-
大量工农业废水和生活污水的排放会致使自然水体氮素污染加剧,引发水体富营养化、城市黑臭水体等重大环境问题[1]。生物脱氮技术因为具有环境友好、适用范围广、废水达标排放可靠性强的优点,已成为当前水中含氮污染物处理应用最广泛的方法[2]。传统生物脱氮过程中(A2O工艺),氨氮首先在好氧硝化微生物作用下被氧化为亚硝氮与硝态氮;在缺氧段,反硝化微生物通过代谢有机碳源最终实现亚硝氮与硝氮向氮气的转化。反硝化过程需充足的有机物作为电子供体以满足氮氧化物的还原要求,为获得较好的脱氮效果,废水C/N比需达到3~5[3]。我国实施的“控源减排”政策有效控制了水中有机污染,这也使废水中C/N比大幅降低,造成反硝化微生物电子供体缺乏问题,因此,需要外加大量碳源(甲醇、乙酸等)以维持反硝化作用。然而,外加碳源不仅极大地增加了运行成本,也容易造成二次污染。
近年来,以无机电子供体如氢、硫、铁为底物的自养反硝化技术收到越来越多的关注。该技术的优势在于避免出水有机碳残留、降低污泥产量,因此,被视为异养反硝化的有效替代方案[4-5]。其中,氢自养反硝化产物清洁、产能高,但由于氢气在水中传质效率低,与空气形成的混合物易燃易爆,因此,难以大规模推广使用[6]。以硫化氢、硫单质、硫代硫酸根等低价硫作为电子供体的硫自养反硝化技术有效避免了氢自养反硝化技术安全性问题,可实现高效反硝化过程[7-8],然而,其仍存在硫酸根过量排放,对生态系统及人类健康造成潜在风险。与氢和硫自养反硝化相比,铁自养反硝化技术具有原料安全易得、价格低廉,且可协同去除磷、砷等污染物的特点,具有广阔的应用前景[9-12]。1996年,STRAUB等[13]首次分离得到厌氧铁氧化反硝化(anaerobic iron-oxidizing denitrification)细菌,其能够在无有机碳源条件下氧化Fe(Ⅱ)并将释放的电子用于硝酸盐还原为氮气(式(1))。1998年,NIELSEN等[14]报道了活性污泥体系中存在铁氧化反硝化过程,预示了以活性污泥作为接种物实现铁自养反硝化的可行性。近年来,以活性污泥接种的铁自养反硝化反应器脱氮速率可达0.07~0.33 kg·(m3·d)−1[1, 4-5],并且,通过定期补充异养反硝化污泥或含铁污泥[15-16],反应器能够实现长期稳定的脱氮效果。虽然铁自养反硝化工艺的性能已被广泛研究,但是由于缺乏对铁自养反硝化过程内在机理的了解,使得该过程中产生的如细胞结壳、释放温室气体N2O、亚硝氮积累等问题,仍难以得到解释和解决。因此,探究铁自养反硝化的机理过程,有利于完善和发展该类型的脱氮工艺,进一步解决水环境中氮素污染问题。
在很长一段时期内,铁氧化反硝化过程被视作完全生物过程[4, 17]。然而,硝氮还原过程所产生的活性中间产物(如亚硝氮等)能通过化学作用直接氧化Fe(Ⅱ)(式(2))。例如,异养硝酸盐还原菌Klebsiella mobilis虽然没有氧化Fe(Ⅱ)的能力,但在还原硝酸盐时,中间产物能够导致Fe(Ⅱ)的化学氧化[18]。因此,铁氧化反硝化过程中,Fe(Ⅱ)的化学氧化和生物氧化作用可能同步发生,各物质的动态变化也使得两种作用紧密联系,难以区分。目前,通过氮同位素分馏[19]、次生矿物表征[19]、铁氮消耗比例[4]和中间产物鉴定[17]等方式,铁氧化反硝化过程中生物和化学作用的共存已被间接证实。由于生物和化学作用的铁壳生成位点[19]、氮还原产物[17]等存在差异,从而对铁氧化反硝化体系的稳定性产生巨大影响,因此,揭示该过程中生物和化学作用的变化规律有利于构建更稳固的铁氧化反硝化体系。然而,目前对于铁自养反硝化过程究竟是化学作用还是生物作用起主导仍存在争议。近年来,大量研究均以纯菌为研究对象对铁自养反硝化过程的生物和化学作用进行机理探究,利用动力学模型,区分了纯培养体系中生物和化学作用的相互关系[20-21]。然而,对纯菌体系的研究并不能反映实际情况中铁自养反硝化过程的表现。对于活性污泥体系,由于其条件更为复杂,且Fe(Ⅱ)对不同铁自养微生物反硝化过程的影响存在差异,故采用动力学模型探究活性污泥铁自养反硝化体系的生物化学作用有利于深入了解其反硝化过程中的内在机理和Fe(Ⅱ)所发挥的作用,衡量铁自养反硝化活性污泥的驯化进程。此外,由于混合培养体系中自养与异养微生物共存,可能存在异养微生物利用微生物代谢产物[22],如胞外聚合物(extracellular polymeric substances, EPS),进行异养反硝化[15, 23]。EPS是由微生物产生的一种高分子聚合物,在饥饿环境中能够分解为小分子可溶性微生物产物(soluble microbial products, SMP)[24],从而为微生物提供碳源和能量。因此,将基质浓度变化与动力学模型相结合,有助于研究和区分铁氧化反硝化过程中的生化过程,了解其内在机理。
本研究以铁自养反硝化污泥为研究对象,分析污泥驯化培养过程中脱氮性能及各项指标的变化;利用批次实验,结合反应动力学模型,定量对比接种污泥与驯化污泥在铁氧化反硝化过程中Fe(Ⅱ)氧化和
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原的生物和化学作用相对贡献,解析该过程涉及的亚铁氧化机制,为铁自养反硝化和混合营养反硝化技术的发展提供参考。
全文HTML
-
实验中采用有机玻璃柱状升流式反应器,直径8 cm,高14.5 cm,有效容积1 L。接种污泥取自合肥市望塘污水处理厂回流段,接种量为700 mL,混合液悬浮固体质量浓度(mixed liquor suspended solid, MLSS)与混合液悬浮挥发性固体质量浓度(mixed liquor volatile suspended volatile solid, MLVSS)分别为12 310 mg·L−1和5 230 mg·L−1。水力停留时间设置为8 h,溶解氧控制在0.25 mg·L−1以下,每隔1 d测定进水和出水中铁、氮、磷质量浓度及pH。实验进水采用人工配水,各物质含量参照以往报道[4],包括0.2 g·L−1 NaHCO3、0.025 g·L−1 KH2PO4、0.025 g·L−1 MgSO4、0.02 g·L−1 CaCl2、1 mL·L−1微量元素,其中微量元素包括14 mg·L−1 H3BO3、190 mg·L−1 NiCl3·6H2O、250 mg·L−1 CuSO4·5H2O、990 mg·L−1 MnCl2·4H2O、240 mg·L−1 CoCl2·6H2O、220 mg·L−1 NaMoO4·2H2O、430 mg·L−1 ZnSO4·7H2O、50 mg·L−1 NaWO4·2H2O。
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和Fe(Ⅱ)以NaNO3和FeSO4·7H2O的形式按需投加,$ {\rm{PO}}_{\rm{4}}^{{\rm{3 - }}}$ -P质量浓度约为5.8 mg·L−1,各阶段基质质量浓度如表1所示。 -
开展批次实验模拟铁自养反硝化体系中可能发生的生化过程。非生物组实验中不添加活性污泥,包括Fe(Ⅱ)+
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N、Fe(Ⅱ)+$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N;分别采用接种污泥和铁自养反硝化反应器中驯化污泥作为添加污泥进行生物组实验,包括$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N+活性污泥、$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N+活性污泥、Fe(Ⅱ)+$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N+活性污泥、Fe(Ⅱ)+$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N+活性污泥。所有批次实验均在丁基胶塞和铝盖密封的100 mL厌氧血清瓶进行,并在黑暗条件下培养,重复3次。Fe(Ⅱ)和N初始物质的量浓度分别为4.8 mmol·L−1和2.4 mmol·L−1,所有批次实验活性污泥的MLVSS为300 mg·L−1,初始pH为7.0±0.1。1)指标测定。污泥MLSS和MLVSS采用国家标准方法测定[25];所有水样经0.45 μm滤膜过滤后进行分析。采用水杨酸-硫酸法测定
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N浓度;采用N-(1-萘基)-乙二胺光度法测定$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度。采用便携式溶氧仪(HQ30d, HACH Co., USA)和台式pH仪(FE-28, MettlerToledo Inc., USA)测量溶解氧浓度和pH。EPS经热提法提取后,采用福林酚法测定蛋白质和腐殖酸浓度,采用苯酚-硫酸法测定多糖浓度。2)反应动力学模拟。将铁氧化硝酸盐还原简化为以下过程[20]:活性污泥通过生物作用将
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N还原为$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N(式(3));产生的一部分$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N被活性污泥进一步生物还原(式(4)),另一部分与Fe(Ⅱ)经化学反应生成气态氮产物(式(5));活性污泥通过生物途径氧化Fe(Ⅱ)(式 (6))。采用动力学常数k表示反应过程中基质降解速率。
1.1. 活性污泥的接种与驯化
1.2. 批次实验
-
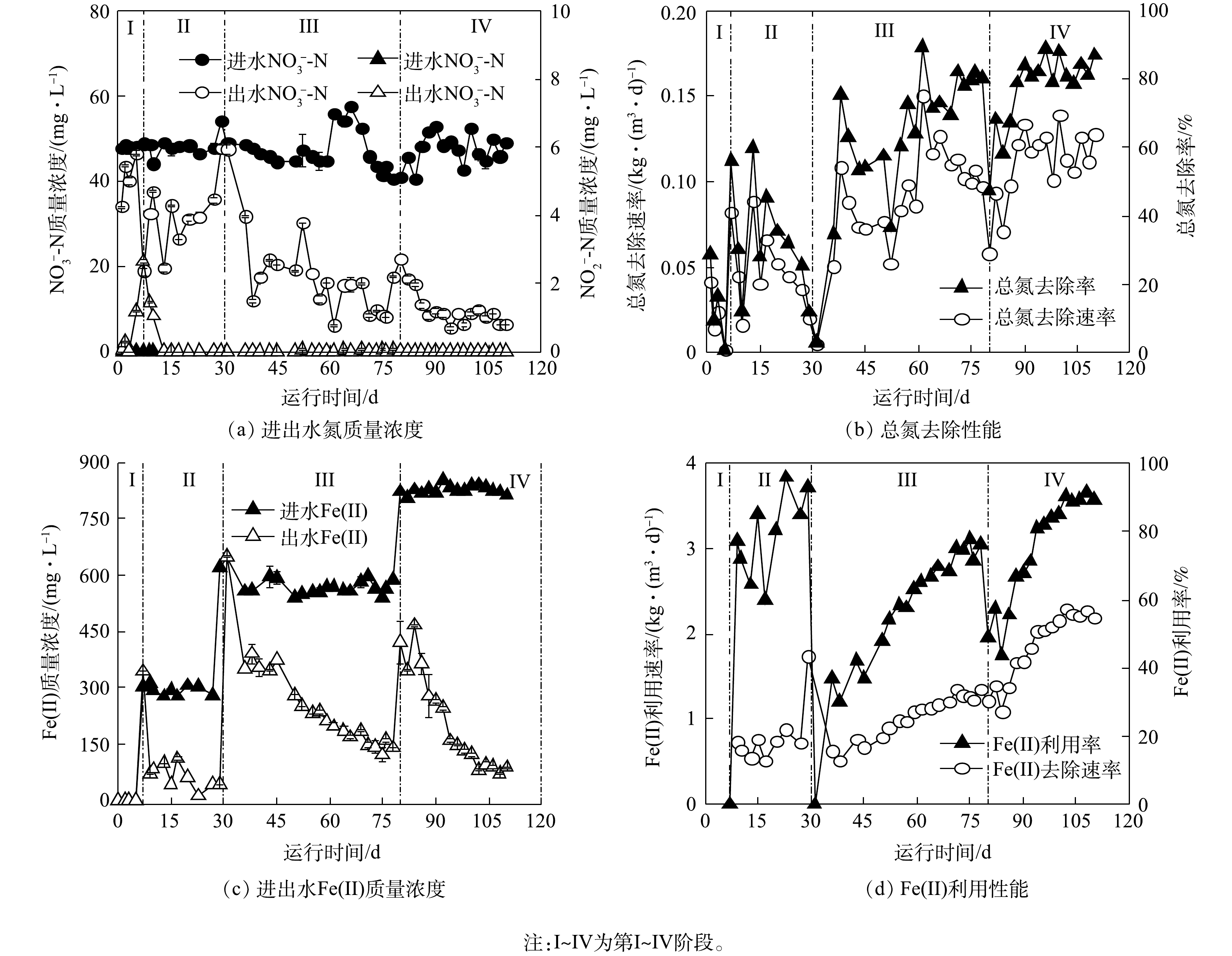
铁自养反硝化反应器运行过程中,进出水水质随时间的变化情况如图1所示。整个运行过程中,进水
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N质量浓度稳定在50 mg·L−1左右,进水Fe(Ⅱ)质量浓度由0 mg·L−1逐步提升至840 mg·L−1。在第I阶段,初始$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除率达到(29.1±1.8)%,随后逐渐降低;与此同时,出水中出现$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N积累,在第7天时最高质量浓度达到2.7 mg·L−1 (图1(a))。初期$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除可能归因于接种污泥中异养反硝化细菌利用污泥残留可利用有机质(包括微生物EPS与SMP)进行反硝化[26],随着有机质含量减少,脱氮效率逐渐降低。第7天时,无反硝化效果说明污泥中残余可利用有机质被耗尽,异养反硝化过程停滞。$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N积累说明在此阶段,污泥残留有机质所能提供电子供体有限,不足以维持全部氮还原过程。在第Ⅱ阶段,进水中添加280 mg·L−1 Fe(Ⅱ),$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除率为(37.6±15.6)%,出水$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N质量浓度逐渐降低。出水Fe(Ⅱ)质量浓度为(78.1±26.2) mg·L−1,Fe(Ⅱ)利用率为(78.6±11.8)% (图1(c)~(d))。在此阶段出水$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和Fe(Ⅱ)质量浓度波动较大,反硝化性能不稳定。与第I阶段的$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N累积现象相比,第Ⅱ阶段$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N质量浓度呈完全相反的趋势,这可能归因于Fe(Ⅱ)与$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N间发生生物或化学反应。反应器运行的前23 d内,出水中检测到氨氮,且最高质量浓度达20 mg·L−1。由于接种污泥中菌群种类丰富,在适应厌氧铁自养反硝化条件过程中,大量异养及好氧细菌可能因无法适应实验条件而裂解,释放胞内有机氮并转化为氨氮,从而造成出水氨氮浓度升高[3, 19]。第23天后未在出水中检测到氨氮,预示着微生物自溶结束,有机氮的释放达到平衡[20]。在第Ⅲ阶段,出水Fe(Ⅱ)浓度降低的同时$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除率上升,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除率和Fe(Ⅱ)利用率分别为(80.2±15.0)%和(76.1±15.1)%。在第Ⅳ阶段,系统效能逐渐趋于稳定,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除率和Fe(Ⅱ)利用率分别为(82.7±8.4)%和(89.2±15.0)%,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除速率和Fe(Ⅱ)利用速率分别为0.12 kg·(m3·d)−1和2.16 kg·(m3·d)−1。与前3个阶段相比,第Ⅳ阶段的$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除率分别增长了53.6%、45.1%和2.5%,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除速率分别增长了0.10、0.07和0.03 kg·(m3·d)−1。此外,自添加Fe(Ⅱ)起,铁氮消耗比由3.2增至4.3,说明随驯化时间增长,体系对Fe(Ⅱ)的利用率也在逐渐提升。当各阶段进水Fe(Ⅱ)浓度增大时,出水$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N质量浓度会在短期内提升(图1(a)),且对Fe(Ⅱ)利用率降低(图1(c))。面对环境中Fe(Ⅱ)浓度提升,系统内微生物群落结构亦发生相应变化,异养反硝化微生物因碳源不足生长受到抑制,铁自养反硝化微生物的丰度可能逐渐上升,进而使反应器性能呈现先降低后逐渐增强的趋势。此外,反应器运行过程中,进水$ {\rm{PO}}_{\rm{4}}^{{\rm{3 - }}}$ -P质量浓度保持在5.8 mg·L−1左右。在第I阶段,出水$ {\rm{PO}}_{\rm{4}}^{{\rm{3 - }}}$ -P质量浓度由1.8 mg·L−1提升至5.8 mg·L−1,除磷效率逐渐降低直至为0;自第Ⅱ阶段进水中加入Fe(Ⅱ)起,出水中未再检测到磷。值得注意的是,第Ⅱ阶段的Fe(Ⅱ)/N消耗比约为3.2,小于铁自养反硝化过程Fe(Ⅱ)/N消耗理论比5(式(1)),因此,可能存在中间副产物如N2O的积累;随着培养时间和亚铁浓度的提升,第Ⅲ阶段和第Ⅳ阶段,Fe(Ⅱ)/N消耗比分别为3.5和4.3,这意味着不同阶段的铁自养反硝化过程生物与化学作用可能存在差异。 -
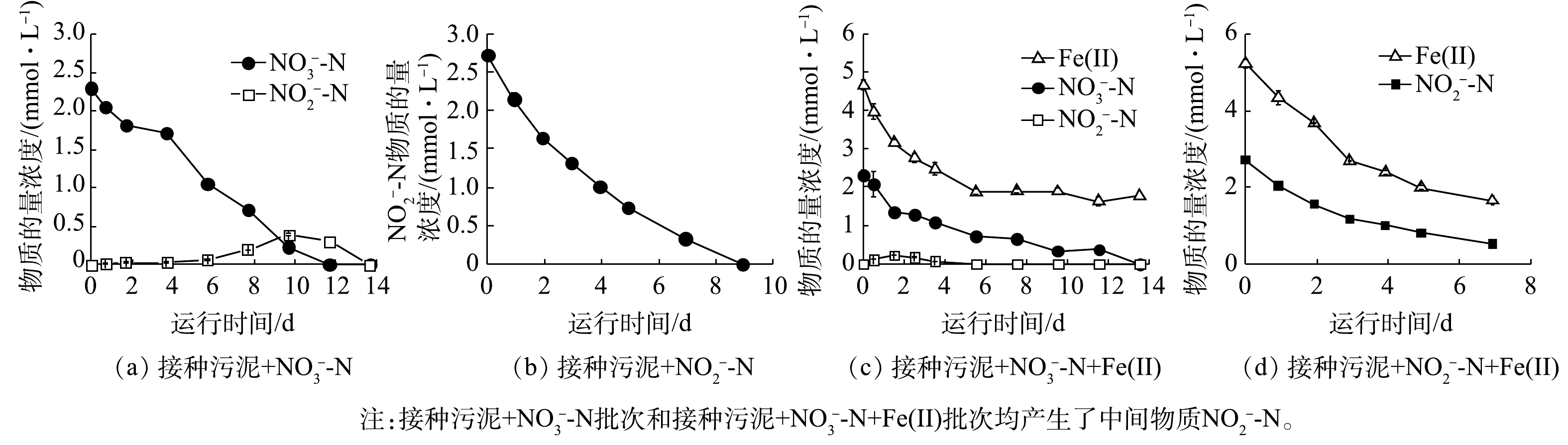
1)活性污泥铁氧化反硝化过程生物作用动力学分析。由于不同阶段活性污泥的反硝化性能存在差异,因此,分别采用接种污泥和铁自养反应器运行后期的驯化污泥进行生物组批次实验,以探究不同阶段活性污泥的反硝化性能。接种污泥在铁自养条件下基质物质的量浓度的变化如图2所示,动力学常数与Fe/N消耗比如表2所示。无外加Fe(Ⅱ)时,接种污泥生物脱氮效果显著,
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N分别在第12天和第9天被完全还原(图2(a)~(b)),$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的质量浓度变化符合准一级动力学。与反应器初期脱氮效果相似,在未添加无机电子供体时,氮的去除可能是由于接种污泥中残余有机物维持异养反硝化过程[19]。外加Fe(Ⅱ)时,14 d内$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N被完全还原,Fe(Ⅱ)利用率为62.0% (图2(c))。7 d内$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N去除率为80.2%,Fe(Ⅱ)利用率为68.3% (图2(d))。Fe(Ⅱ)氧化与$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N、$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原同样符合准一级动力学,kbio,1、kbio,2、kbio,3分别为6.5×10−3、1.0×10−2、4.9×10−3 h−1 (表2)。接种污泥还原$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N过程中,由于有机电子供体存在,优先发生了异养反硝化过程,铁自养反硝化过程受限,因此Fe/N消耗比实际值(1.25)远小于铁自养反硝化Fe/N消耗比的理论值(5);而对于$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原,生物作用与化学作用共存,Fe/N消耗比实际值(1.6)接近Fe(Ⅱ)与$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N之间化学作用比值(2)。铁自养反硝化驯化污泥在无外加Fe(Ⅱ)时,对
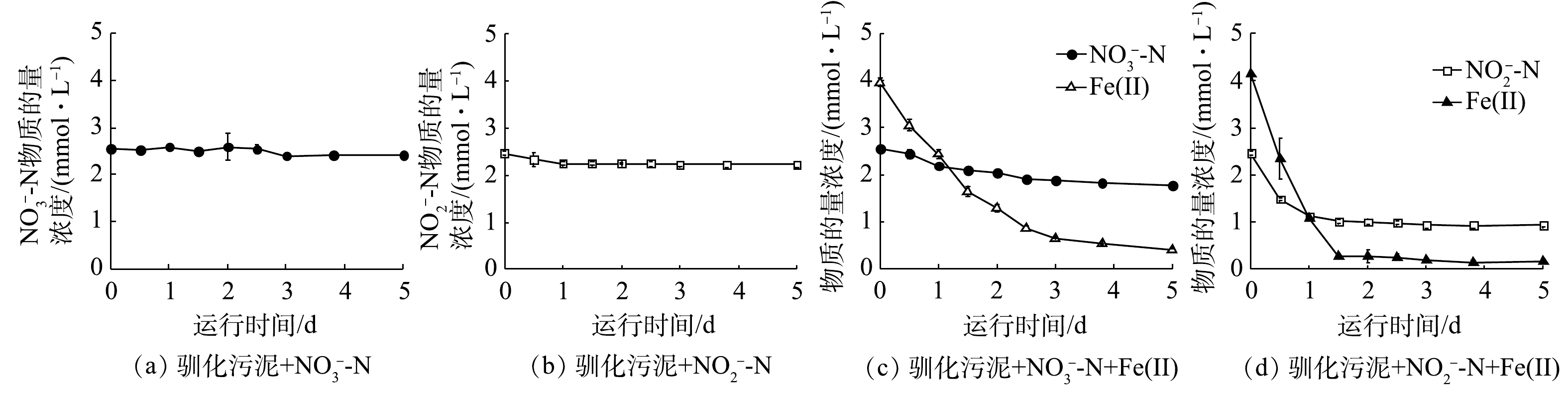
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N几乎没有反硝化能力(图3(a)~(b)),这说明体系中没有可供利用的无机或有机电子供体。当外加Fe(Ⅱ)时,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N在5 d内浓度缓慢降低,硝氮去除率为30.9%,Fe(Ⅱ)利用率为90%(图3(c));$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N在5 d内的去除率为62.0%,Fe(Ⅱ)利用率为95.9% (图3(d))。与接种污泥相比,驯化污泥的脱氮效率低于接种污泥。由于驯化污泥组在反应末期Fe(Ⅱ)几乎被耗尽,因此,可能是Fe(Ⅱ)供给不足导致。如表2所示,驯化污泥$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N与$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原的Fe/N消耗比分别为4.5和2.6,远大于接种污泥。由于铁自养反硝化过程中存在$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N与Fe(Ⅱ)的化学作用,驯化污泥Fe/N消耗比的增加预示着化学反应的减少,这有利于减少温室气体N2O的释放。驯化污泥在铁氧化反硝化过程中的基质降解速率也符合准一级动力学,kbio,1、kbio,2、kbio,3分别为3.1×10−3、3.3×10−2、2.1×10−2 h−1。除硝氮还原速度低于接种污泥外,驯化污泥对Fe(Ⅱ)的利用效率大大提升,Fe(Ⅱ)氧化和亚硝氮还原速度均远大于接种污泥,此时硝氮还原为亚硝氮成为脱氮的主要限速步骤[1]。微生物群落演替导致的铁自养反硝化微生物的富集、异养微生物的衰亡,可能是促成这一结果的原因之一。2)活性污泥铁氧化反硝化过程化学作用动力学分析。铁自养反硝化过程中存在氮素与Fe(Ⅱ)间化学氧化还原。在化学组批次实验中,当
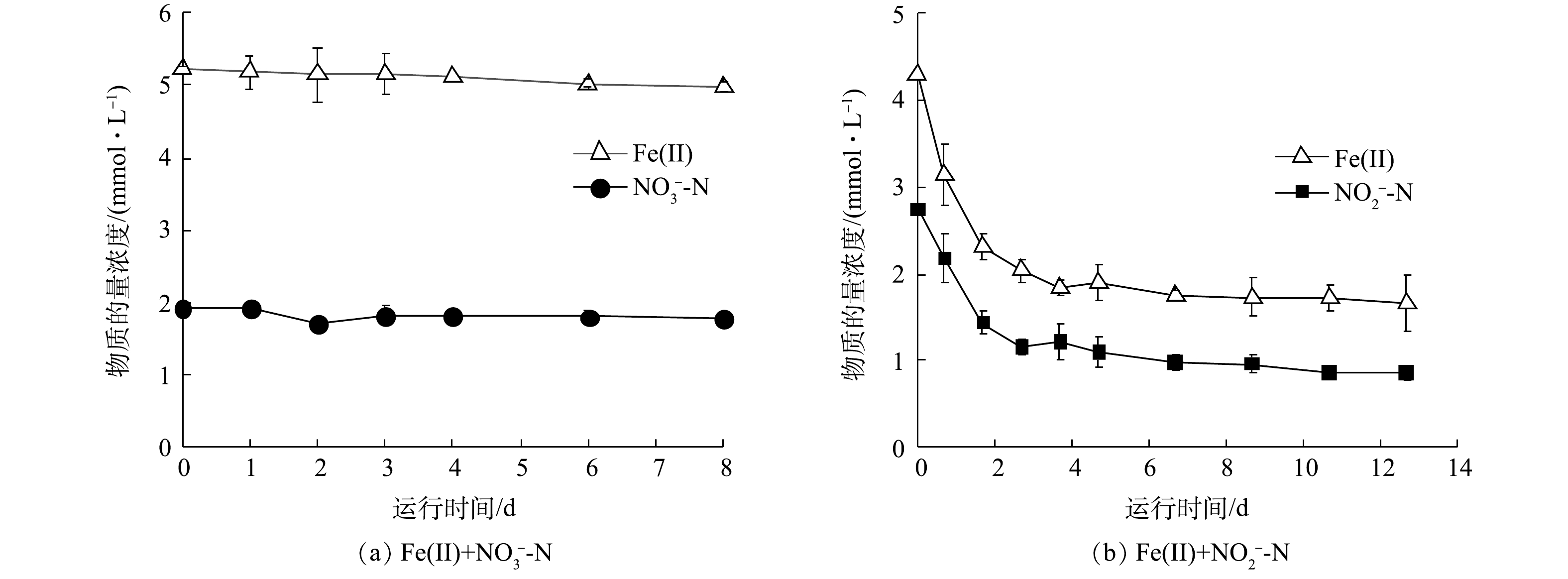
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N与Fe(Ⅱ)共存时,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N浓度无明显变化,Fe(Ⅱ)的浓度由5.2 mmol·L−1略降至5.0 mmol·L−1 (图4(a)),体系中形成绿色絮凝沉淀。中性环境下亚铁离子发生水解会形成灰绿色的氢氧化亚铁,这在一定程度上解释了Fe(Ⅱ)浓度降低的原因。$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和Fe(Ⅱ)的化学反应结果与以往的报道一致,即在中性条件且没有催化剂的条件下,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N难以氧化Fe(Ⅱ)[27]。当$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N与Fe(Ⅱ)共存时,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N能够以较高的速率氧化Fe(Ⅱ) (图4(b)),初始阶段$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度迅速降低,而后速度减慢,前4 d内,Fe(Ⅱ)和$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的浓度分别由4.3 mmol·L−1和2.7 mmol·L−1降至1.8 mmol·L−1和1.1 mmol·L−1。整个过程中$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N与Fe(Ⅱ)化学反应基质降解速率符合二级动力学方程,动力学常数为2.9×10−3 mol·(L·h)−1。值得注意的是,在前4 d内Fe(Ⅱ)/N消耗比仅为1.6,然而体系最终Fe(Ⅱ)/N消耗比为2,与$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N与Fe(Ⅱ)化学反应的计量比相等(式(2)),因此,反应初期可能存在氮还原中间体。大量$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N消耗会导致NO积累,如式(7)~式(9)所示[26],其中$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N先被还原为NO,再被还原为N2O。若前4 d非生物组实验的最终产物为N2O,理论上需要更多Fe(Ⅱ)提供电子,这说明在$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原为N2O的过程中氮素中间体(NO)未向N2O转化,因此,减少了电子供体需求。在4 d后,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度较低,产生的NO进一步转化为N2O,Fe/N消耗比增至2,主要氮产物为N2O。因此,不同阶段Fe/N比可能影响化学反应产物的种类。为探究不同Fe/N比对化学反应产物的影响,通过改变
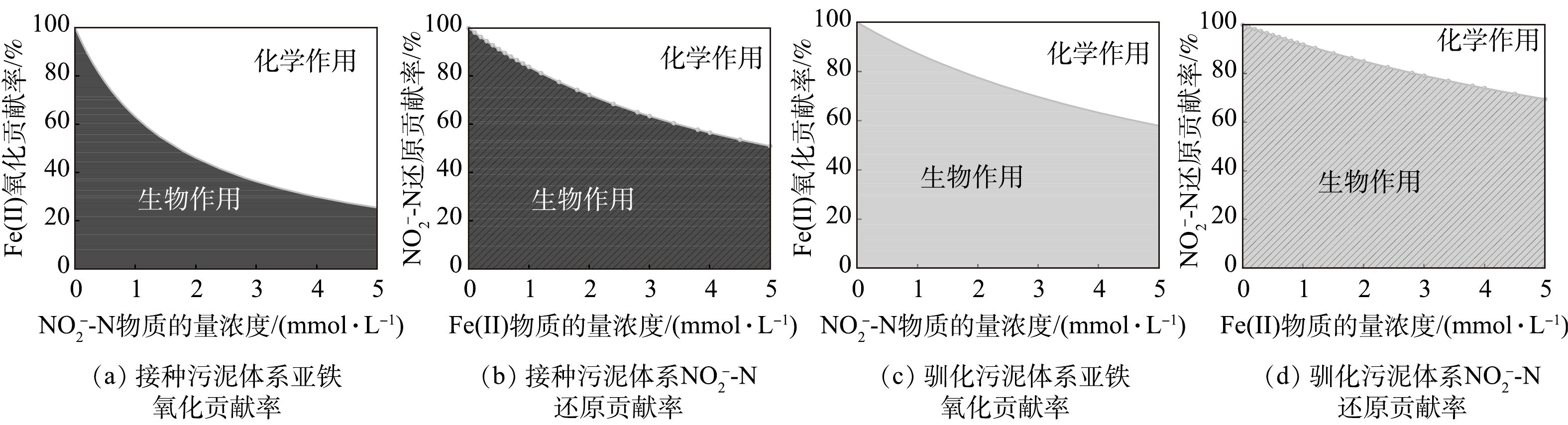
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度将初始Fe(Ⅱ)/N分别调整为1∶1、2∶1和4∶1,其中Fe(Ⅱ)的浓度始终为4.8 mmol·L−1。4 d内各组Fe(Ⅱ)/N消耗比见表3,当初始Fe(Ⅱ)/N为1∶1时,Fe(Ⅱ)/N消耗比为1.1,随着初始Fe(Ⅱ)/N比的增加,Fe/N消耗比分别为1.6和2.1。根据物质守恒定律和电荷守恒定律推算NO和N2O产物的生成比例,结果如表3所示。当反应物浓度相等时,NO的产率比N2O高13倍。当初始Fe(Ⅱ)/N比为2时,NO/N2O比值为1.2,产物中NO与N2O含量相当。当初始Fe(Ⅱ)/N为4时,NO/N2O的比例接近0,N2O为主要产物。基质的初始比例对产物有很大影响,Fe(Ⅱ)含量越高,Fe(Ⅱ)与$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的氮还原产物更易由NO向N2O转化。因此,在自养反硝化体系中,当$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的浓度低于Fe(Ⅱ)浓度的1/4时,化学作用的产物为N2O。3)铁自养反硝化过程中生物与化学作用间的相互关系。根据上述生物及化学批次实验结果,在铁自养反硝化过程中,微生物参与的过程(式(3)~(6)),基质降解速率均符合准一级动力学,化学反应(式(5))基质降解速率符合二级动力学。利用各级动力学常数计算铁自养反硝化过程中生物和化学作用的相对贡献[20](式(10)~(13)),结果如图5所示。对于接种污泥,Fe(Ⅱ)氧化主要由
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的化学氧化作用主导,只有当$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N低于1.7 mmol·L−1时,生物作用的贡献率大于化学作用(图5(a));对于$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原,接种污泥的生物还原起主导作用(图5(b))。对于铁型自养反硝化污泥,Fe(Ⅱ)氧化与$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原均主要由生物作用完成(图5(c)和图5(d))。接种污泥与驯化污泥在铁自养条件下的反硝化过程中,Fe(Ⅱ)氧化的主导作用完全相反,体系内微生物及可利用有机质的含量差异可能是导致这一结果的原因。对于Fe(Ⅱ)氧化作用,接种污泥中,自养、异养微生物共存且体系内存在剩余有机物。有研究[17, 28-29]表明,约90%的硝酸盐还原菌能够在Fe(Ⅱ)和有机质共存条件下氧化Fe(Ⅱ),但是在没有任何有机质的条件下,仅少数细菌能够进行铁氧化硝酸盐还原[30-32]。接种污泥以EPS为电子供体的异养反硝化过程与以Fe(Ⅱ)为电子供体的自养反硝化过程存在竞争,且自养反硝化细菌丰度较低。此外,EPS中多糖的存在也可能对Fe(Ⅱ)的化学氧化过程起促进作用[33]。对于驯化污泥,其自养微生物在驯化过程中富集,而异养微生物被淘汰,因此,主要以Fe(Ⅱ)为电子供体,Fe(Ⅱ)氧化过程由生物作用主导。对于亚硝氮还原过程,2种污泥的生物作用贡献率均占50%以上。驯化污泥在富集培养过程中增强了对Fe(Ⅱ)的生物利用能力,且无法在不含Fe(Ⅱ)条件下通过异养反硝化实现亚硝氮还原(图3(b))。因此,驯化污泥生物反硝化主要为自养过程。通过铁自养条件的驯化,有利于增强自养微生物对Fe(Ⅱ)的利用,从而提升铁氧化硝酸盐还原过程中的生物作用,减少亚铁与亚硝氮的化学作用占比。
虽然目前仍缺少生物作用氧化Fe(Ⅱ)的直接酶证据,但铁氧化硝酸盐还原过程中生物、化学作用的共存已在多个研究体系得到证实[19]。近年来,有研究者采用模型方法对其生化作用进行了区分。JAMIESON等[21]认为,60%~75%的Fe(Ⅱ)氧化通过生物酶促过程发生;LIU等的研究表明,Fe(Ⅱ)氧化由化学作用主导[20]。上述2项研究采用的菌种不同,且前者所分析的铁氧化硝酸盐还原过程体系中存在有机络合态亚铁,后者则采用纯自养体系,然而2项研究中使用的模式菌株均为兼养细菌,有机质的存在可能影响其生化过程。目前,对于混菌体系中生化作用的区分尚未见报道,本研究以2种活性污泥为研究对象,发现二者亚硝氮还原过程生化作用与以往报道类似。对于接种污泥体系的亚铁氧化过程,得出的结论与LIU等[20]的研究结论类似,这可能归因于本文同样采用了纯自养培养体系;驯化污泥的生物作用较接种污泥大大提升,混养体系菌群丰富,在驯化过程中筛选了优势菌群,且菌群间形成互作关系,这可能是生物作用氧化Fe(Ⅱ)贡献率提升的原因之一。
式中:
$ {N_{{\rm{bio}},{\rm{Fe}}\left( {{\rm{II}}} \right)}} $ 为Fe(Ⅱ)氧化过程中生物作用的相对贡献率;$ {N_{{\rm{abi}},{\rm{Fe}}\left( {{\rm{II}}} \right)}} $ 为Fe(Ⅱ)氧化过程中非生物作用的相对贡献率;$ {N}_{{\rm{bio}},{{\rm{N}}{\rm{O}}}_{2}^{-}} $ 为$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ 还原过程中生物作用的相对贡献率;$ {N_{{\rm{abi}},{\rm{NO}}_2^ - }}$ 为$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ 还原过程中非生物作用的相对贡献率;k为反应动力学常数;[Fe(Ⅱ)]、[$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ ]为基质的物质的量浓度。 -
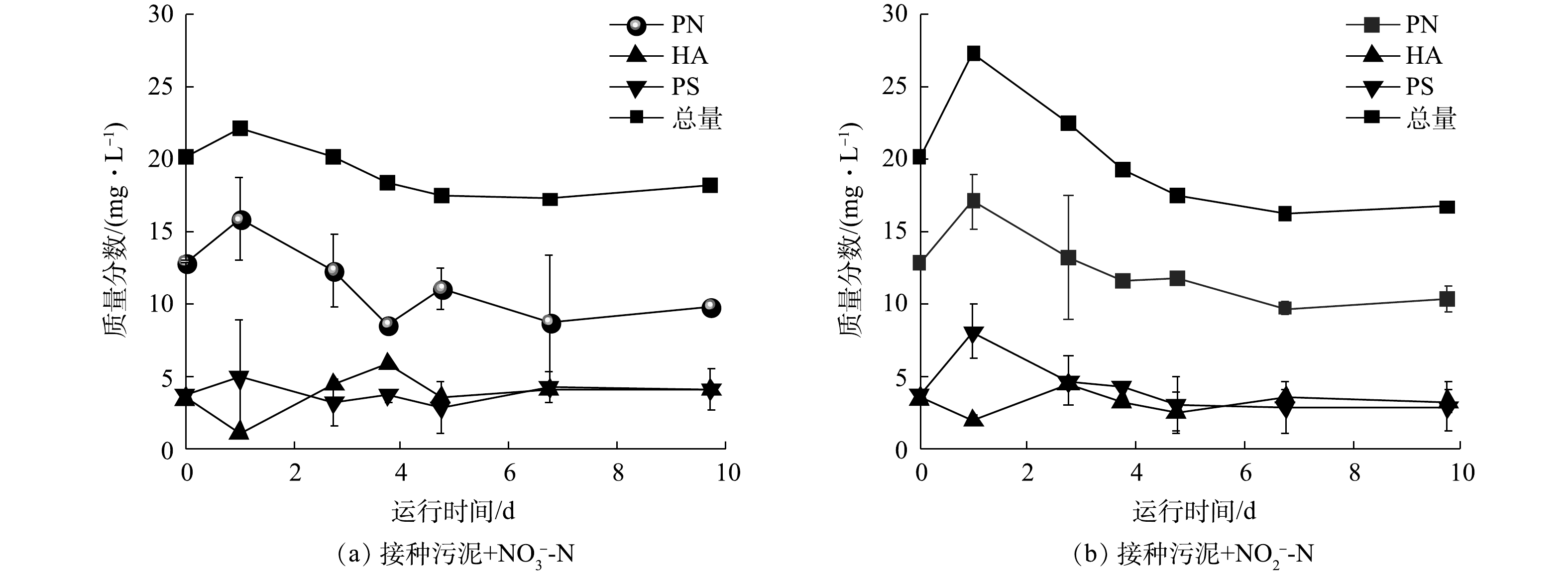
对于接种污泥和驯化污泥,除铁自养条件下反硝化过程的生化作用相对贡献存在差异外,在缺乏外加电子供体时,二者的反硝化性能也截然不同。接种污泥在没有Fe(Ⅱ)供给的条件下仍能还原硝氮和亚硝氮(图2(a)~(b)),存在以有机质为底物的异养反硝化过程。此外,反应器外加亚铁初期时铁氮消耗比(3.2)高于铁氮化学反应理论比值(2),说明存在部分Fe(Ⅱ)被铁自养微生物的生物作用氧化。然而,驯化污泥在此条件下则对硝氮和亚硝氮均没有还原效果(图3(a)~(b))。由于接种污泥中自养、异养微生物共存,而驯化污泥经自养条件长期培养,异养菌可能被淘汰且EPS含量降低。因此,接种污泥的生物反硝化过程应被进一步划分为自养反硝化和异养反硝化,驯化污泥则仅存在自养反硝化过程。为验证这一推测的可能性,研究了接种污泥在无Fe(Ⅱ)条件下反硝化过程中EPS含量的变化。如图6所示,接种污泥在无外源Fe(Ⅱ)提供的反硝化过程中,每克MLVSS中EPS含量总体呈降低趋势且与氮还原趋势一致。第1天2组实验中的总EPS含量均呈上升趋势,之后的9 d内,EPS中蛋白质(protein, PN)、多糖(polysaccharide, PS)和腐殖酸(humic acid, HA)的质量分数逐渐降低,分别由22.0 mg·g−1和27.2 mg·g−1下降至17.2 mg·g−1和16.2 mg·g−1。其中,蛋白质含量最高且质量分数变化最为显著,而多糖和腐殖酸质量分数较低且无明显变化。因此,推测EPS能够被接种污泥用于异养反硝化过程,且主要被利用的物质为蛋白质。
分别提取接种污泥和驯化污泥的EPS并测定其总有机碳(total organic carbon, TOC)含量,结果显示,接种污泥每克MLVSS所含EPS的TOC值为304.9 mg·g−1,为自养反硝化污泥EPS的30倍(10.3 mg·g−1)。较高的EPS含量可为接种污泥中的异养反硝化微生物提供充足的有机碳源,从而促进反硝化过程的发生。而驯化污泥经自养条件下的长期驯化,不仅淘汰了部分异养和兼养微生物,还减少了微生物胞外聚合物的产生,导致可利用有机质含量少,使得异养反硝化作用停滞。因此,接种污泥中的反硝化过程可能由异养微生物起主导作用,而驯化污泥以自养微生物主导且EPS含量较低,在没有Fe(Ⅱ)存在时无法进行反硝化作用。
由于不同阶段活性污泥中微生物的种类与丰度可能存在差异,进而影响了铁自养反硝化性能,因此,采用多种来源的接种污泥,增加微生物的组成和丰度,可能有助于提高铁自养反硝化微生物的丰度,提升驯化效率和脱氮性能。通过分析混菌体系中微生物群落及其相互关系,有助于进一步了解铁自养反硝化体系的过程机理。此外,由于铁自养反硝化已被证明具有较好的脱氮性能,且能够同步去除多种污染物,产物具有二次利用价值,因此,未来还有必要通过生命周期评价对其进行深入的经济分析与评价,为其应用于实际工程的可行性提供科学依据。
2.1. 铁自养反硝化反应器的启动与运行
2.2. 铁型自养反硝化过程机理分析
2.3. 接种污泥与驯化污泥脱氮过程差异分析
-
1)经铁自养条件驯化后的活性污泥展现出较强的反硝化和亚铁利用能力。其硝氮去除率和硝氮去除速率为(87.0±1.8)%和0.12 kg·(m3·d)−1,分别比未经驯化的活性污泥体系高出53.6%和0.10 kg·(m3·d)−1,且无
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N积累;其Fe(Ⅱ)利用速率达到2.16 kg·(m3·d)−1,比添加亚铁初期时高出1.48 kg·(m3·d)−1。2)在铁自养条件下,在接种污泥介导的反硝化过程中,Fe(Ⅱ)氧化由化学作用主导,
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原由生物作用主导;对于驯化污泥,经富集培养后其生物利用Fe(Ⅱ)的能力显著增强,Fe(Ⅱ)氧化和$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N还原均以生物作用为主导。与接种污泥相比,驯化污泥能够实现更高的Fe(Ⅱ)利用率,更完全的氮素还原,进而减少化学反硝化产物N2O的释放。3)接种污泥在铁自养条件下的生物反硝化作用可被进一步划分为异养反硝化和自养反硝化,其中异养反硝化主要以EPS中的蛋白质作为有机电子供体;驯化污泥的生物反硝化过程仅存在自养反硝化作用。







 下载:
下载: