-
近年来,基于
${\rm{SO}}_4^ - \cdot $ (E0=2.5~3.1 V)的高级氧化技术(sulfate radical based advanced oxidation processes, SR-AOPs)由于其具有氧化性强、使用范围广等优点越来越广泛的被应用于污水和地下水的处理[1]。特别是由于过硫酸盐稳定性强、溶解性好和不易挥发,而且具有适用pH范围广和产生的${\rm{SO}}_4^ - \cdot $ 的比HO·寿命长等优点,更有利于降解高浓度有机污染物[2]。氯离子(Cl−)是影响污水和地下水处理的最重要的影响因素之一。水体中含有大量的盐类(主要是NaCl),其中湖水<1 000 mg·L−1、苦咸水为1 000 ~10 000 mg·L−1和海水为10 000~35 000 mg·L−1[3]。同时,工厂的生产废水中也含有大量Cl−,例如在食品加工厂、石油精炼工艺厂、反渗透工艺出水中均会含有大量的Cl−[3],故在实际水处理中需要考虑Cl−的影响。已有研究[4]表明,Cl−对SR-AOPs有较大的影响,Cl−作为自由基的淬灭剂和金属络合剂可能会影响氧化反应的途径、动力学和反应效率[5]。此外,Cl−能够与过硫酸盐氧化过程中产生的活性物质发生链式反应[6]。在修复沿海含水层和各种受污染的水体时,经常会遇到高浓度Cl−的问题。因此,在应用SR-AOPs氧化处理实际废水中有机物时,需要考虑Cl−的影响。关于Cl−对SR-AOPs氧化过程的影响,目前已有部分研究报道。CHEN等[7]通过热活化过硫酸钠(PS)对对羟基苯甲酸甲酯(MeP)和羟苯乙酯(EtP)进行降解,结果表明,加入10 mmol·L−1的Cl−后,MeP和EtP的降解率分别下降了22.8%和26.0%。MA等[8]通过对热活化PS氧化苯酚时发现,当加入1、10和100 mmol·L−1的Cl−后,苯酚的去除率基本没有发生变化。PENG等[9]利用硫化铜活化PS降解农药阿特拉津(ATZ)时发现,当分别加入4、8和20 mmol·L−1 Cl−后,对ATZ的去除率有较弱的提升,其中加入20 mmol·L−1的Cl−后,ATZ去除率仅提高了约10%。目前关于Cl−对PS氧化过程影响的研究主要考察了Cl−浓度对目标有机物去除率的影响,而对相关的反应机理和反应过程中生成的活性氧化物质尚未有深入的研究。
苯胺是重要的有机化工原料和精细化工中间体,广泛应用于染料、农药、医药、军工、香料和橡胶硫化等行业[10]。苯胺类化合物有毒,有致癌、致畸、致突变“三致”作用,如不经有效处理就直接排放,将会对周围环境产生严重污染[10]。基于此,本研究选择苯胺(AN)作为目标污染物,通过Fe2+活化PS氧化体系去除AN,对不同pH下Cl−对AN去除的影响和作用机理进行了研究,并通过添加淬灭剂对反应过程中的活性物质进行识别,通过对总有机碳(TOC)的测定探究其矿化效果,利用GC-MS对反应过程的中间产物进行了鉴定并依此推断出其可能的反应路径。
全文HTML
-
过硫酸钠(≥99%)、氢氧化钠(≥99%)和硫酸(95%~98%)均购于天津光复精细化工研究所。氯化钠(≥99.8%)和七水硫酸亚铁(FeSO4·7H2O,98%)购于天津市大茂化学试剂厂。苯胺购于上海TCI试剂公司。甲醇(MeOH,色谱纯)和叔丁醇(TBA,色谱纯)购自Fisher公司。硫酸铵[(NH4)2·SO4]、N, N -二乙基-1,4-苯二胺(DPD)和对苯醌(BQ)均为分析纯购于国药集团化学试剂有限公司。所用超纯水电导率为18.2 Ω·cm−1。
-
所有实验均在50 mL棕色玻璃瓶中进行,通过恒温磁力搅拌装置控制搅拌速度600 r·min−1和反应温度25 ℃。在棕色玻璃瓶加入0.1 mmol·L−1的AN溶液和一定浓度的PS溶液。通过使用10 mmol·L−1的H2SO4或NaOH溶液将其调节至所需的pH。然后加入一定浓度的FeSO4·7H2O溶液,反应过程中不对溶液pH进行调节。在一定的时间间隔下取1 mL样品,用MeOH终止反应,然后用0.45 µm滤膜过滤后放入液相小瓶中分析。为了进一步研究Cl−对Fe2+活化PS过程的反应活性物质,使用MeOH、TBA和BQ作为自由基淬灭剂。MeOH与
${\rm{SO}}_4^ - \cdot $ ((1.6~7.7)×107 L·(mol·s)−1)和HO·((1.2~2.8)×109 L·(mol·s)−1)反应速率较快,TBA与HO·反应很快((3.8~7.6)×108 L·(mol·s)−1),但与${\rm{SO}}_4^ - \cdot $ ((4.0~9.1)×105 L·(mol·s)−1)反应速率较慢[11]。因此,MeOH可以同时清除${\rm{SO}}_4^ - \cdot $ 和HO·,而TBA可选择性地清除HO·,利用这2种自由基淬灭剂对自由基的选择性不同,可识别溶液中生成的${\rm{SO}}_4^ - \cdot $ 和HO·。BQ可以用做超氧自由基${\rm{O}}_2^ - \cdot $ ((0.9~1)×109 L·(mol·s)−1)的淬灭剂[12]。所有实验至少进行2次,取平均值计算去除率。 -
AN浓度的测定均采用高效液相色谱(LC-2010型,日本岛津)直接进样的方式测定,反相C18柱(250 mm×4.6 mm),流动相为乙腈/缓冲盐((v∶v) =65∶35),柱温为25 ℃,流速为1.0 mL·min−1,紫外检测波长为280 nm。溶液pH采用pHS-3C型pH计(上海雷磁)测定;总有机碳(TOC)的测定采用TOC-L型TOC分析仪(日本岛津)进行测定;对于游离氯的测定,采用DPD与游离氯反应生成红色化合物,用分光光度法测量其吸光度的方法测定其浓度;苯胺中间产物的测定采用三重四级杆气质联用仪(GC-MS-TQ8040)测定中间产物,以高纯氦气为载气,进样口温度280 ℃,分流比50∶1,色谱柱为Rtx-5MS毛细管柱,柱流量为1.0 mL·min−1(恒流模式),线速度36.1 cm·s−1,吹扫流量为5.0 mL·min−1,柱温箱升温程序为初始柱温40 ℃,保持1 min,以10 ℃·min−1的速率升至280 ℃,保持5.0 min,进样体积1.0 μL,分流比10∶1。MS条件:离子源为电子轰击电离源(EI),电子能量为70 eV,离子源温度230 ℃,接口温度250 ℃,采集模式为Q3 扫描模式,扫描范围为35~450,扫描速率为0.25 s·次−1。
1.1. 材料和试剂
1.2. 实验装置和实验方法
1.3. 分析方法
-
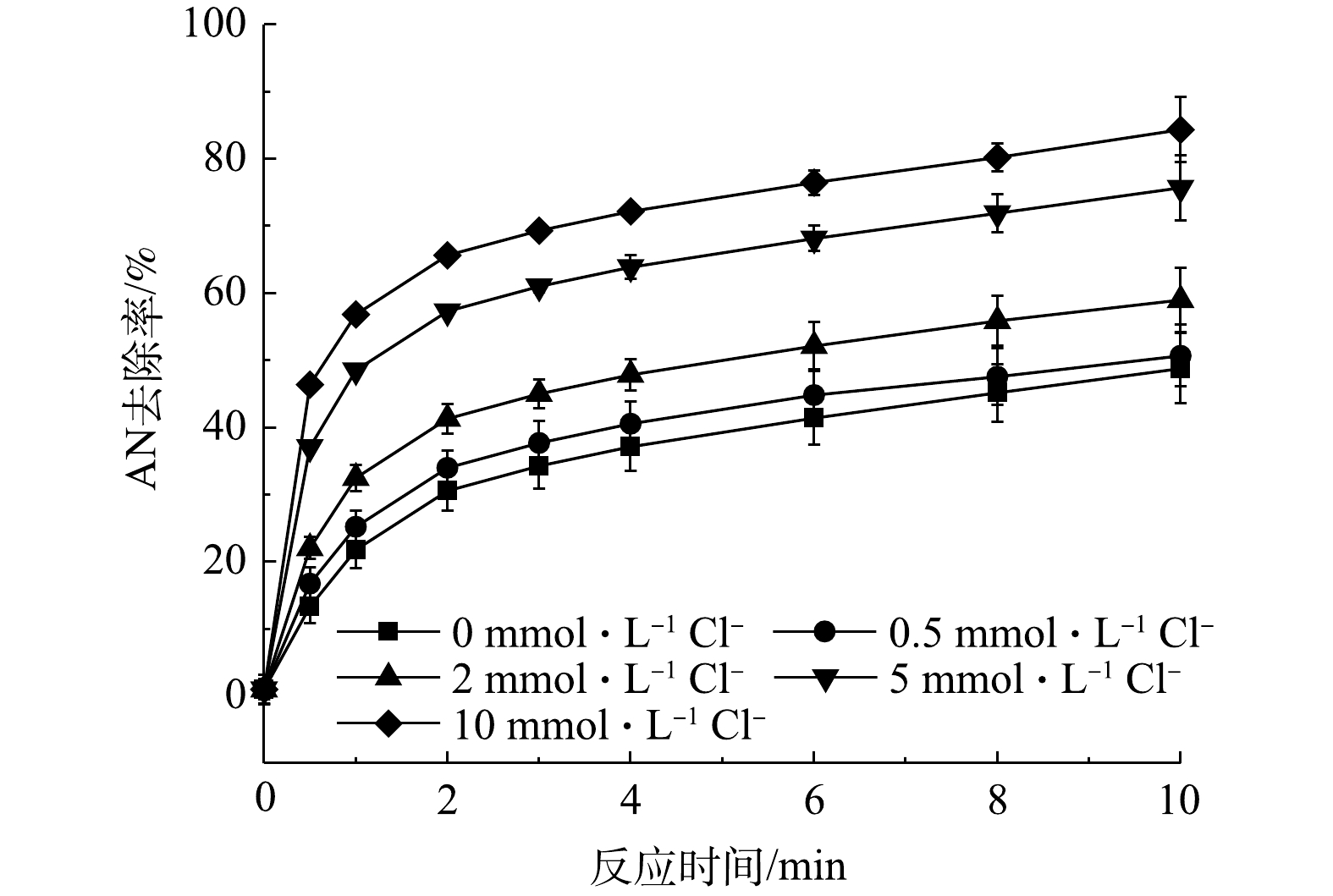
当AN浓度为0.1 mmol·L−1,Fe2+和PS浓度分别为1 mmol·L−1和8 mmol·L−1时,不同Cl−浓度对Fe2+/PS氧化AN的影响见图1,反应过程中未对pH调节(反应初始pH=2.5)。由图1可知,当Cl−浓度分别为0.5、2、5和10 mmol·L−1时,AN的去除率逐渐升高。当Cl−浓度为10 mmol·L−1时,反应10 min后AN的去除率由48.77%升高至84.36%。Cl−可以与
${\rm{SO}}_4^ - \cdot $ 和HO·(式(1)~式(2))发生反应生成氯自由基(Cl·、$ {\rm{Cl}}_2^ - \cdot $ 和HOCl−·)(式(3)~式(11))和游离氯(HClO、Cl2和ClO−)(式(12)~式(15))[13]。因此,在Fe2+/PS氧化AN的过程中加入Cl−后可能会形成3类氧化活性物质:${\rm{SO}}_4^ - \cdot $ 和HO·;Cl·,$ {\rm{Cl}}_2^ - \cdot $ 以及其他氯自由基;ClO−、HClO和Cl2。已有研究表明,Cl·、$ {\rm{Cl}}_2^ - \cdot $ 以及其他氯自由基的氧化活性相比${\rm{SO}}_4^ - \cdot $ 和HO·更弱[14],所以对AN去除的促进作用不是由于Cl−与${\rm{SO}}_4^ - \cdot $ 或HO·反应生成氯自由基造成的。由式(10)~(11)知,Cl−可以在不消耗${\rm{SO}}_4^ - \cdot $ 和HO·情况下生成HClO或ClO−,而HClO和ClO−能够对有机物有效去除。由式(15)可知,在酸性条件下游离氯主要以HClO形式存在。因此,Cl−之所以可以促进PS对AN的去除,可能是因为生成了HClO。YUAN等曾研究Cl−对Co2+活化过一硫酸盐(PMS)降解偶氮染料酸性橙7(AO7)的影响,发现Cl−可能会与PMS反应生成HClO,使得AO7的降解速率大大提高[15]。LEI等[16]同样发现,利用PMS氧化亚甲蓝(MB)时,当加入50 mmol·L−1 Cl−后MB的反应速率提高了66倍,这可能是由于Cl−与PMS反应生成HClO或Cl2所致。 -
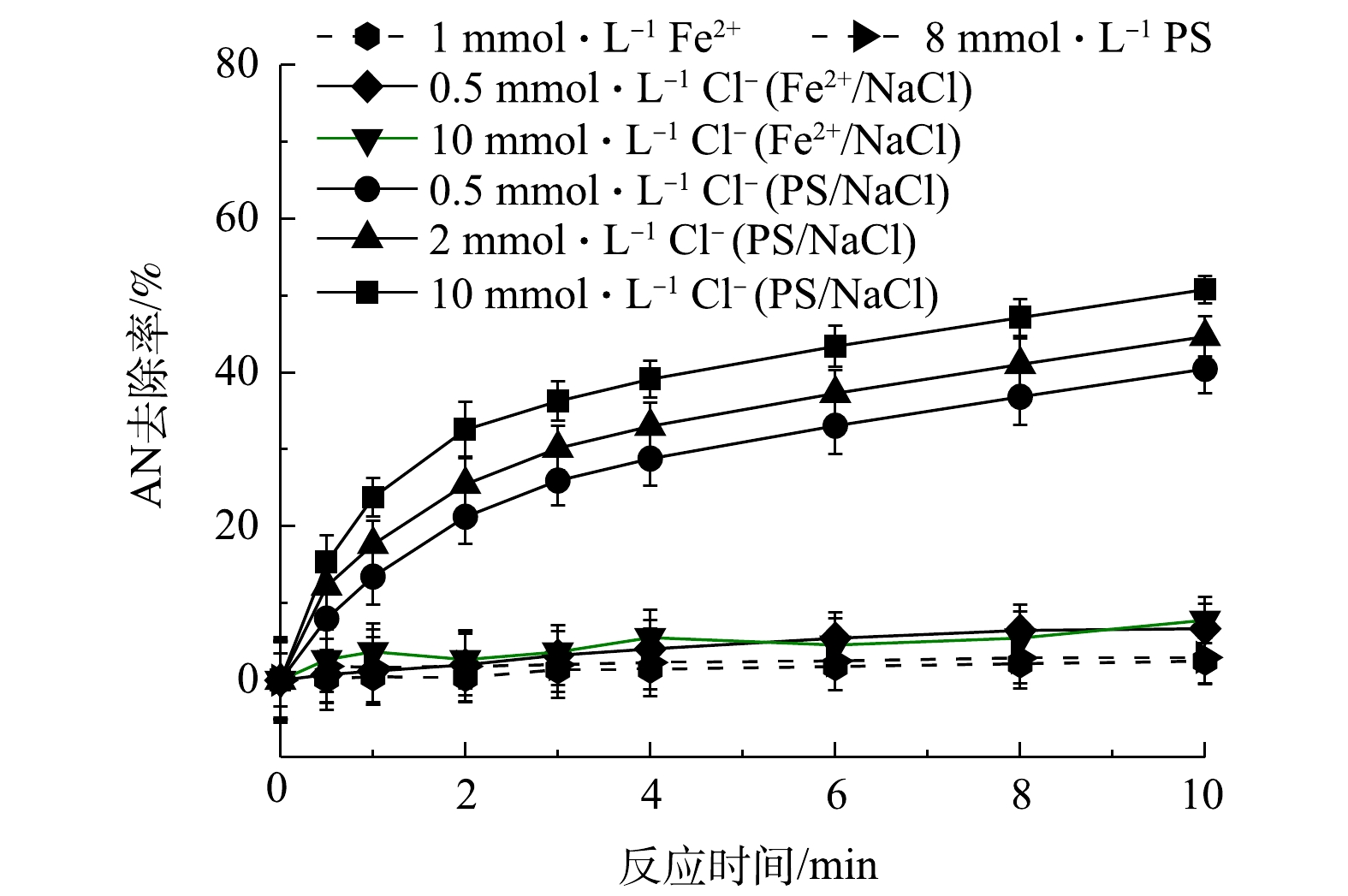
1) Fe2+/NaCl和PS/NaCl对AN去除率的影响。为了验证Cl−加入Fe2+/PS过程中生成了HClO,分别考察了在Fe2+/NaCl和PS/NaCl体系中AN的去除率变化情况,结果见图2。由图2可知,当只有PS或Fe2+存在时,AN基本不发生去除,可见在常温下PS非常稳定,不能对有机物进行氧化。TSITONAKI等[17]的研究表明,常温下PS与有机物的反应速率较低,且Fe2+和NaCl同时存在对AN的去除也基本没有影响。而当只有NaCl和PS同时存在时,可以促进AN的去除,且随着Cl−浓度的升高,AN的去除率也逐渐提高,当AN浓度为0.1 mmol·L−1、PS浓度为8 mmol·L−1、Cl−浓度为0、0.5、2和10 mmol·L−1时,反应10 min后AN的去除率由2.95%上升至50.77%。以上结果表明,Cl−促进Fe2+/PS对AN的去除是由于Cl−与PS反应生成了氧化活性物质。
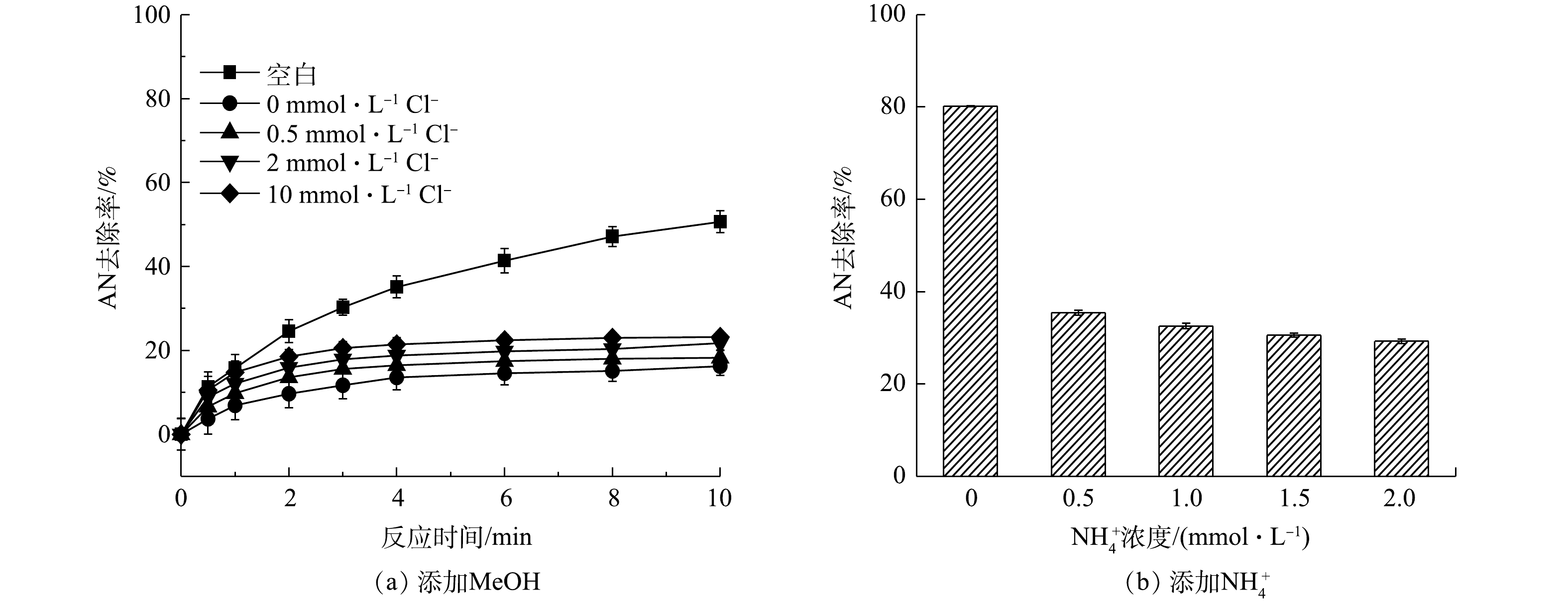
2)活性物质的淬灭实验。在Fe2+/PS/NaCl反应过程中添加自由基淬灭剂MeOH,过量的MeOH会淬灭反应过程中生成的
${\rm{SO}}_4^ - \cdot $ 和HO·。分别添加0.1 mmol·L−1AN,1 mmol·L−1 Fe2+和8 mmol·L−1 PS,然后加入0.5、2和10 mmol·L−1 NaCl,结果如图3所示。其中空白表示只添加Fe2+和PS。由图3(a)可知,当加入MeOH后,Fe2+/PS体系对AN的去除率迅速降低,反应10 min后AN去除率由50.65%降低至16.24%,可见Fe2+/PS体系中起主要作用的是${\rm{SO}}_4^ - \cdot $ 和HO·,这与CHEN等[18]的研究结果一致。CHEN等在利用Fe2+活化PS氧化去除2,4-二氯苯氧乙酸(2,4-D)时同样发现,2,4-D的降解主要是${\rm{SO}}_4^ - \cdot $ 和HO·的作用。但由图3(a)也可以看出,在Fe2+/PS体系中加入MeOH和Cl−后,AN的去除率逐渐升高,且随着Cl−浓度的增大而升高,这表明Fe2+/PS/NaCl体系中不仅生成了${\rm{SO}}_4^ - \cdot $ 和HO·,同时生成了非自由基类的氧化物(如HClO)。CHOI等[19]利用水动力空化活化过硫酸盐降解双酚A(BPA)时发现,Cl−的加入可以促进PS降解BPA,这可能是反应过程中Cl−与PS反应生成的氯自由基通过促进PS的链式反应生成更多的${\rm{SO}}_4^ - \cdot $ 。由于本研究中加入MeOH后Cl−仍能够促进AN的去除,显然并不是${\rm{SO}}_4^ - \cdot $ 作用的结果。在Fe2+/PS/NaCl中加入${\rm{NH}}_4^ + $ (可用作HClO的淬灭剂[20]),由于${\rm{NH}}_4^ + $ 可以与HClO发生反应生成氯胺,而氯胺的氧化活性弱于HClO。当反应过程中加入1 mmol·L−1 Fe2+,8 mmol·L−1 PS和10 mmol·L−1的NaCl,并添加不同浓度的${\rm{NH}}_4^ + $ ,反应8 min后结果见图3(b)。由图3(b)可知,当加入不同浓度的${\rm{NH}}_4^ + $ 后,AN的去除率降低。以上结果表明,在Fe2+/PS体系中加入Cl−可促进AN的去除,这是由于Cl−与PS在反应过程中生成了HClO,有利于AN的去除。3)反应过程中游离氯的测定。为了进一步验证Cl−与PS反应过程中生成的活性物质是HClO,对反应过程中的游离氯(包括Cl2、HClO、ClO−)的生成量(以Cl2计)进行测定,在8 mmol·L−1 PS中添加浓度为0.5、2、5、10 mmol·L−1的Cl−和0.1 mmol·L−1的AN(未添加Fe2+),反应后游离氯浓度如图4所示。由图4可以看出,随着Cl−浓度升高,游离氯含量也逐渐增多,这直接证明Cl−与PS反应过程中生成了HClO。可见,在酸性条件下时,Cl−能够促进PS氧化去除AN是由于Cl−与PS反应生成了非自由基形态的HClO。
-
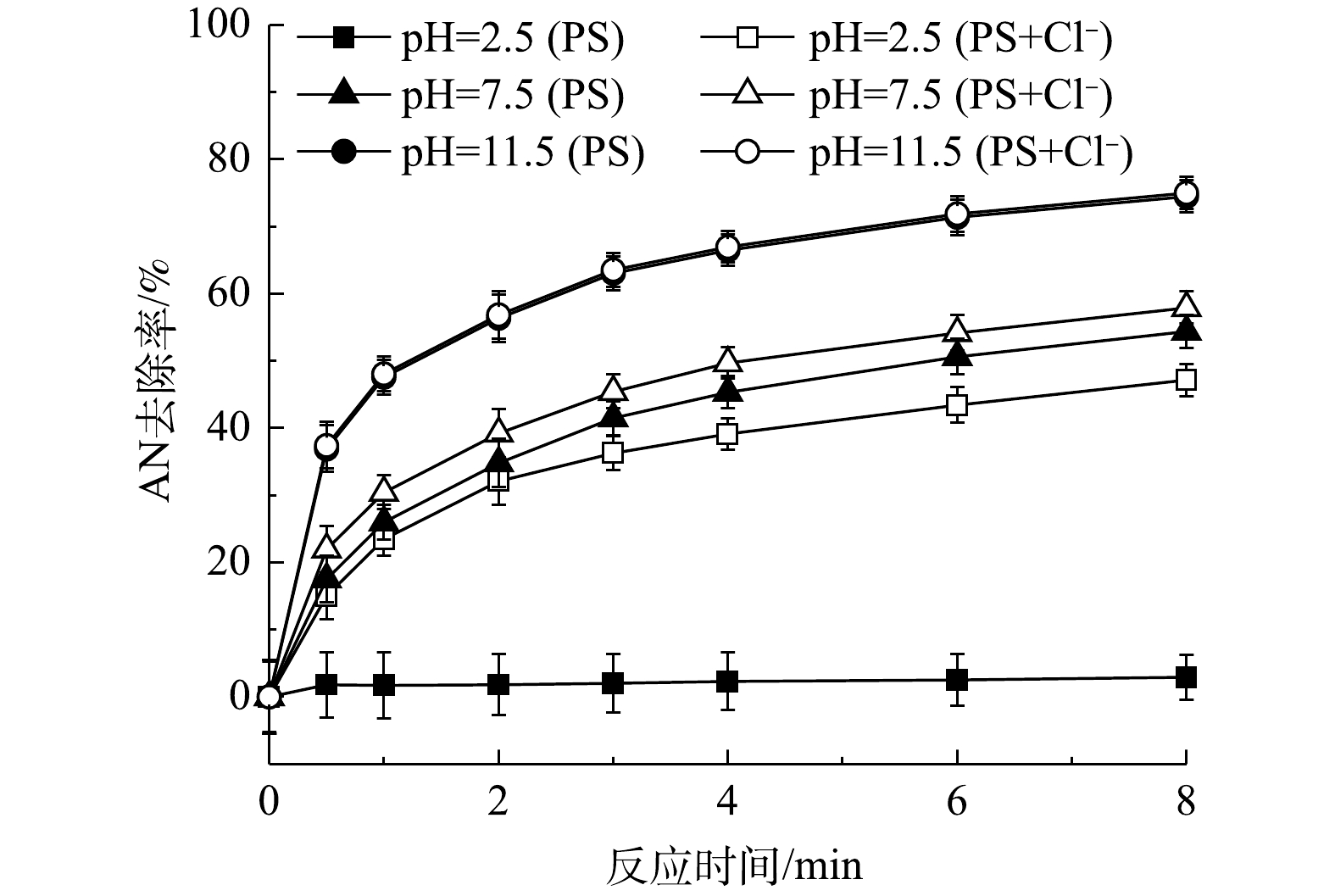
pH可以影响氧化活性物质的生成(
${\rm{SO}}_4^ - \cdot $ 和HO·)以及污染物的形态,因此,溶液pH为PS氧化有机物的重要影响因素。以上研究结果表明,当未调节初始pH(pH为2.5)时,Cl−可以与PS反应生成HClO。本研究继续对在其他pH条件下Cl−对PS氧化过程的影响进行研究。为避免加入的缓冲溶液(PBS缓冲液)中的阴离子对反应过程造成影响,本实验采用NaOH和H2SO4调节反应溶液的pH。已有研究[21]表明,Fe2+/PS在酸性条件下对有机物的氧化效率更高;而当在碱性条件时(pH>4.0),由于Fe2+和Fe3+会发生沉淀而减少,且会形成Fe2+和Fe3+的羟基化合物,从而导致PS的氧化活性降低。当AN浓度为0.1 mmol·L−1、PS浓度为8 mmol·L−1、Cl−浓度为10 mmol·L−1、pH分别为2.5、7.5和11.5时,AN的去除效果见图5。由图5可知,在不投加Fe2+时,碱性条件有利于AN的去除,与Fe2+存在时pH的作用效果完全相反,有研究[22-24]也表明,碱性条件能够活化PS降解有机物。由2.2节可知,在酸性条件下Cl−能够促进AN的去除是由于反应过程中生成了HClO,而HClO是微弱酸性物质,随着pH的升高会电离成ClO−和H+,且ClO−的氧化性(E0=0.94 V)要小于HClO (E0=1.49 V)[25],而本实验中发现碱性条件下更有利于AN的氧化,可见并不是HClO或ClO−作用的结果。进一步对不同pH下NaCl/PS反应过程中的自由氯含量进行测定,结果如图6。由图6中可以看出,当pH为7.5时,反应过程中仍然有自由氯的生成,而在pH=11.5时反应过程中没有自由氯的生成。同时,由图5可见,在pH为11.5时Cl−对AN的去除率远大于pH为2.5时的去除率。以上结果均表明,在中性和碱性条件下,Cl−与PS生成了除活性氯之外的氧化性物质。利用自由基淬灭剂与不同自由基反应速率的不同,可以用来识别反应体系中可能存在的自由基。自由基淬灭剂的存在可以降低自由基对目标化合物降解的反应活性。因此,自由基淬灭剂可以用来间接推测反应体系中可能存在的优势自由基。为了验证反应过程中的活性物质,在pH为11.5时向反应中添加淬灭剂MeOH和TBA,反应结果见图7。由图7可以看出,加入TBA后AN的去除率由74.96%下降至23.61%,可见反应过程中HO·对AN的去除有51.35%的贡献率。加入MeOH后,AN的去除率下降至12.36%,可见反应过程中
${\rm{SO}}_4^ - \cdot $ 对AN的去除有11.25%的贡献率。此外,当同时加入MeOH和BQ后基本完全抑制了AN的去除。由此可见,在该反应体系中的活性氧化物质有${\rm{SO}}_4^ - \cdot $ 、HO·和${\rm{O}}_2^ - \cdot $ 。经计算可得${\rm{O}}_2^ - \cdot $ 对AN的去除率为9.79%,而由图7得到的加入BQ后${\rm{O}}_2^ - \cdot $ 对AN的去除贡献率为24.36%。这可能是由于BQ与PS之间的反应所致[12]。由图5可以得出,在酸性条件下,对AN去除起主要作用的是Cl−。而在中性或碱性条件下,对AN去除起主要作用的是pH,而Cl−的作用影响很弱,在pH=11.5时AN的去除率在加Cl−前后基本不发生改变。FURMAN等[26]同样发现,碱活化PS氧化去除有机物的主要作用的是
${\rm{SO}}_4^ - \cdot $ 、HO·和${\rm{O}}_2^ - \cdot $ 。而对于${\rm{SO}}_4^ - \cdot $ 、HO·和${\rm{O}}_2^ - \cdot $ 的生成,目前认为主要有2种路径:在碱性条件下PS水解生成${\rm{HO}}_2^ - $ (式(16)),${\rm{HO}}_2^ - $ 继续与PS反应生成${\rm{SO}}_4^ - \cdot $ 和${\rm{SO}}_4^{2 - } $ (式(17)),与此同时,${\rm{HO}}_2^ - $ 被氧化生成${\rm{O}}_2^ - \cdot $ ,生成的${\rm{SO}}_4^ - \cdot $ 大部分与HO−反应生成HO·(式(18))[27];PS水解生成${\rm{SO}}_4^{2 - } $ 和H2O2(式(19)),H2O2与HO−反应生成${\rm{HO}}_2^ - $ (式(20)),${\rm{HO}}_2^ - $ 再与H2O2反应生成HO·和${\rm{O}}_2^ - \cdot $ (式(21))[28]。 -
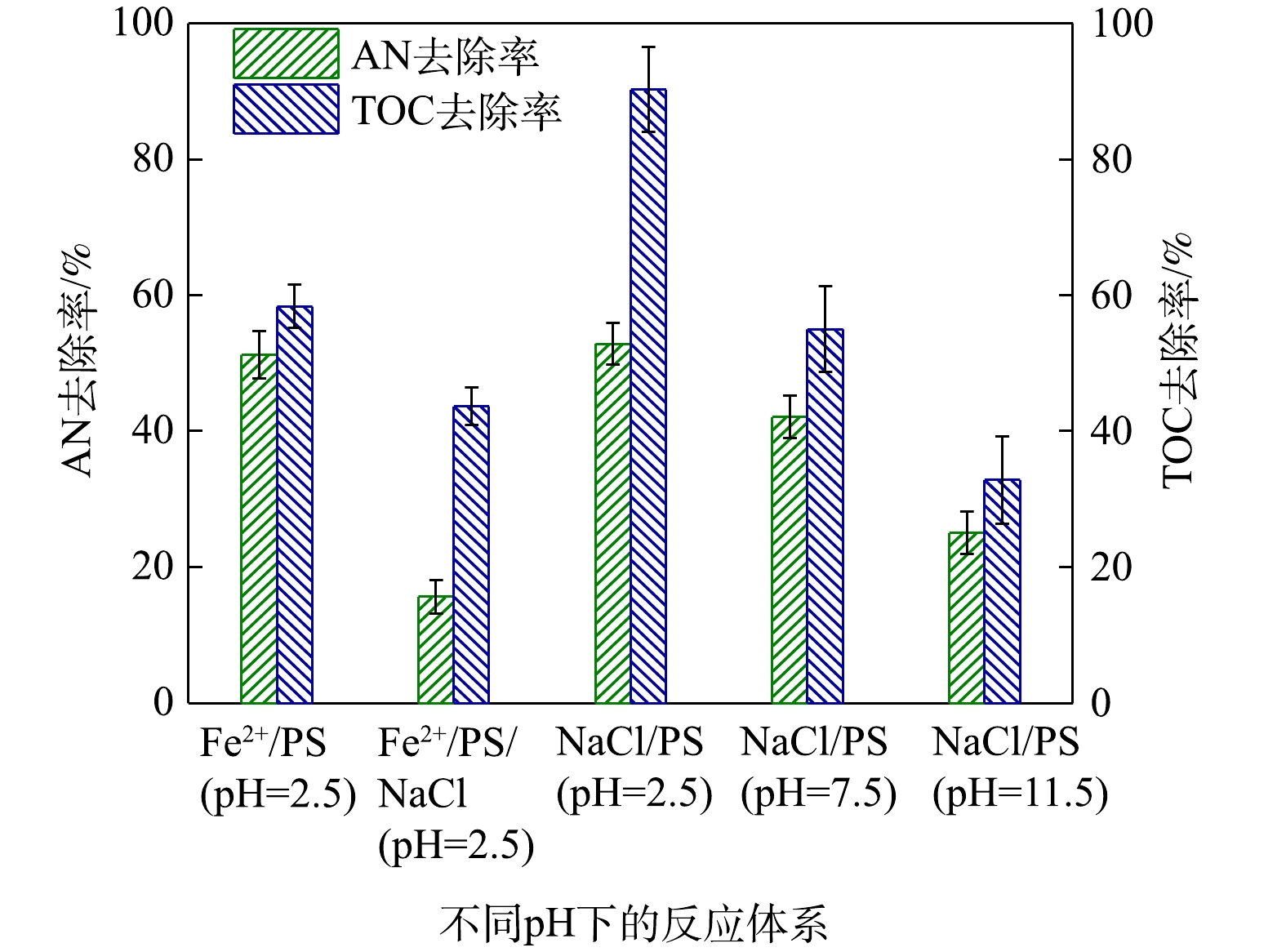
为了进一步研究Cl−促进AN去除的同时,其对AN矿化程度,对反应过程中的TOC进行了测定,其中反应条件为添加0.1 mmol·L−1 AN、1 mmol·L−1 Fe2+、8 mmol·L−1 PS和10 mmol·L−1 NaCl,反应时间为8 min,实验结果如图8所示。由图8可以计算得出,Fe2+/PS对AN的去除率为48.77%,TOC去除率为41.66%,说明
${\rm{SO}}_4^ - \cdot $ 和HO·能矿化AN。而在NaCl/Fe2+/PS去除AN过程中,AN的浓度虽然降低了84.36%,但其中TOC只降低了56.35%。这表明虽然Cl−可以促进对Fe2+/PS对AN的去除,但并不能将其彻底矿化为CO2和H2O。CHEN等[29]利用碳纳米管活化PMS时同样发现,当在反应过程中加入一定量的Cl−后能够提高偶氮染料的去除率,但反应体系中的TOC并不会降低。由图8同样可以得出,当在酸性条件下,AN的去除率为61.77%,TOC的去除率仅为9.78%,可见,PS/NaCl体系不能对AN有效矿化。而在碱性和中性条件下PS可以对AN矿化较为彻底,在中性条件(pH=7.5)下,AN的去除率为57.90%,TOC的去除率为44.98%;在碱性条件(pH=11.5)下,AN的去除率为74.96%,TOC的去除率为67.15%,可见${\rm{O}}_2^ - \cdot $ 同样能够对AN进行有效矿化去除。 -
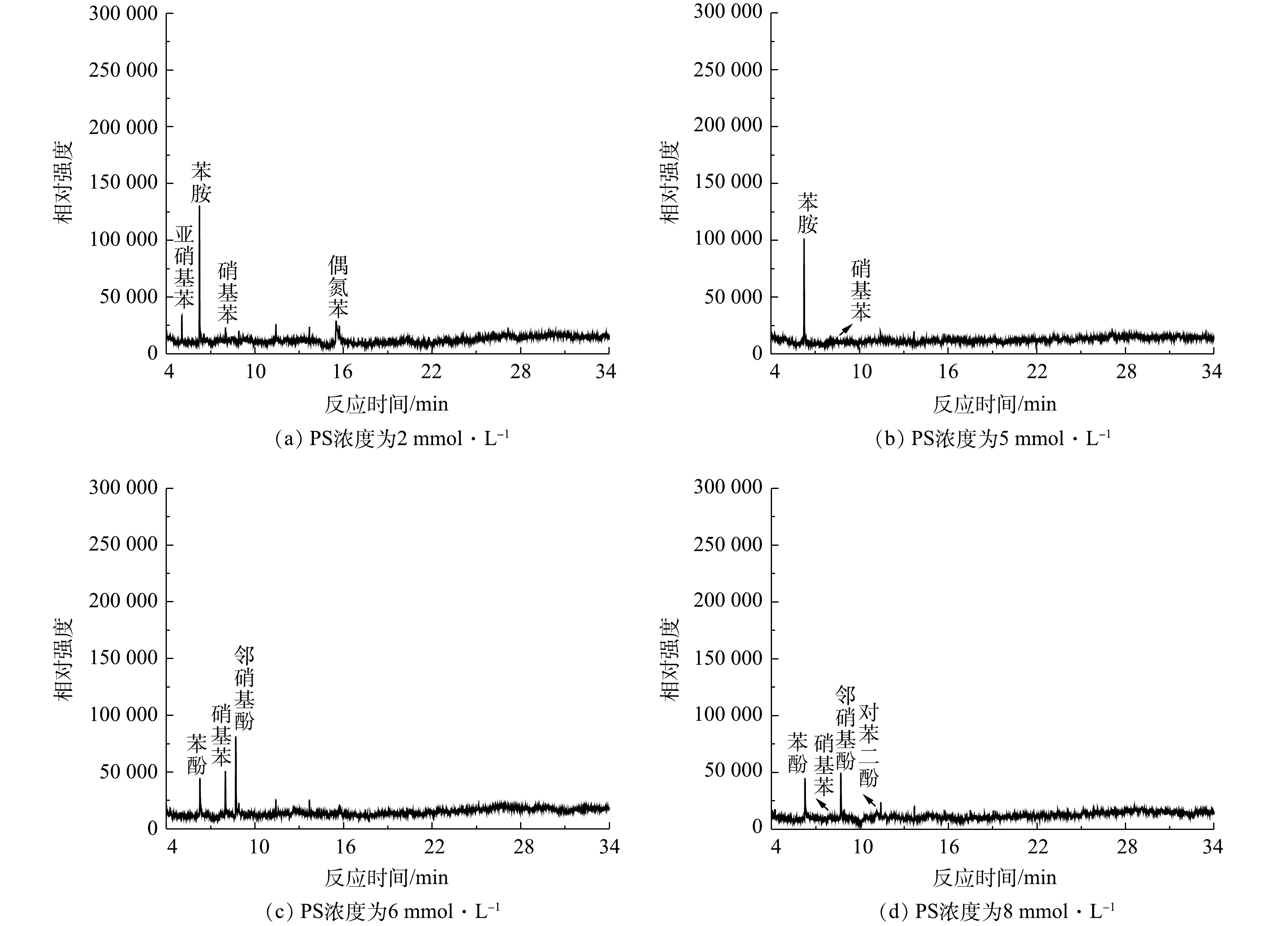
为了进一步研究当Cl−存在时PS的反应机理,我们利用GC-MS对NaCl/PS和Fe2+/PS去除AN的反应产物进行了检测。已有研究表明,卤素离子会导致过硫酸盐氧化过程中生成有害的卤代副产物[7]。ANIPSITAKIS等[30]的研究表明,在利用金属钴复合物活化PMS降解苯酚时,Cl−的加入会使反应过程中生成2-氯苯酚、4-氯苯酚、2,4-二氯苯酚、2,6-二氯苯酚等氯代副产物。CHEN等[29]利用碳纳米管活化PMS降解偶氮染料过程中同样发现,Cl−的加入会导致7-氯-2-萘酚、2,4-二氯-1-萘酚、5-氯异苯呋喃-1,3-二酮等有毒卤代副产物的生成。WALDEMER等[31]研究发现,在处理全氯乙烯(PCE)过程中,由于活性氯的存在,甚至可以形成六氯乙烷。虽然这些氯代副产物生成量很少,但由于其毒性很大,故可对环境造成危害。本研究的结果表明,在苯胺的降解过程中,苯胺溶液的颜色由无色逐渐变为黄色和棕色,最后变为无色,可见,在反应过程中生成了一些复杂的有机产物,从而造成了溶液颜色的变化。图9为加入0.1 mmol·L−1 AN、1 mmol·L−1 Fe2+且反应8 min后,含有不同PS浓度的Fe2+/PS体系在反应过程中的降解产物生成情况。由图9可知,在Fe2+/PS去除AN过程中降解产物有偶氮苯、硝基苯、亚硝基苯、邻硝基酚、苯酚、对苯二酚。有研究[32]表明,HO·和
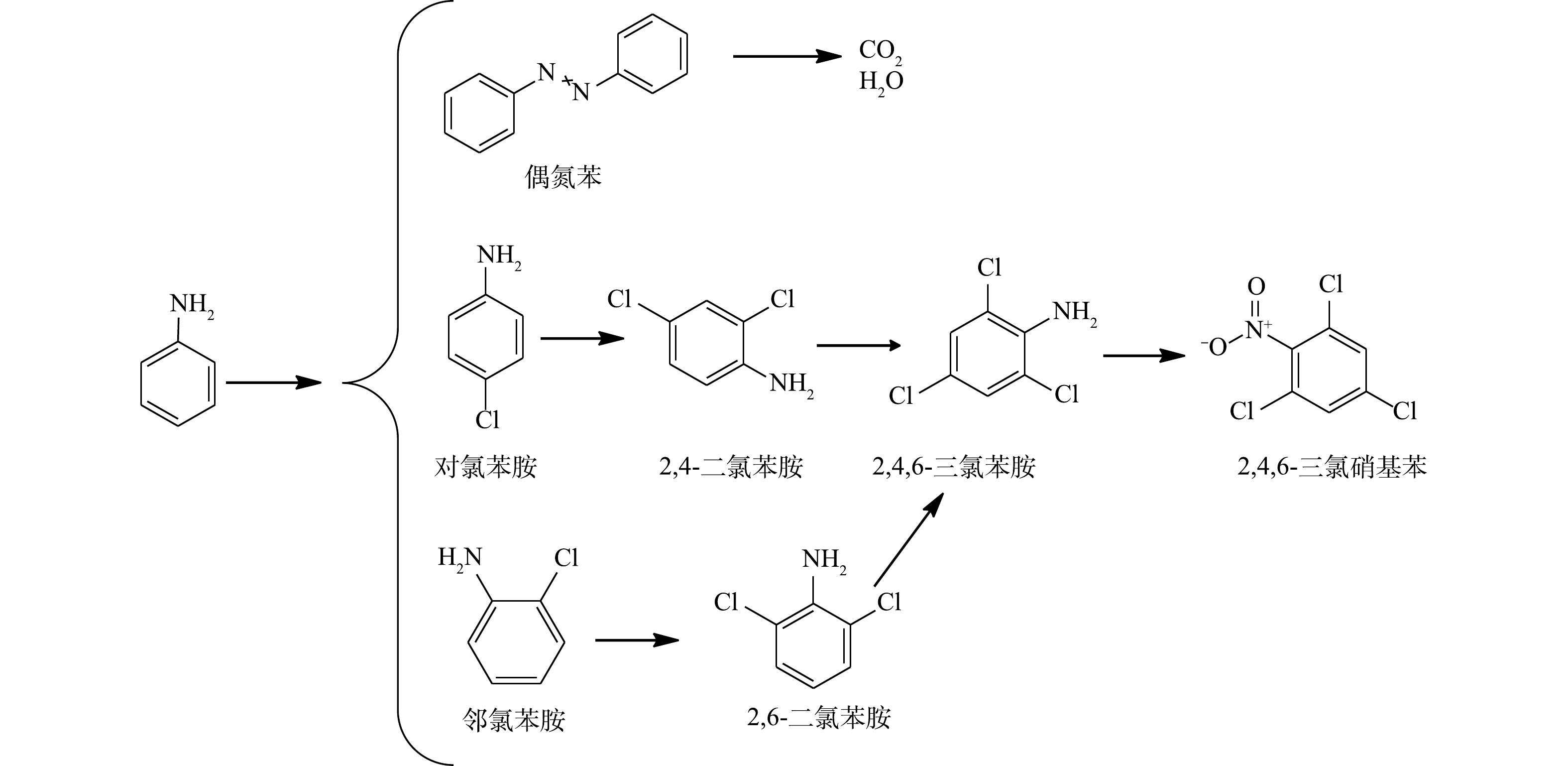
${\rm{SO}}_4^ - \cdot $ 会对AN的芳香环进攻发生脱质子形成苯胺自由基。苯胺自由基可进一步被氧化成硝基苯和亚硝基苯,硝基苯可以氧化为邻硝基酚。邻硝基酚通过脱硝反应生成苯酚,然后苯酚氧化产生对苯二酚,这与BRILLAS等的研究结果一致[33]。反应过程中生成的偶氮苯同样可能是苯胺自由基的反应产物。有研究[34]表明,活化PS降解AN的过程中生成了对苯醌。但在本研究中并未发现对苯醌的生成,可能是对苯醌在该反应体系中容易被氧化,故难以被检测到。最终,AN被矿化生成CO2、H2O和${\rm{NO}}_3^ - $ (在313 nm波长的紫外-可见分光光度计验证)。根据在水溶液中确定的反应中间体,可以推断Fe2+活化过硫酸盐法降解苯胺的合理途径(图10)。进一步对NaCl/PS(pH=2.5)反应过程的反应产物进行测试,发现生成了多种含氯有毒副产物(2,6-二氯苯胺,2,4,6-三氯苯胺,2,4,6-三氯硝基苯等)的生成。图11为加入0.1 mmol·L−1 AN和8 mmol·L−1 PS后,在反应8 min时不同浓度NaCl下NaCl/PS体系中的降解产物生成情况。由图11可知,随着Cl−浓度的增加,AN的浓度逐渐降低,表明AN被逐步降解并转化为其他物质。邻氯苯胺和对氯苯胺的浓度先升高后逐渐降低,这表明邻氯苯胺和对氯苯胺是AN反应过程的中间产物。2,6-二氯苯胺和2,4-二氯苯胺分别随着邻氯苯胺和对氯苯胺的减少而增加;2,4,6-三氯苯胺随着2,6-二氯苯胺和2,4-二氯苯胺的降低而逐渐生成;2,4,6-三氯硝基苯的含量随着2,4,6-三氯苯胺含量的降低而逐渐增加。以上结果表明,邻氯苯胺和对氯苯胺氧化为2,6-二氯苯胺和2,4-二氯苯胺;2,6-二氯苯胺和2,4-二氯苯胺进一步氧化生成2,4,6-三氯苯胺,然后2,4,6-三氯苯胺氧化生成2,4,6-三氯硝基苯,该结论与之前的报道结果一致[35]。根据以上推断,对苯胺的降解途径如图12所示。以上结果表明,在酸性条件下Cl−可以与PS反应生成HClO进而降解AN,但该过程不能彻底矿化AN,且会生成有害的氯代副产物。
2.1. Cl−浓度对Fe2+/PS氧化苯胺的影响
2.2. Cl−在Fe2+/PS反应体系中生成HClO的验证
2.3. pH对PS /NaCl反应过程的影响
2.4. Cl−对PS反应过程中AN和TOC去除的影响
2.5. Fe2+/PS和NaCl/PS反应途径的推断
-
1) Fe2+/PS在酸性条件下可以高效氧化去除AN,且能矿化AN,在反应过程中起主要作用的是
${\rm{SO}}_4^ - \cdot $ 和HO·。2)在酸性条件下,Cl−可以与PS反应生成HClO,其可促进AN的去除,但不能对AN有效矿化;当在PS溶液中加入10 mmol·L−1 Cl−后,AN的去除率升高了47.82%,但TOC去除率仅提高了9.78%,且会生成2,4,6-三氯苯胺等有害的氯代副产物。
3)在中性或碱性条件下,PS能够有效去除AN,而Cl−的作用不显著,主要作用为PS自身在碱性条件下生成
${\rm{SO}}_4^ - \cdot $ 、HO·和${\rm{O}}_2^ - \cdot $ ,且起主导作用的是HO·,并发现该过程能够对AN较彻底矿化;在碱性条件下,苯胺的去除率为74.96%时,HO·、${\rm{SO}}_4^ - \cdot $ 和${\rm{O}}_2^ - \cdot $ 对苯胺的去除率分别为51.36%、11.25%和9.79%。4)在不同pH下Cl−对PS的作用机理不同,氯化物在活性过硫酸盐中发挥重要作用,不应简单的认为Cl−是自由基的淬灭剂,更好地了解氯化物的影响可降低其负面影响。











 下载:
下载: