment and batch experiment. On the one hand, the changes of water quality in the pipe under different initial chlorine concentrations were compared through the actual pipe section soaking experiment. The result showed that turbidity and adenosine triphosphate increased with the increase of initial chlorine concentration, which indicated the increase of disinfectants may increase microbiological risk. On the other hand, the influence of chlorine on the structure of particles from the pipe was further studied through batch experiments by using the particles collected from the pipe section in advance. It was found that chlorine can reduce the surface charge of particles resulting low electrostatic repulsion, which reduced the agglomeration of particles and increased particle size, lead to the increase of turbidity of the particle suspension. Moreover, it would have certain enrichment and accumulation effect on the disinfection by-product trihalomethane, thus causing chemical risks of water quality. This study provides an important basis for a comprehensive understanding of the discoloration risk caused by chlorine in the water supply network.
-
Key words:
- drinking water distribution system /
- particles /
- yellow water /
- water quality risks
-

-
-
[1] YE B X, WANG W Y, YANG L S, et al. Factors influencing disinfection by-products formation in drinking water of six cities in China[J]. Journal of Hazardous Materials, 2009, 171(1): 147-152. [2] DING S K, DENG Y, BOND T, et al. Disinfection byproduct formation during drinking water treatment and distribution: A review of unintended effects of engineering agents and materials[J]. Water Research, 2019, 160: 313-329. doi: 10.1016/j.watres.2019.05.024 [3] 孙慧芳, 石宝友, 吴永丽, 等. 硫酸根、溶解氧和余氯对管垢铁释放的影响[J]. 中国给水排水, 2013, 29(22): 58-63. doi: 10.19853/j.zgjsps.1000-4602.2013.22.016 [4] DOUTERELO I, DUTILH B. E, ARKHIPOVA K, et al. Microbial diversity, ecological networks and functional traits associated to materials used in drinking water distribution systems[J]. Water Research, 2020, 173: 115586. doi: 10.1016/j.watres.2020.115586 [5] LIU G, ZHANG Y, KNIBBE W J, et al. Potential impacts of changing supply-water quality on drinking water distribution: A review[J]. Water Research, 2017, 116: 135-148. doi: 10.1016/j.watres.2017.03.031 [6] LIU S, GUNAWAN C, BARRAUD N, et al. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems[J]. Environmental Science & Technology, 2016, 50(17): 8954-8976. [7] NESCERECKA A, JUHNA T, HAMMES F. Behavior and stability of adenosine triphosphate (ATP) during chlorine disinfection[J]. Water Research, 2016, 101: 490-497. doi: 10.1016/j.watres.2016.05.087 [8] VREEBURG J. H. G, BOXALL J. B. Discolouration in potable water distribution systems: A review[J]. Water Research, 2007, 41(3): 519-529. doi: 10.1016/j.watres.2006.09.028 [9] 张剑桥, 袁媛, 迟惠中, 等. 管材对氯/二氧化氯消毒过程中氯衰减的影响及机理[J]. 中国给水排水, 2018, 34(13): 71-75. doi: 10.19853/j.zgjsps.1000-4602.2018.13.015 [10] 庄媛, 张堯, 于影, 等. 给水管网疏松沉积物的结构特征及其风险识别[J]. 环境工程学报, 2021, 15(10): 3455-3462. doi: 10.12030/j.cjee.202104121 [11] CHU W H, LI X, BOND T, et al. Copper increases reductive dehalogenation of haloacetamides by zero-valent iron in drinking water: Reduction efficiency and integrated toxicity risk[J]. Water Research, 2016, 107: 141-150. doi: 10.1016/j.watres.2016.10.047 [12] LIU J, WANG J, ZHANG J, et al. Iodo-trihalomethanes formation during chlorination and chloramination of iodide-containing waters in the presence of Cu2+[J]. Science of the Total Environment, 2019, 671: 101-107. doi: 10.1016/j.scitotenv.2019.03.356 [13] LI W Y, TIAN Y, CHEN J P, et al. Synergistic effects of sodium hypochlorite disinfection and iron-oxidizing bacteria on early corrosion in cast iron pipes[J]. Frontiers of Environmental Science & Engineering, 2021, 16(6): 72. [14] 陈灏琳, 田一梅, 郭浩, 等. NaClO对再生水球墨铸铁管道腐蚀行为的影响[J]. 腐蚀科学与防护技术, 2017, 29(1): 41-47. doi: 10.11903/1002.6495.2016.212 [15] 钟丹, 袁一星, 马文成, 等. 供水管网内生物膜与余氯衰减交互作用[J]. 哈尔滨工业大学学报, 2017, 49(8): 49-54. doi: 10.11918/j.issn.0367-6234.201608050 [16] PAN R J, ZHANG K J, CEN C, et al. Characteristics of biostability of drinking water in aged pipes after water source switching: ATP evaluation, biofilms niches and microbial community transition[J]. Environmental Pollution, 2021, 271: 116293. doi: 10.1016/j.envpol.2020.116293 [17] Harimawan A, Ting YP. Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion[J]. Colloids Surf B Biointerfaces, 2016, 146: 459-467. doi: 10.1016/j.colsurfb.2016.06.039 [18] LI J J, JIANG Z Q, CHEN S S, et al. Biochemical changes of polysaccharides and proteins within EPS under Pb(II) stress in Rhodotorula mucilaginosa[J]. Ecotoxicology and Environmental Safety, 2019, 174: 484-490. doi: 10.1016/j.ecoenv.2019.03.004 [19] GOMEZ-ORDONEZ E, RUPEREZ P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds[J]. Food Hydrocolloids, 2011, 25(6): 1514-1520. doi: 10.1016/j.foodhyd.2011.02.009 [20] PEREIRA L, SOUSA A, COELHO H, et al. Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids[J]. Biomolecular Engineering, 2003, 20(4/5/6): 223-228. [21] DEMMIN T. R, SWERDLOFF M. D, ROGIC M. M. Copper(II)-Induced oxidations of aromatic substrates: Catalytic conversion of catechols to o-Benzoquinones. Copper phenoxides as intermediates in the oxidation of phenol and a single-step conversion of phenol, ammonia, and oxygen into muconic acid mononitrile[J]. Journal of the American Chemical Society, 1981, 103(19): 5795-5804. doi: 10.1021/ja00409a030 [22] ZHAO Y, YANG H W, LIU S T, et al. Effects of metal ions on disinfection byproduct formation during chlorination of natural organic matter and surrogates[J]. Chemosphere, 2016, 144: 1074-1082. doi: 10.1016/j.chemosphere.2015.09.095 [23] CHEN R Y, ZHUANG Y, YU Y, et al. Enhanced perfluorooctanoic acid (PFOA) accumulation by combination with in-situ formed Mn oxides under drinking water conditions[J]. Water Research, 2021, 190: 116660. doi: 10.1016/j.watres.2020.116660 [24] 苏乐, 朱延平, 舒诗湖, 等. 管网输配系统中消毒副产物生成及控制技术研究[J]. 中国给水排水, 2022, 38(14): 42-46. doi: 10.19853/j.zgjsps.1000-4602.2022.14.007 -



 下载:
下载: