-
近年来,药品和个人护理产品(PPCPs)的消费量逐年增加[1],引起的环境污染越来越受到关注[2-3]。大多数PPCPs在污水处理厂无法完全去除,其会随着污水厂出水进入受纳水体,导致其在自然水环境中的暴露。例如,被广泛用作止疼和抗风湿的双氯芬酸(DCF),在地表水甚至自来水中都被检测出[4],DCF的基本理化性质见表1[5]。此外,低剂量的PPCPs即可对生物产生生理效应[1, 6],这使得处理含有PPCPs的工业废水和生活污水尤为重要。
高级氧化工艺(AOPs)被认为是降解PPCPs的有效方法之一,常用的氧化剂包括过氧化氢(H2O2)、过一硫酸盐(PMS)和过硫酸盐(PS)等[7-9]。过氧乙酸(PAA, CH3C(=O)OOH)是一种类似H2O2的新型氧化剂,近年来在AOPs中得到广泛研究[10-12]。此前,PAA因其良好的消毒性能和副产物的低毒性而被广泛用于废水的消毒[13-15]。PAA的过氧键键能仅为159 kJ·mol−1,低于H2O2的213 kJ·mol−1[16],这意味着PAA更容易被活化[17]。迄今为止,已报道活化PAA的方法有紫外(UV)、过渡金属、热等[15, 18-22]。UV能激活PAA产生活性物质进而降解污染物[18],然而UV在水中的穿透力有限,如果没有足够的UV强度,这一过程很难实现。过渡金属活化PAA的反应条件简单,能够活化PAA的过渡金属离子包括Co2+、Mn2+、Fe2+、Cu2+等[15, 19-21],然而Co2+和Mn2+具有一定毒性,进而可能造成二次污染;Fe2+虽然无毒性,但在非酸性条件下易被氧化而降低活化效率;Cu2+的毒性较低,但低剂量的Cu2+活化PAA效果不佳。热能激活PAA产生活性物质,从而快速降解磺胺甲恶唑(SMX)[22],如公式(1)所示,但断裂过氧键需要消耗大量能量。
根据预实验发现,Cu(Ⅱ)和热联用活化PAA (Cu(Ⅱ)-heat/PAA)只需要较低浓度的Cu(Ⅱ)(低至μmol·L−1级别)和消耗较少的能量(与单独热激活PAA比较);相比于单独Cu(Ⅱ)活化PAA(Cu(Ⅱ)/PAA)和热激活PAA(heat/PAA)体系,Cu(Ⅱ)-heat/PAA体系显著加速了DCF的去除。因此,本文拟对该现象进行深入探讨研究,考察该协同效应的作用机理;调查常见水基质(Cl−、SO42−、NO3−、HCO3−、天然有机质(NOM))对Cu(Ⅱ)-heat/PAA体系降解DCF的影响;评估该技术对实际水体中DCF的去除效果。
-
双氯芬酸钠(≥ 99%)、富里酸(FA)和叔丁醇(TBA,≥ 99.5%)购买于上海阿拉丁生化科技有限公司(中国);过氧乙酸(PAA,质量分数15%,H2O2/PAA的摩尔比 = 1.4)、五水合硫代硫酸钠(Na2SO3·5H2O)、五水合硫酸铜(CuSO4·5H2O)、硝酸钾(KNO3)、氢氧化钠(NaOH)、硫酸钠(Na2SO4)、氯化钠(NaCl)和硫酸(H2SO4)购买于成都科隆化学试剂有限公司。甲醇(MeOH,LC/MS级)购买于Fisher公司。除了实际水体,实验用水均使用超纯水(18.2 MΩ·cm)。
-
实验均在烧杯(250 mL)中进行,通过恒温水浴锅控制反应温度,转速定为350 r·min−1。具体步骤如下:首先配制5 μmol·L−1 DCF反应液100 mL,然后加入指定量的Cu(Ⅱ),再加入一定量的5% H2SO4或10 g·L−1 NaOH溶液调节反应体系初始pH值;当溶液达到指定温度后,加入PAA启动反应。在给定时间点,取样1 mL加入到含有0.1 mL 0.2 mol·L−1 Na2SO3溶液的液相小瓶中进行DCF定量分析。在自由基清除实验和水基质实验中,先向反应液加入清除剂(TBA或MeOH)或者常见水基质(Cl−、SO42−、NO3−和NOM),再加入DCF。所有实验至少进行3次重复,图中的数据为带有误差棒的平均值。
-
使用配备光电二极管阵列检测器(Waters 2998)的Waters ACQITY UPLC I-Class测定DCF浓度。固定相为ACQUITY-UPLC-BEH-C18柱(2.1 mm× 50 mm,1.7 μm);流动相为甲醇和0.1 %醋酸的混合物(体积比 61:39);进样量设为10 μL;波长设置为276 nm;柱温30 ℃。通过滴定法[23]定期测量PAA储备液的浓度。使用pH计(PHS-3C,上海雷磁)测量溶液的pH值。
-
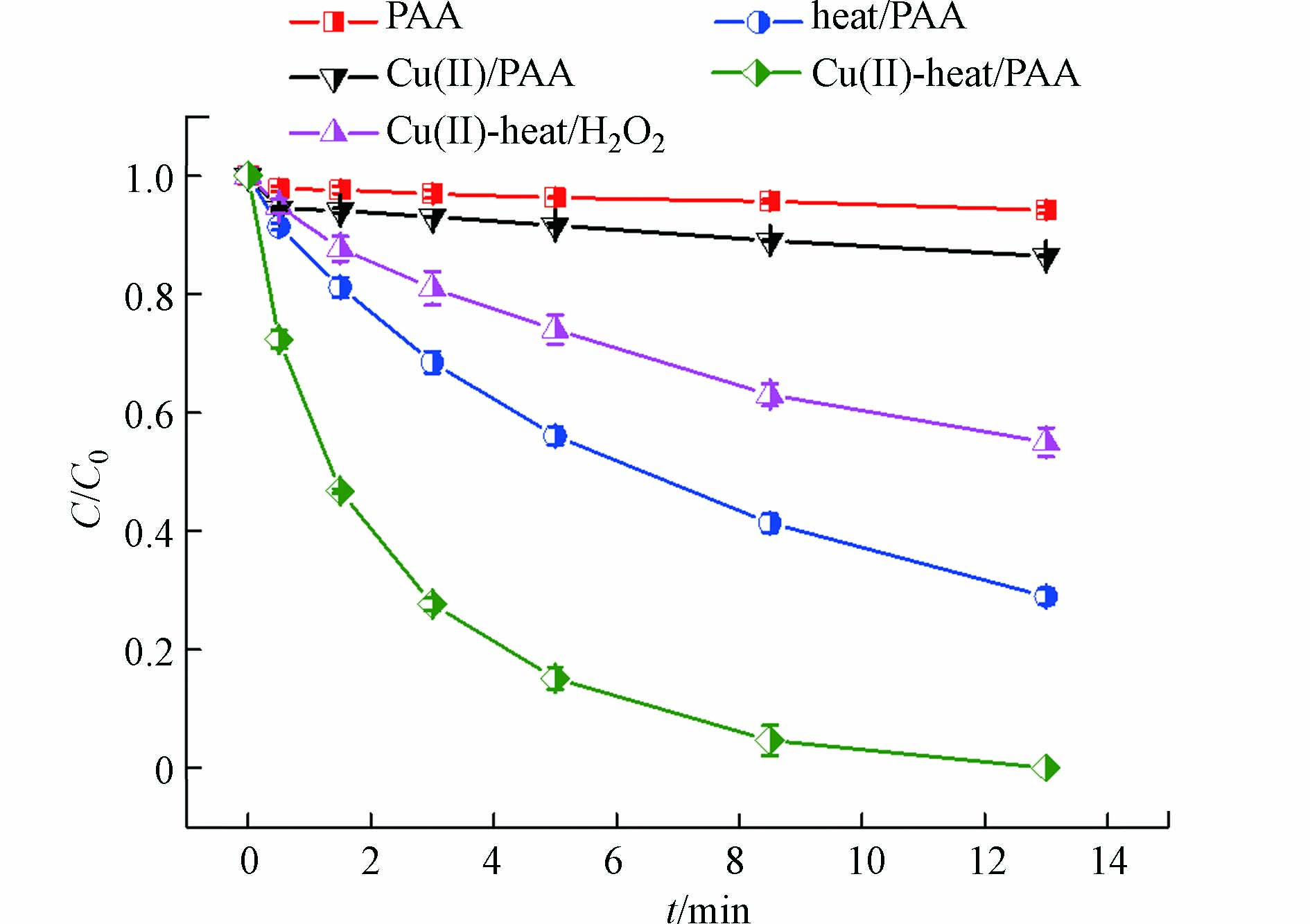
如图1所示,反应13 min后,在单独PAA体系中仅有6% DCF被降解;而在Cu(Ⅱ)/PAA体系中,DCF去除率增加至13%,这说明Cu(Ⅱ)对PAA有一定的活化效果。PAA能被热激活产生活性物质[22],故在60 ℃时,heat/PAA体系对DCF的降解率为72%。而在相同的温度下,Cu(Ⅱ)-heat/PAA体系能够完全消除DCF,远大于heat/PAA体系和Cu(Ⅱ)/PAA体系单独作用,这表明Cu(Ⅱ)和热联用活化PAA存在协同效应。
据报道,在Cu(Ⅱ) 协同热活化H2O2(Cu(Ⅱ)-heat/H2O2)过程中也有类似现象[24-25]。在温度高于35 ℃后,Cu(Ⅱ)-heat/H2O2体系对硝基苯的降解速率快速增加,表观活化能由12.6 kcal·mol−1(20—35 ℃)增加到27.3 kcal·mol−1(35—50 ℃),由此作者认为Cu(Ⅱ)被还原成Cu(I)是Cu(Ⅱ)-heat/H2O2体系降解硝基苯的限速步骤之一。因此,在Cu(Ⅱ)-heat/PAA体系中,Cu(Ⅱ)被PAA还原为Cu(I)也可能是限速步骤之一,体系温度升高能够明显提高该限速反应,从而加速了Cu(Ⅱ)的还原和Cu(I)的生成。Cu(I)能够有效激活PAA产生CH3C(=O)O·和·OH,如式(2)—(4)所示,进而加速了DCF的去除。由于实验使用的PAA溶液是PAA和H2O2的混合物,因此有必要考察Cu(Ⅱ)-heat/PAA体系中H2O2对DCF降解的贡献。由图1可知,在相同条件下Cu(Ⅱ)-heat/H2O2体系对DCF的去除率仅为45%,故H2O2在Cu(Ⅱ)-heat/PAA体系中的贡献较小,主要活性氧化剂仍为PAA。DCF在各个体系中降解符合伪一级反应动力学模型,其表观降解速率常数(kobs)可以通过式(5)计算得出,结果如表2所示。
式中,kobs为表观降解速率常数(min−1);C和C0分别为指定时间和初始时间时DCF的摩尔浓度(μmol·L−1);t为反应时间(min)。
-
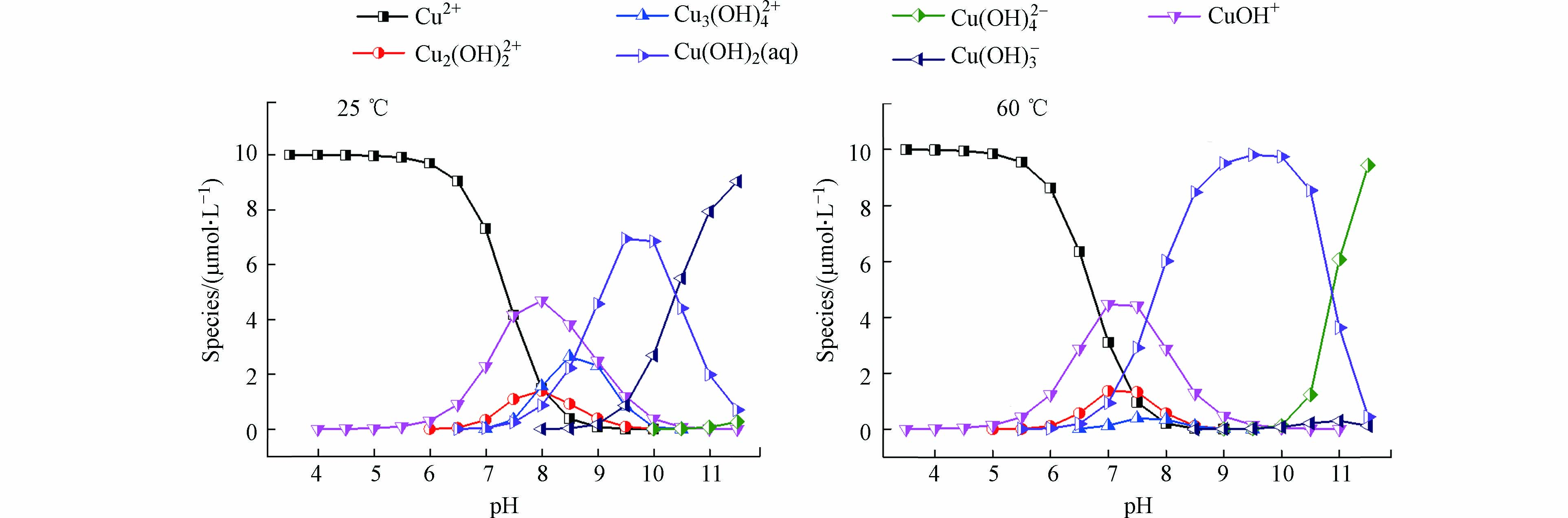
在不同pH或温度下,物质存在的形态可能不同,利用环境水化学平衡软件(Visual MINTEQ 3.0)[26]模拟了25 ℃和60 ℃时不同pH条件下Cu(Ⅱ) 的存在形态,所用参数均为数据库默认值。如图2所示,在pH 8时,从25 ℃升高至60 ℃过程中,虽然Cu(OH)+浓度有所降低,但其仍在Cu(Ⅱ)存在形态中占有较高比例,故Cu(OH)+可能是被PAA还原的Cu(Ⅱ)形态之一。此外,Cu(OH)2 (aq)浓度随温度升高而增大,在60 ℃时已成为Cu(Ⅱ)的主要存在形态,因此Cu(OH)2 (aq)也可能是被PAA还原的Cu(Ⅱ)形态。Millero等[27]分别在pH 6—9和温度5—45 ℃条件下测量了海水中H2O2对Cu(Ⅱ)的还原率,认为Cu(OH)2 (aq)是被H2O2还原的活性物质。这与模拟Cu(OH)2 (aq)是被PAA还原的Cu(Ⅱ)形态结论类似。综上所述,Cu(OH)2 (aq)和Cu(OH)+可能是被PAA还原的活性Cu(Ⅱ)形态。
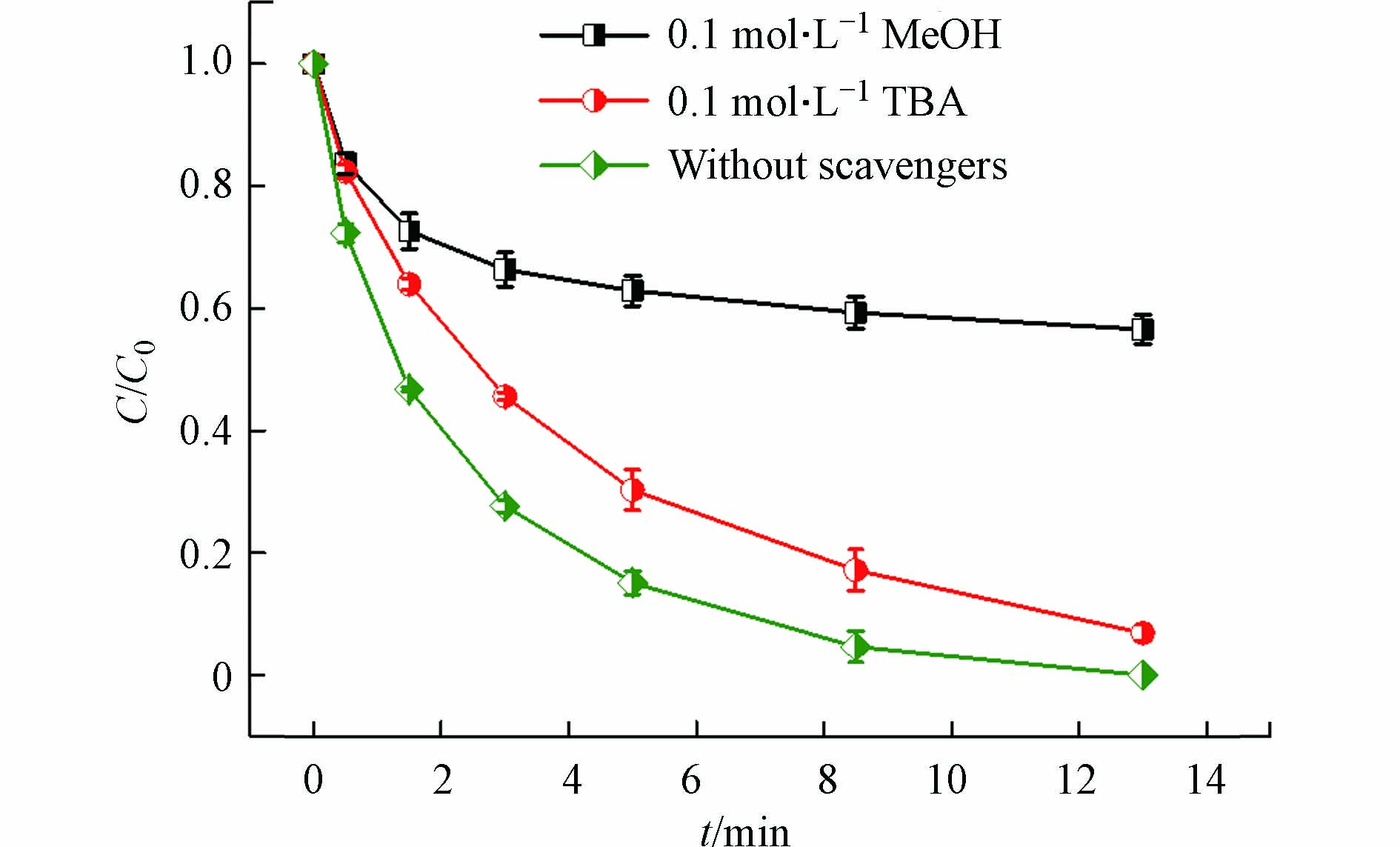
如上所述,热能激活PAA产生CH3C(=O)O·和·OH。此外,温度升高能加速Cu(Ⅱ)/PAA体系中Cu(Ⅱ)的还原和Cu(I)的生成,Cu(I)也能够有效活化PAA产生CH3C(=O)O·和·OH。产生的CH3C(=O)O·和·OH均可与PAA反应生成CH3C(=O)OO·,如式(6)和(7)所示[20]。而CH3C(=O)O·可进一步分解为·CH3和CO2,形成的·CH3可与O2反应生成CH3OO·,如式(8)和(9)所示[18]。因此,在Cu(Ⅱ)-heat/PAA体系中,可能产生·OH和有机自由基R-O·(CH3C(=O)O·、CH3C(=O)OO·、·CH3和CH3OO·)。为了识别该体系中负责DCF降解的活性自由基,分别将TBA和MeOH添加到反应体系中。TBA是一种常用的·OH淬灭剂(7.6×108 L·mol−1·s−1)[28],但不能有效淬灭R-O·[29]。而MeOH不仅可以清除·OH(9.7×108 L·mol−1·s−1)[30],也可以抑制R-O·[31]。如图3所示,当TBA投加到Cu(Ⅱ)-heat/PAA体系中,DCF的降解部分被抑制;然而加入MeOH后,抑制作用明显增强,这表明·OH和R-O·都是Cu(Ⅱ)-heat/PAA体系中的活性物质,且R-O·对DCF去除的作用更大。由于CH3COO·和·CH3氧化性较弱[22],它们对DCF降解的作用可能较小,因此CH3C(=O)O·和CH3C(=O)OO·可能是主要活性的有机自由基。类似的结论在Co2+/PAA体系降解SMX中也有报道[31]。综上,在Cu(Ⅱ)-heat/PAA体系中,·OH和R-O·(CH3C(=O)O·和CH3C(=O)OO·)是主要的活性物质,且R-O·对DCF降解的贡献更大。
-
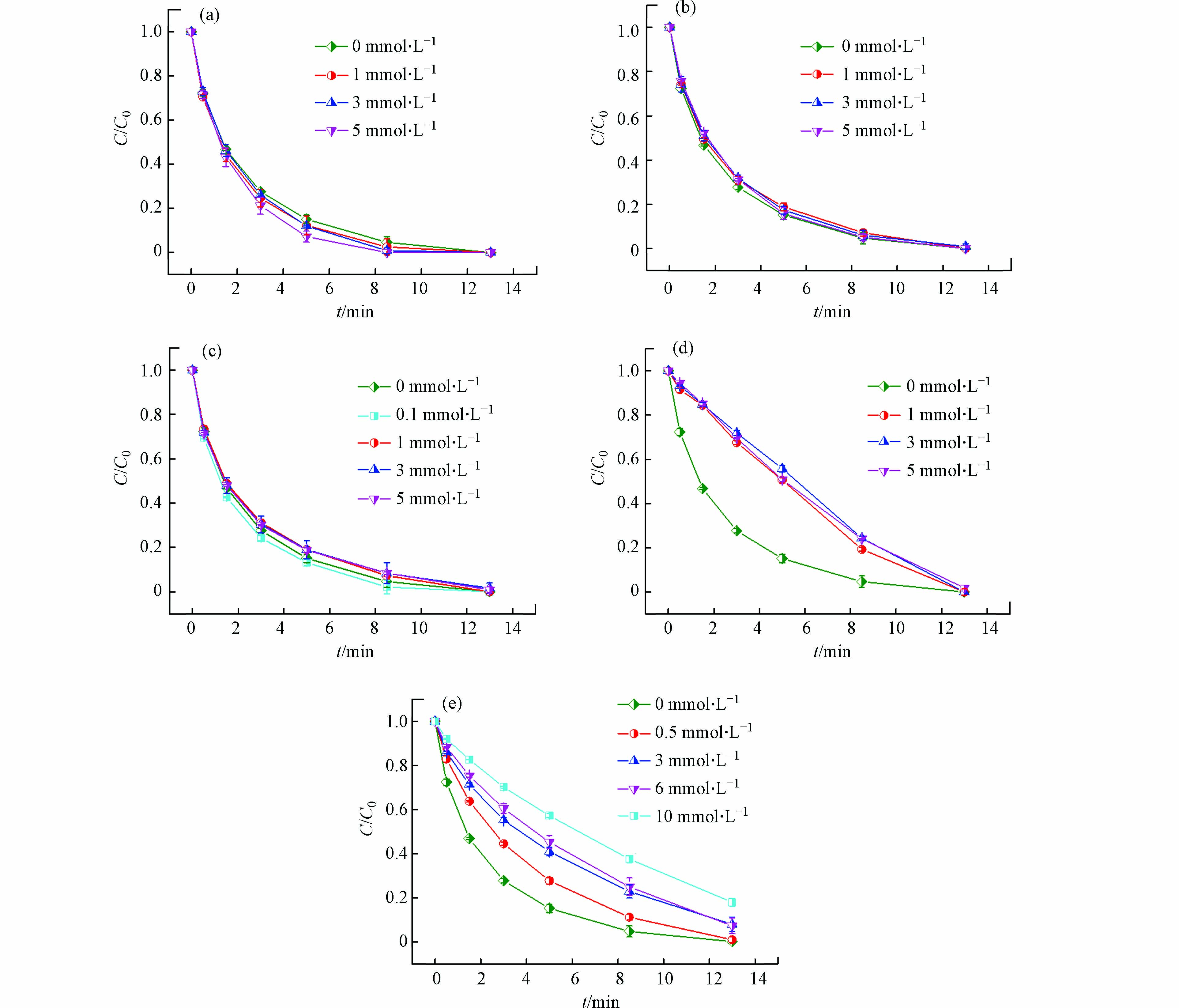
如图4(a)所示,1—5 mmol·L−1 Cl−会微弱促进Cu(Ⅱ)-heat/PAA体系降解DCF。
由于Cl−和Cu(Ⅱ)/Cu(I)能够形成氧化还原电位较高的Cu(Ⅱ)/Cu(I)-Cl络合物,故Cu(Ⅱ)-heat/PAA体系中Cl−的存在可能会促进Cu(Ⅱ)还原为Cu(I)[32]。此外,虽然Cl−能与CH3C(=O)OO·和·OH反应生成氧化性较弱的含氯自由基[18, 33],如式(10)—(13)所示,但是这些含氯自由基(Cl·和Cl2·−)也可能氧化降解DCF [18]。如图4(b)和4(c)所示,SO42− 和NO3−对Cu(Ⅱ)-heat/PAA体系降解DCF几乎没有影响,这可能是因为它们不与体系中的活性物质发生反应[33, 34]。HCO3−是常见的·OH淬灭剂,其可能会抑制·OH氧化降解污染物。如图4(d)所示,HCO3−加入后虽然会减缓DCF的降解速率,但是反应结束后其去除率基本不受影响。这可能是因为HCO3−能够与Cu(Ⅱ)形成配合物,从而提高了Cu(Ⅱ)催化PAA的能力[35],其促进作用可能刚好与HCO3−对·OH的抑制作用相抵消,结果表现出HCO3−不影响DCF的降解率。本研究使用富里酸(FA)代表NOM,考察其对DCF降解的影响,结果如图4(e)所示。NOM的存在抑制了Cu(Ⅱ)-heat/PAA体系对DCF的降解,且NOM浓度越高,抑制作用越显著。NOM能够与DCF竞争反应体系中的活性自由基(·OH和R-O·)[11],从而导致DCF去除率的下降.
-
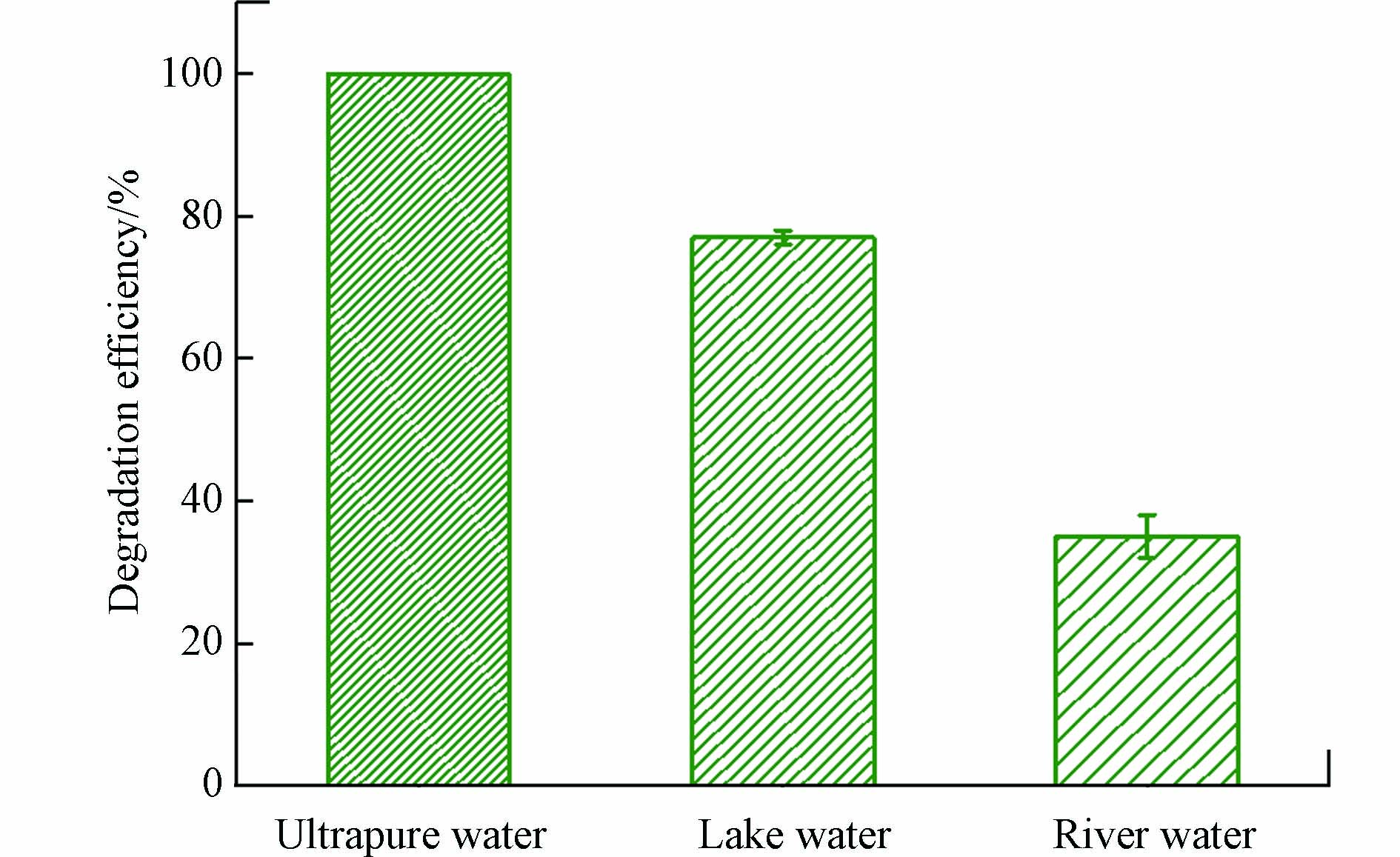
为了评估Cu(Ⅱ)-heat/PAA体系在实际运用中对有机污染物的去除能力,考察了其对实际水体中DCF的降解,结果如图5所示。与超纯水相比,DCF在湖水和河水中均受到抑制。两种实际水体的水质参数见先前的报道[36]。湖水和河水的CODcr、UV254分别为20.8 mg·L−1、0.0365和22.4 mg·L−1、0.0672,表明两种实际水体中都含有一定量的NOM,而NOM会抑制Cu(Ⅱ)-heat/PAA体系降解DCF,造成DCF在两种实际水体中的降解均受到抑制。由于河水中的NOM浓度高于湖水,故DCF在河水中的降解效率低于其在湖水中的去除。
-
(1) Cu(Ⅱ)-heat/PAA体系降解DCF明显高于heat/PAA和Cu(Ⅱ)/PAA体系,表明Cu(Ⅱ)和热联用活化PAA存在协同效应,这主要是由于温度升高能够加快体系中Cu(Ⅱ)向Cu(I)的还原。
(2) 在pH 8时,通过自由基清除实验证明·OH和R-O·(CH3C(=O)O·和CH3C(=O)OO·)是Cu(Ⅱ)-heat/PAA体系降解DCF的主要活性物质,且R-O·对DCF去除的贡献更大。
(3) 由于Cl−与Cu(Ⅱ)/Cu(I)可能形成氧化还原电位较高的Cu(Ⅱ)/Cu(I)-Cl络合物,从而微弱促进了DCF的降解;SO42−、NO3−和HCO3−对DCF的去除几乎没有影响;而NOM抑制DCF降解,导致DCF在河水和湖水中的降解受到抑制。
Cu(II)协同热活化过氧乙酸降解水中双氯芬酸
Degradation of diclofenac in water by Cu(II)-combined with heat activation of peracetic acid
-
摘要: 采用Cu(Ⅱ)协同热活化过氧乙酸(Cu(Ⅱ)-heat/PAA)降解水中双氯芬酸(DCF),识别了Cu(Ⅱ)-heat/PAA体系中的主要活性物质;考察了常见水基质(Cl−、SO42−、NO3−、HCO3−、天然有机质(NOM))对DCF去除的影响;探讨了该体系对天然水体中DCF的去除。结果表明,Cu(Ⅱ)-heat/PAA体系去除DCF的效率明显高于Cu(Ⅱ)/PAA和heat/PAA体系,表明热与Cu(II)两者结合对PAA的活化具有协同作用。在pH 8时,羟基自由基(·OH)和有机自由基R-O·(CH3C(=O)O·和CH3C(=O)OO·)是Cu(Ⅱ)-heat/PAA体系去除DCF的主要活性物质,且R-O·对DCF去除的作用更大。在Cu(Ⅱ)-heat/PAA 体系中,SO42−、NO3−和HCO3−对DCF的去除几乎没有影响;Cl−对DCF去除有微弱的促进效果;而NOM抑制DCF的降解,导致DCF在湖水和河水中的去除受到抑制。Abstract: Removal of diclofenac (DCF) in water by Cu(Ⅱ)-combined with heat activation peracetic acid (Cu(Ⅱ)-heat/PAA) was investigated, and the main active species in Cu(Ⅱ)-heat/PAA system were identified. The effects of common water matrix (Cl−, SO42−, NO3−, HCO3− and natural organic matter (NOM)) on the removal of DCF were explored and the degradation of DCF in natural water was studied. As a result, the removal efficiency of DCF in Cu(Ⅱ)-heat/PAA process presented a relatively faster rate than those in Cu(Ⅱ)/PAA and heat/PAA systems, indicating that the combination of heat and Cu(Ⅱ) had a synergistic effect on the activation of PAA. At pH 8, hydroxyl radical (·OH) and organic radicals (R-O·, CH3C(=O)O· and CH3C(=O)OO·) were the main active substances for DCF removal in Cu(Ⅱ)-heat/PAA system and R-O· played a major role in DCF removal. Presence of SO42−, NO3− and HCO3− had little effect on DCF degradation; Cl− could weakly promote the degradation of DCF; NOM inhibited DCF removal, which resulted in the inhibition on DCF removal in lake water and river water.
-
Key words:
- diclofenac /
- peracetic acid /
- Cu(II) /
- heat /
- organic radicals /
- advanced oxidation process
-

-
表 1 DCF的理化性质
Table 1. Physicochemical properties of DCF
分子式
Formula溶解度
Solubility分子量
Molecular weight结构式
StructureC14H11Cl2NO2 2.37 mg·L−1(25 ℃) 296.16 
表 2 不同体系的kobs和R2值
Table 2. kobs and R2 in different systems
体系 PAA Cu(Ⅱ)/PAA Cu(Ⅱ)-heat/H2O2 heat/PAA Cu(Ⅱ)-heat/PAA kobs /min−1 0.004 0.008 0.054 0.094 0.394 R2 0.910 0.945 0.990 0.990 0.996 -
[1] KOSTICH M S, BATT A L, LAZORCHAK J M. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation [J]. Environmental Pollution, 2014, 184: 354-359. doi: 10.1016/j.envpol.2013.09.013 [2] EBELE A J, ABOU-ELWAFA ABDALLAH M, HARRAD S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment [J]. Emerging Contaminants, 2017, 3(1): 1-16. doi: 10.1016/j.emcon.2016.12.004 [3] YANG Y, OK Y S, KIM K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review [J]. Science of the Total Environment, 2017, 596/597: 303-320. doi: 10.1016/j.scitotenv.2017.04.102 [4] 王鸿斌, 王群, 刘义青, 等. 亚铁活化过硫酸盐降解水中双氯芬酸钠 [J]. 环境化学, 2020, 39(4): 869-875. doi: 10.7524/j.issn.0254-6108.2019040806 WANG H B, WANG Q, LIU Y Q, et al. Degradation of diclofenac by ferrous activated persulfate [J]. Environmental Chemistry, 2020, 39(4): 869-875(in Chinese). doi: 10.7524/j.issn.0254-6108.2019040806
[5] LONAPPAN L, BRAR S K, DAS R K, et al. Diclofenac and its transformation products: Environmental occurrence and toxicity - A review [J]. Environment International, 2016, 96: 127-138. doi: 10.1016/j.envint.2016.09.014 [6] CHEN W P, XU J, LU S D, et al. Fates and transport of PPCPs in soil receiving reclaimed water irrigation [J]. Chemosphere, 2013, 93(10): 2621-2630. doi: 10.1016/j.chemosphere.2013.09.088 [7] 张楠, 刘国光, 刘海津, 等. 双氯芬酸在水环境中光降解的初步研究 [J]. 环境化学, 2013, 32(1): 42-47. doi: 10.7524/j.issn.0254-6108.2013.01.007 ZHANG N, LIU G G, LIU H J, et al. Photodegration mechanism of diclofenac in aqueous environment [J]. Environmental Chemistry, 2013, 32(1): 42-47(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.01.007
[8] 谷得明, 郭昌胜, 冯启言, 等. 基于硫酸根自由基的高级氧化技术及其在环境治理中的应用 [J]. 环境化学, 2018, 37(11): 2489-2508. doi: 10.7524/j.issn.0254-6108.2018012102 GU D M, GUO C S, FENG Q Y, et al. Sulfate radical-based advanced oxidation processes and its application in environmental remediation [J]. Environmental Chemistry, 2018, 37(11): 2489-2508(in Chinese). doi: 10.7524/j.issn.0254-6108.2018012102
[9] 刘萌, 胡莉敏, 张广山, 等. Co/Zn双金属氧化物活化过一硫酸盐降解双酚A的性能研究 [J]. 环境化学, 2018, 37(4): 753-760. doi: 10.7524/j.issn.0254-6108.2017081605 LIU M, HU L M, ZHANG G S, et al. Activation of peroxymonosulfate by the Co/Zn bimetallic oxide for the degradation of bisphenol A [J]. Environmental Chemistry, 2018, 37(4): 753-760(in Chinese). doi: 10.7524/j.issn.0254-6108.2017081605
[10] SUN P Z, ZHANG T Q, MEJIA-TICKNER B, et al. Rapid disinfection by peracetic acid combined with UV irradiation [J]. Environmental Science & Technology Letters, 2018, 5(6): 400-404. [11] WANG Z R, SHI H L, WANG S X, et al. Degradation of diclofenac by Fe(II)-activated peracetic acid [J]. Environmental Technology, 2021, 42(27): 4333-4341. doi: 10.1080/09593330.2020.1756926 [12] ZHANG L, FU Y S, WANG Z R, et al. Removal of diclofenac in water using peracetic acid activated by zero valent copper [J]. Separation and Purification Technology, 2021, 276: 119319. doi: 10.1016/j.seppur.2021.119319 [13] KITIS M. Disinfection of wastewater with peracetic acid: A review [J]. Environment International, 2004, 30(1): 47-55. doi: 10.1016/S0160-4120(03)00147-8 [14] KOIVUNEN J, HEINONEN-TANSKI H. Peracetic acid (PAA) disinfection of primary, secondary and tertiary treated municipal wastewaters [J]. Water Research, 2005, 39(18): 4445-4453. doi: 10.1016/j.watres.2005.08.016 [15] LUUKKONEN T, HEYNINCK T, RÄMÖ J, et al. Comparison of organic peracids in wastewater treatment: Disinfection, oxidation and corrosion [J]. Water Research, 2015, 85: 275-285. doi: 10.1016/j.watres.2015.08.037 [16] BIANCHINI R, CALUCCI L, LUBELLO C, et al. Intermediate free radicals in the oxidation of wastewaters [J]. Research on Chemical Intermediates, 2002, 28(2/3): 247-256. [17] da SILVA W P, CARLOS T D, CAVALLINI G S, et al. Peracetic acid: Structural elucidation for applications in wastewater treatment [J]. Water Research, 2020, 168: 115143. doi: 10.1016/j.watres.2019.115143 [18] CHEN S A, CAI M Q, LIU Y Z, et al. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process [J]. Water Research, 2019, 150: 153-161. doi: 10.1016/j.watres.2018.11.044 [19] ROTHBART S, EMBER E E, van ELDIK R. Mechanistic studies on the oxidative degradation of Orange II by peracetic acid catalyzed by simple manganese(ii) salts. Tuning the lifetime of the catalyst [J]. New J Chem, 2012, 36(3): 732-748. doi: 10.1039/C2NJ20852K [20] KIM J, ZHANG T Q, LIU W, et al. Advanced oxidation process with peracetic acid and Fe(II) for contaminant degradation [J]. Environmental Science & Technology, 2019, 53(22): 13312-13322. [21] KIM J, DU P H, LIU W, et al. Cobalt/peracetic acid: Advanced oxidation of aromatic organic compounds by acetylperoxyl radicals [J]. Environmental Science & Technology, 2020, 54(8): 5268-5278. [22] WANG J W, WAN Y, DING J Q, et al. Thermal activation of peracetic acid in aquatic solution: The mechanism and application to degrade sulfamethoxazole [J]. Environmental Science & Technology, 2020, 54(22): 14635-14645. [23] ZHANG K J, ZHOU X Y, DU P H, et al. Oxidation of β-lactam antibiotics by peracetic acid: Reaction kinetics, product and pathway evaluation [J]. Water Research, 2017, 123: 153-161. doi: 10.1016/j.watres.2017.06.057 [24] IBOUKHOULEF H, AMRANE A, KADI H. Removal of phenolic compounds from olive mill wastewater by a Fenton-like system H2O2/Cu(II)—thermodynamic and kinetic modeling [J]. Desalination and Water Treatment, 2016, 57(4): 1874-1879. doi: 10.1080/19443994.2014.978385 [25] NICHELA D A, BERKOVIC A M, COSTANTE M R, et al. Nitrobenzene degradation in Fenton-like systems using Cu(Ⅱ) as catalyst. Comparison between Cu(Ⅱ)- and Fe(Ⅲ)-based systems [J]. Chemical Engineering Journal, 2013, 228: 1148-1157. doi: 10.1016/j.cej.2013.05.002 [26] WANG H B, DENG J W, LU X H, et al. Rapid and continuous degradation of diclofenac by Fe(Ⅱ)-activated persulfate combined with bisulfite [J]. Separation and Purification Technology, 2021, 262: 118335. doi: 10.1016/j.seppur.2021.118335 [27] MILLERO F J, SHARMA V K, KARN B. The rate of reduction of copper(Ⅱ) with hydrogen peroxide in seawater [J]. Marine Chemistry, 1991, 36(1/2/3/4): 71-83. [28] LIU Y Q, HE X X, DUAN X D, et al. Significant role of UV and carbonate radical on the degradation of oxytetracycline in UV-AOPs: Kinetics and mechanism [J]. Water Research, 2016, 95: 195-204. doi: 10.1016/j.watres.2016.03.011 [29] CAI M Q, SUN P Z, ZHANG L Q, et al. UV/peracetic acid for degradation of pharmaceuticals and reactive species evaluation [J]. Environmental Science & Technology, 2017, 51(24): 14217-14224. [30] WANG H B, WANG S X, LIU Y Q, et al. Degradation of diclofenac by Fe(Ⅱ)-activated bisulfite: Kinetics, mechanism and transformation products [J]. Chemosphere, 2019, 237: 124518. doi: 10.1016/j.chemosphere.2019.124518 [31] WANG Z P, WANG J W, XIONG B, et al. Application of cobalt/peracetic acid to degrade sulfamethoxazole at neutral condition: Efficiency and mechanisms [J]. Environmental Science & Technology, 2020, 54(1): 464-475. [32] HUANG Y, JIANG Q J, YU X B, et al. A combined radical and non-radical oxidation processes for efficient degradation of Acid Orange 7 in the homogeneous Cu(II)/PMS system: Important role of chloride [J]. Environmental Science and Pollution Research International, 2021, 28(37): 51251-51264. doi: 10.1007/s11356-021-14262-1 [33] LIU Y Q, HE X X, FU Y S, et al. Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes [J]. Chemical Engineering Journal, 2016, 284: 1317-1327. doi: 10.1016/j.cej.2015.09.034 [34] WANG S X, WANG H B, LIU Y Q, et al. Effective degradation of sulfamethoxazole with Fe2+-zeolite/peracetic acid [J]. Separation and Purification Technology, 2020, 233: 115973. doi: 10.1016/j.seppur.2019.115973 [35] WANG Z R, FU Y S, PENG Y L, et al. HCO3-/CO32- enhanced degradation of diclofenac by Cu(Ⅱ)-activated peracetic acid: Efficiency and mechanism [J]. Separation and Purification Technology, 2021, 277: 119434. doi: 10.1016/j.seppur.2021.119434 [36] DENG J W, WANG H B, FU Y S, et al. Phosphate-induced activation of peracetic acid for diclofenac degradation: Kinetics, influence factors and mechanism [J]. Chemosphere, 2022, 287: 132396. doi: 10.1016/j.chemosphere.2021.132396 -



 下载:
下载: