-
持久性自由基(persistent free radicals, PFRs)是相较活性氧自由基(reactive oxygen radicals, ROS)等瞬时自由基提出的一类长寿命自由基,瞬时自由基如·OH自由基半衰期通常以纳秒计,而现有报道中PFRs半衰期长达数小时、数天甚至数月[1 − 3]. 目前已在大气颗粒物、有机物污染的土壤、香烟焦油等介质中检测到PFRs的存在[4 − 14]. PFRs不仅可以诱导生物体氧化应激造成损伤[5, 12,15 − 22],还可作为多种持久性有机污染物(persistent organic pollutants, POPs)生成的重要中间体[23 − 33],带来潜在环境风险. 作为一类全球污染物,目前已有186个国家或地区加入[11]《关于持久性有机污染物的斯德哥尔摩公约》(以下简称《POPs公约》),以实现对POPs问题的共同管控,中国已于2004年开始履约. 2022年5月,国务院办公厅发布了《新污染物治理行动方案》,POPs也作为一类新污染物列在其中. 源头管控是POPs治理工作的重要策略,识别主要排放源、解析生成转化机制、开发有效POPs控制减排方法,是我国《POPs公约》履约行动及新污染物治理重点工作的迫切要求.
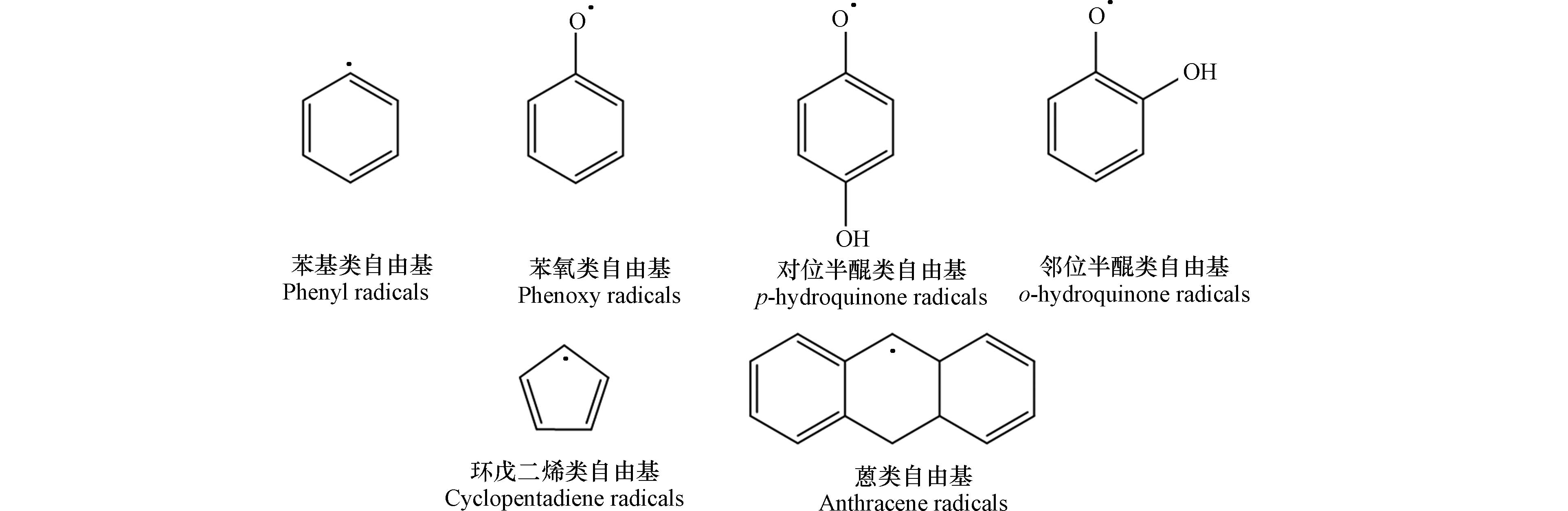
从控制PFRs的角度阻滞POPs的生成有望成为POPs源头管控的新途径. 再生金属冶炼、垃圾焚烧、炼焦、水泥窑协同处理固废等工业热过程是我国POPs的主要排放源[34 − 41]. 近年来研究者发现多氯代二苯并-对-二噁英/呋喃(polychlorinated dibenzo-p-dioxins/furans, PCDD/Fs)等的前驱体生成过程也伴随着PFRs的生成,二者的生成转化存在一定的协同行为. 阐明这些典型工业热过程中PFRs与POPs的生成及转化的相互关系,可为实现新型有效POPs控制减排技术提供理论基础. 与POPs生成相关的PFRs有苯基类自由基、苯氧类自由基、半醌类自由基、环戊二烯类自由基、蒽类自由基等,其结构如图1所示. 识别热化学生成的PFRs,阐明工业热过程中PFRs与POPs的协同行为机制,对从控制PFRs角度开发POPs源头控制减排新策略具有重要意义. 本研究概述了热化学过程PFRs的生成与识别研究,综述了热化学过程PFRs对POPs生成的促进作用研究进展,并从PFRs与POPs的协同控制角度对现有研究进行汇总,从PFRs分析识别、PFRs参与的POPs生成与控制机制等方面提出展望,旨在为我国典型工业热过程排放POPs等新污染物治理工作提供依据和思路.
-
工业热过程是PCDD/Fs、多氯联苯(polychlorinated biphenyls, PCBs)、多氯萘(polychlorinated naphthalenes, PCNs)、氯代多环芳烃(chlorinated polycyclic aromatic hydrocarbons, Cl-PAHs)等的重要排放源,且研究表明这些热化学过程中PFRs是生成POPs的重要中间体,识别关键PFRs中间体的结构是阐明PFRs参与的POPs生成机制的重要环节[42 − 47]. 由于存在结构解析的困难,目前对于热化学过程生成PFRs的研究主要在实验室内完成,针对实际工业热过程样品的研究相对较少. 解析热化学过程生成PFRs的结构,阐明PFRs生成转化机制是完善POPs生成机制理论体系的前提.
-
苯类、酚类、多环芳烃(PAHs)及其卤代物不仅是POPs生成的重要前驱体,也是PFRs生成的重要前驱体. 如苯酚中O—H键断裂可生成苯氧自由基,进一步氧化生成半醌自由基,苯氧自由基脱CO生成环戊二烯自由基等[29, 32, 48 − 51]. 体系中过渡金属的存在可以大大增加PFRs的产率,如氯苯、氯酚前驱体先吸附到Cu(Ⅱ)、Al(Ⅲ)、Fe(Ⅲ)等金属氧化物表面,进一步发生从前驱体向金属的电子转移,形成PFRs-金属共振稳定结构,同时金属被还原,发生化学吸附时也伴随着脱HCl或H2O反应[1, 24, 27, 52]. Zn(Ⅱ)也可促进PFRs的生成及稳定,但电子转移的方向为从金属至前驱体[53]. 共振结构可极大增加PFRs的稳定性,延长PFRs的半衰期,如2,4-二氯-1-萘酚加热后在Al2O3表面生成的PFRs半衰期长达108 d[54]. 此外,温度、氧含量也是影响PFRs生成的重要因素,不同自由基对温度和氧气的敏感度不同,如环戊二烯自由基具有较强的抗热分解能力,而半醌类自由基和苯氧类自由基具有较强的抗氧化能力[55]. 固体表面羟基化程度也会影响PFRs的产率,早期研究认为固体表面羟基是前驱体吸附并发生电子转移生成PFRs的重要位点[1, 27, 30, 48],而近期一项针对前驱体-CuO-SiO2模拟体系生成PFRs的研究发现,1,2-二氯苯前驱体生成PFRs产率与表面羟基化程度成正相关,而一氯酚生成PFRs产率受表面羟基化程度的影响不大,这是由于一氯酚的酚羟基与表面氧或羟基之间有强氢键或范德华相互作用,因此一氯酚相较1,2-二氯苯而言更易吸附到表面羟基位点,进而发生电子转移生成PFRs[56].
-
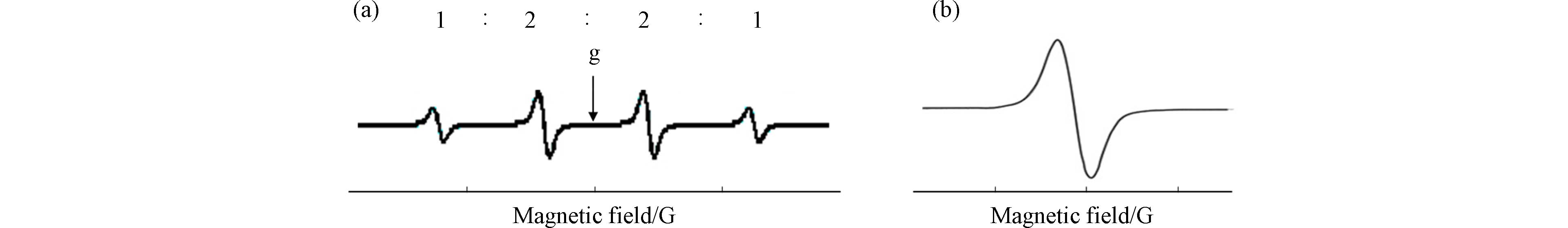
电子顺磁共振波谱仪(electron paramagnetic resonance spectroscopy, EPR)是实现自由基直接检测的有效手段,也是分析检测模拟工业热过程实验中固相体系表面PFRs的常用方法. 通常采用固定微波频率,在一定范围内扫描磁场强度的方式对谐振腔内的顺磁性物质进行检测,谱图以二阶微分的形式呈现,横坐标为磁场强度,纵坐标为响应信号强度,也可将横坐标转换为g因子. 二阶微分谱峰的精细分裂特征和g因子是进行谱图解析、自由基结构识别的关键信息(如图2所示). 精细分裂特征可反映周边自旋对电子自旋的影响,g值是自由基的本征值. 自由电子的g值为
2.0023 ,通常认为C中心自由基的g值小于2.003,O中心自由基的g值大于2.004,C中心且周围有O的自由基g值介于2.003和2.004之间[57].目前对于PFRs的定性及定量均存在困难. 研究对PFRs浓度的报道仅能从数量级进行比较,如CuO质量含量约为1%的硅胶-CuO基质中,230 ℃下2-氯酚气体生成的PFRs约为3×1019 spins·g−1,苯酚气体生成的PFRs约为1018 spins·g−1[58],柴油机燃烧排放的颗粒物中PFR约为1019 spins·g−1[59],大气PM2.5中的PFRs约为1016—1018 spins·g−1[3, 60-61]. 当前仪器仅能做到对PFRs的半定量,仪器状态、样品状态、定量操作等均对定量结果有影响,不同EPR厂家仪器的定量方式亦有区别,因此在进行不同研究之间PFRs浓度比较时需更加谨慎[62]. 定性识别方面,EPR原位无损检测可最大程度还原PFRs的实际存在状态,但无论是模拟工业热过程的含PFRs基质,还是含PFRs的大气颗粒物样品,其中PFRs种类多,且PFRs中自由电子周围通常有多个磁性核,精细分裂特征复杂,导致直接检测得到的EPR谱图往往呈现为一个展宽的单峰(如图2b所示),结构解析困难.
近年来,研究者将理论计算、质谱检测等方法与EPR检测相结合,开发了针对固相体系中PFRs的识别策略. 如在一项模拟再生铜冶炼过程PFRs与POPs协同行为的研究中,通过热过程模拟实验EPR检测与量子化学理论计算结合,计算并绘制体系中潜在生成PFRs的理论谱图,以谱峰宽度、g因子等为依据,将理论谱图与实验样品中PFRs的检测谱图比对拟合,最终确定2-氯酚加热生成的PFRs为高氯代苯氧自由基[57]. Liu等[33]也通过模拟实验与理论计算的结合,基于PFRs理论谱图对实验谱图的拟合迭代,识别出热氧化条件下五氯酚在CuO、ZnO、α-Al2O3、γ-Al2O3分别存在时生成的PFRs种类,CuO主要催化生成高氯代苯氧自由基,ZnO主要催化生成半醌类自由基,而Al2O3主要催化生成甲基取代的苯氧类自由基. 此外,Zhong等[63]首先识别出大气颗粒物中与O中心PFRs具有最强化学转化相关关系的含氧PAHs为醌类,进一步开发了基于FT-ICR-MS的含氧PAHs筛查检测方法,用于大气颗粒物中O中心PFRs的替代识别,并通过热源生成PFRs与光解生成PFRs的比较,提出大气颗粒物上O中心PFRs的溯源方法. 当前的PFRs识别方法对研究体系、PFRs种类等有一定要求,未来可在现有基础上开发更为普适性的PFRs分析检测方法.
-
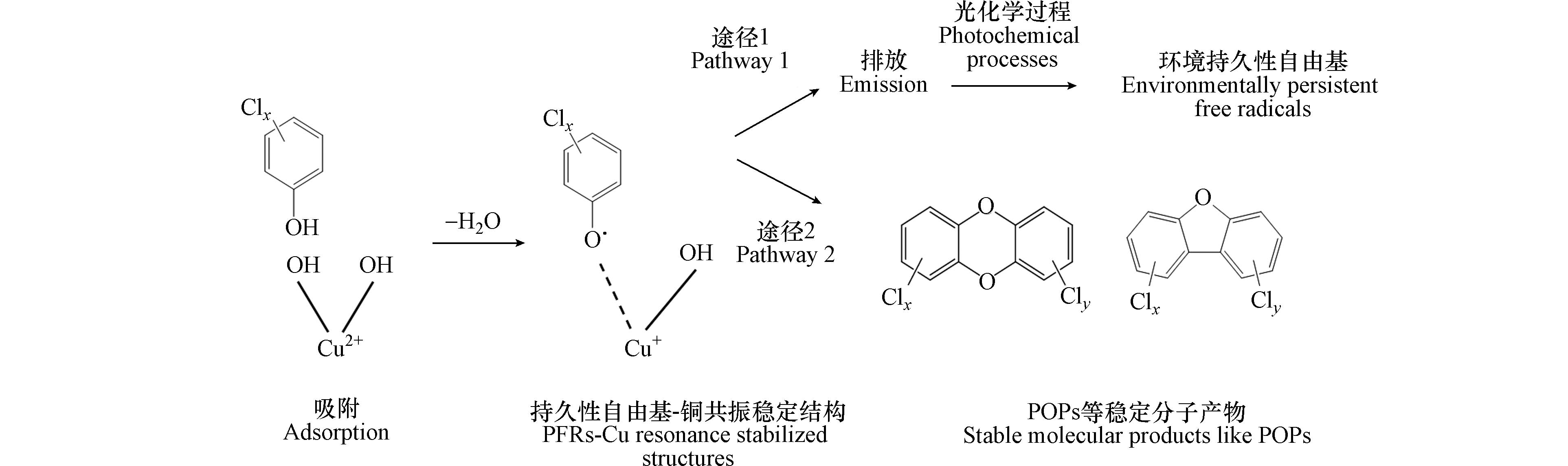
热化学过程中,PFRs与POPs具有相似的生成行为,即以苯、酚、PAHs及其卤代物等为前驱体,经金属催化生成. PFRs是多种POPs生成的中间体,相关反应的可行性通过理论计算得到印证[44, 64-65]. 而另一方面,PFRs与POPs均可作为前驱体的热化学产物存在,且反应条件改变时,PFRs与POPs的种类及浓度变化不尽相同,表明二者的热化学生成转化行为可能存在一定竞争关系. 如图3所示,以氯酚为例,氯酚吸附在Cu(Ⅱ)表面,经电子转移生成氯代苯氧自由基-Cu(Ⅰ)共振稳定结构,若该结构随飞灰、烟气等排放进入环境(途径1),进一步发生“醌-半醌自由基-氢醌”转化[30, 66]等光化学反应,形成大气颗粒物上的环境持久性自由基(environmentally persistent free radicals, EPFRs). 此外,氯代苯氧自由基也可进一步发生耦合、重排等反应生成PCDDs、PCDFs等稳定分子产物(途径2). 目前关于二者竞争关系的研究结果较少,本文主要总结了PFRs作为中间体参与POPs生成的相关研究进展.
Dellinger课题组[67, 68]对于PFRs与POPs生成的关系研究起步较早,在氯酚-CuO体系气相热解和氧化实验中,提出氯酚在金属表面生成氯代苯氧自由基,在空间位阻作用下,PCDFs通过固体表面的自由基-自由基起始反应生成、PCDDs通过固体表面的自由基-气相分子起始反应生成的分子机制,并通过理论计算给出各基元反应并验证了可行性. Evans等[69]探究并提出了溴酚-CuO体系经热化学过程生成多溴代二苯并-对-二噁英/呋喃的机制,与氯酚生成PCDD/Fs机制相似. Kim等[70 − 71]也针对氯酚-CuO体系热化学反应过程的研究,提出了两个一氯代苯氧自由基耦合为二聚体,二聚体经烯醇异构化反应、脱H2O生成PCDFs,或二聚体脱去两分子CO生成二氯代二氢富瓦烯,再经自由基重排及环反应生成PCNs的分子机制. 此外,也有纯理论计算对氯代苯氧自由基生成PCNs、PCDFs等的机理阐释,研究指出一氯酚生成PCNs的过程中优先脱氯而非脱氢,生成PCDFs时则相反,且PCDFs生成的决速步能垒低于PCNs生成的决速步能垒,解释了PCNs的氯代程度低于PCDFs,且PCDFs产物浓度高于PCNs浓度的现象[72 − 74]. 这些早期研究中对PFRs的结构确定主要通过对分子产物检测及对反应机制的推理获得.
如前文“1.2”所述,固相体系中PFRs识别方法的开发为PFRs与POPs生成转化机制的阐明提供了直接的检测证据. Liu等[57]基于对2-氯酚模拟再生铜冶炼过程生成的高氯代苯氧自由基中间体及POPs产物的筛查识别,提出了低氯酚生成高氯代苯氧自由基,高氯代苯氧自由基与低氯酚进一步耦合生成1,2,3,4,7,8-六氯代二噁英的机制,通过相互作用区域指示函数分析计算并解释了PFRs的高氯代带来的空间位阻在两环耦合形成平面结构这一关键步骤中的阻碍作用. 此外,不同金属对PFRs与POPs热化学生成的催化作用亦有差异,在五氯酚-金属氧化物-SiO2体系的加热模拟实验研究中,通过对PFRs的谱图拟合识别及分子产物的筛查分析,研究发现CuO体系的PFRs及POPs产物主要为高氯代苯氧自由基和高氯代PCDDs,ZnO体系主要生成半醌自由基及含氧分子产物,Al2O3体系中则是甲基化苯氧自由基及长链分子产物,表明不同金属催化条件下体系中发生的主要反应类型和稳定分子产物有所不同,CuO体系中易发生高氯代苯氧自由基与高氯酚耦合生成高氯代PCDDs的反应,ZnO体系中易发生氧化反应,Al2O3体系中易发生链增长反应,有助于PAHs的生成[33, 75].
PAHs作为工业热过程烟气、飞灰中的重要成分,也是多种POPs生成的前驱体. Lin等[76]以蒽为前驱体,模拟再生铜冶炼过程,基于对PFRs检测和分子产物筛查,提出蒽生成蒽自由基(图1所示),进一步氧化成含氧蒽自由基及蒽醌的反应机制. 蒽自由基经氯化可生成Cl-PAHs,蒽醌经重排等反应可生成PCDD/Fs、PCBs等.
-
CaO、尿素、硫脲等含Ca、S、N阻滞剂已被发现具有良好的POPs生成抑制效果,且部分已在实际工业生产过程中投入使用以减少POPs生成及排放. 研究表明CaO的加入对氯苯、氯酚生成PCDD/Fs的抑制效果高达99%,抑制机理为Ca优先与体系中Cl结合,进而减少POPs等氯代有机产物的生成,含S物质可以通过发生S对O的取代以及使催化剂失活减少PCDD/Fs的生成,含N物质可通过分解出NH3消耗Cl源和使催化剂失活实现抑制效果[77 − 80].
在对PFRs参与的POPs生成机制研究基础上,探究POPs阻滞剂对PFRs生成的影响可进一步完善POPs生成阻滞机制理论,同时为开发工业热过程PFRs与POPs协同阻滞技术提供基础. 研究表明PCDD/Fs阻滞剂对PFRs的生成也有阻滞效果. Elisabeth等[81]探究了含Ca、S的阻滞剂对PFRs生成的影响,向模拟飞灰中引入一定量硝酸钙和硫酸铵,发现可有效抑制PFRs的生成,并结合元素分析及表面分析手段指出抑制机理为生成的SO2与金属表面结合形成硫酸盐,减少了金属表面活性位点,降低了金属表面对有机前驱体的吸附能力. Lin等[76]关注了热化学过程中CaO对PFRs及多种POPs生成的抑制作用,以蒽为前驱体,CuCl2为催化剂设计模拟加热实验,CaO的加入对PFRs、PCDD/Fs、PCBs、PCNs生成的抑制率分别为85%、97%、93%、97%,当以CuO为催化剂时,此时体系中无添加Cl源,CaO的加入对PFRs生成的抑制率为45%. 有氯源体系中CaO的加入比无氯源体系中CaO的加入对PFRs抑制率高约40%,推测这部分高的抑制来自于CaO对氯源消耗,进而抑制氯参与的PFRs的生成,表明消耗Cl源可能是CaO抑制PFRs生成的路径之一. 为进一步说明不同阻滞剂对PFRs及POPs生成的影响,Wang等[82]以蒽为前驱体,CuCl2为催化剂,CaO,CaS,CH4N2S,(NH4)2SO4为阻滞剂,设计加热模拟实验,探究Ca、S、N的阻滞效果及机制. 结果表明四种阻滞剂对PFRs及POPs的生成均有明显抑制效果,其中含S、N阻滞剂对PFRs抑制效果最好,而CaO对POPs抑制效果最好. 该研究进一步讨论了PFRs与POPs阻滞的协同性,相关性分析结果表明,PFRs浓度与PCDD/Fs、PCBs浓度具有正相关性,尤其是与高氯代产物的浓度相关性更强,而PFRs浓度与PCNs浓度相关性较弱,合理推测PCDD/Fs、PCBs的生成抑制主要由PFRs的生成抑制导致,PCNs生成抑制主要由Cl源被消耗导致.
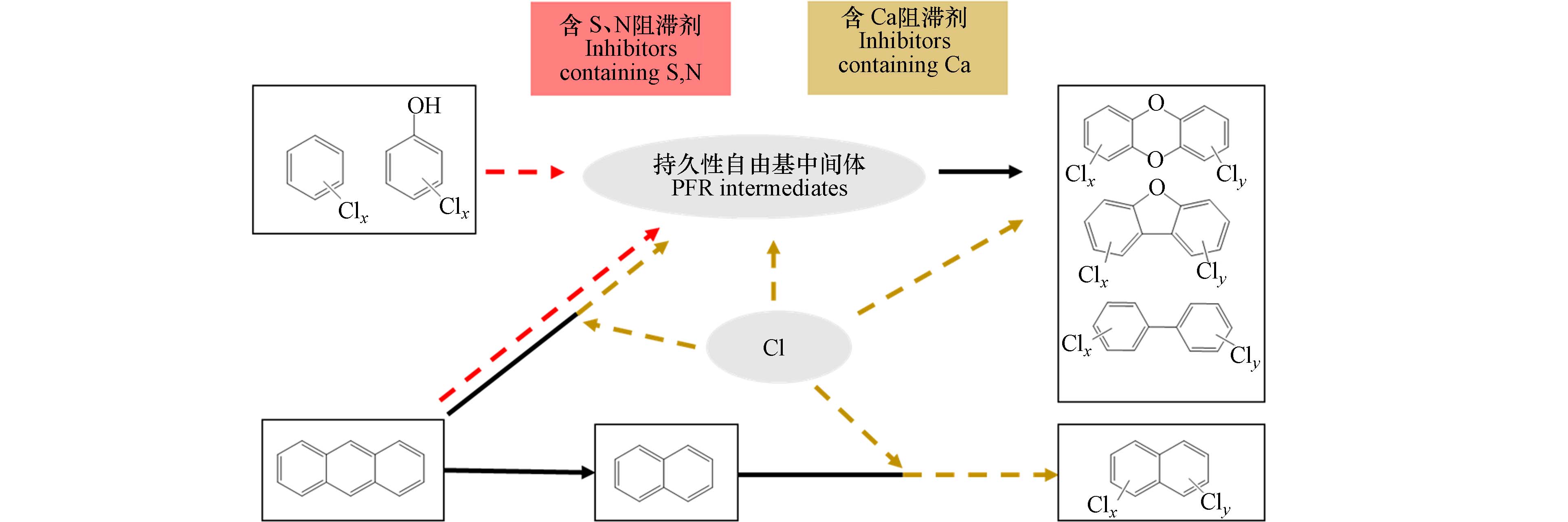
图4对现有研究中PFRs与POPs的协同控制途径进行了梳理,含S、N阻滞剂可以通过降低金属的催化活性减少PFRs中间体的生成,进而抑制后续POPs母环结构的生成,含Ca阻滞剂可以与Cl结合以无机氯形式存在,减少有机氯产物的生成. 氯苯、氯酚为主要前驱体的体系中,含S、N阻滞剂可有效实现PFRs与PCDD/Fs、PCBs的协同控制,蒽为前驱体的体系中,含Ca阻滞剂可有效减少PCNs生成的氯化反应. 此外,PFRs与POPs的协同生成研究表明氯苯、氯酚前驱体可以通过生成PFRs中间体进而生成PCNs,该过程是否为PCNs的主要生成机制,以及常见阻滞剂对该过程PFRs与POPs的协同控制效果及机制仍待研究. 氯苯、氯酚、蒽是工业热过程飞灰基质中的典型前驱体,后续可在现有研究基础上,基于对实际飞灰有机组分及金属组成的分析识别,完善常见阻滞剂对多种典型前驱体生成PFRs及POPs的效果及抑制机制理论体系,为PFRs及POPs协同控制方法开发提供依据.
-
再生金属冶炼等工业热过程是我国POPs的主要排放源,解析POPs生成机制,开发POPs生成阻滞技术是我国《POPs公约》履约行动和新污染物治理工作的需求. PFRs是POPs热生成过程的重要中间体,二者的生成行为具有一定的协同性,为开发PFRs与POPs的源头协同控制技术提供了条件,本文对现有PFRs与POPs的热化学生成及阻滞的协同行为研究进行了综述,在此基础上,未来的研究可从以下方向进行深入探讨:
(1)PFRs的定性及定量研究:当前PFRs的半定量结果仅能用于数量级之间的比较,且不同测试之间数据可比性较差,未来可通过开发标准物质,建立内标法等方式实现定量结果的校正. 此外,应继续开发更为普适性的PFRs结构识别方法,用于实际工业热过程飞灰中PFRs的检测,为PFRs及POPs的生成及阻滞研究提供依据.
(2)PFRs中间体对POPs及EPFRs的生成贡献研究:再生金属冶炼等工业热过程具备PFRs生成的前驱体、金属催化剂、温度等条件,生成的PFRs可进一步生成POPs,或随飞灰排放进入环境大气,以EPFRs的形式存在. 未来可在PFRs准确定量的基础上,结合同位素示踪等手段,探究不同条件下PFRs对POPs及EPFRs的生成贡献及机制,进而开发不同工业热过程中POPs、EPFRs的针对性控制技术.
(3)PFRs参与的多种POPs生成机制及影响因素研究:当前对POPs的自由基生成机制研究主要关注PCDD/Fs,对PCNs、PCBs等有部分研究,对工业热过程排放的其他有机卤代物如Cl-PAHs等的自由基生成机制关注较少,未来可进一步深入探究多种POPs生成的自由基机制,完善多种POPs及类似物的热化学生成机制理论体系,解释不同工业热过程中多种POPs及类似产物的关键生成机理.
(4)阻滞剂对PFRs和POPs生成的协同抑制研究:碱性、含S、含N等POPs阻滞剂对PFRs的生成也表现出明显的抑制效果,但PFRs的生成抑制机理及PFRs生成抑制对POPs控制的作用仍待阐明,PFRs与不同POPs的协同抑制行为及关键影响因素仍待进一步探究,为针对不同工业热过程选择最优阻滞剂配比、开发针对性PFRs与POPs阻滞方法提供数据支撑.
持久性自由基的热化学生成及其与持久性有机污染物的协同行为
Persistent free radicals formation during thermochemical processes and their synergistic behavior with persistent organic pollutants
-
摘要: 工业热过程是我国POPs的重要排放源,热化学过程中持久性自由基(persistent free radicals,PFRs)与持久性有机污染物(persistent organic pollutants,POPs)的生成行为具有协同性. 阐明PFRs的生成机制及其对POPs生成和控制的关键作用,是开发高效PFRs与POPs协同控制技术的理论基础,也是我国履约行动及新污染物治理工作的重要支撑. 本文从热化学过程PFRs的生成及识别方法、PFRs与POPs的协同生成及协同控制行为方面对现有研究进行了综述,并对未来研究方向进行了展望.Abstract: Industrial thermal processes are significant sources of persistent organic pollutants (POPs) in China. The generation of persistent free radicals (PFRs) during thermochemical processes exhibits synergistic behavior with the formation of POPs. Clarifying the mechanisms of PFR generation and their critical role in forming and controlling POPs is crucial for developing effective technologies for the simultaneous control of PFRs and POPs. It serves as both a theoretical foundation for the implementation of international conventions and a support for managing new pollutants. This paper reviews existing research on the generation and identification methods of PFRs in thermochemical processes, as well as the synergistic behaviors and control strategies involving PFRs and POPs. Additionally, it provides an outlook on future research directions.
-

-
-
[1] DELLINGER B, LOMNICKI S, KHACHATRYAN L, et al. Formation and stabilization of persistent free radicals[J]. Proceedings of the Combustion Institute, 2007, 31(1): 521-528. doi: 10.1016/j.proci.2006.07.172 [2] VEJERANO E, LOMNICKI S, DELLINGER B. Lifetime of combustion-generated environmentally persistent free radicals on Zn(II)O and other transition metal oxides[J]. Journal of Environmental Monitoring, 2012, 14(10): 2803-2806. doi: 10.1039/c2em30545c [3] GEHLING W, DELLINGER B. Environmentally persistent free radicals and their lifetimes in PM2.5[J]. Environmental Science & Technology, 2013, 47(15): 8172-8178 [4] VEJERANO E, LOMNICKI S M, DELLINGER B. Formation and stabilization of combustion-generated, environmentally persistent radicals on Ni(II)O supported on a silica surface[J]. Environmental Science & Technology, 2012, 46(17): 9406-9411. [5] GEHLING W, KHACHATRYAN L, DELLINGER B. Hydroxyl radical generation from environmentally persistent free radicals (EPFRs) in PM2.5[J]. Environmental Science & Technology, 2014, 48(8): 4266-4272. [6] CHEN Q C, SUN H Y, WANG M M, et al. Environmentally persistent free radical (EPFR) formation by visible-light illumination of the organic matter in atmospheric particles[J]. Environmental Science & Technology, 2019, 53(17): 10053-10061. [7] YANG L L, LIU G R, ZHENG M H, et al. Highly elevated levels and particle-size distributions of environmentally persistent free radicals in haze-associated atmosphere[J]. Environmental Science & Technology, 2017, 51(14): 7936-7944. [8] ZHAO S, GAO P, MIAO D, et al. Formation and evolution of solvent-extracted and nonextractable environmentally persistent free radicals in fly ash of municipal solid waste incinerators[J]. Environmental Science & Technology, 2019, 53(17): 10120-10130. [9] DELA CRUZ A L, GEHLING W, LOMNICKI S, et al. Detection of environmentally persistent free radicals at a superfund wood treating site[J]. Environmental Science & Technology, 2011, 45(15): 6356-6365. [10] DELA CRUZ A L, COOK R L, DELLINGER B, et al. Assessment of environmentally persistent free radicals in soils and sediments from three Superfund sites[J]. Environmental Science. Processes & Impacts, 2014, 16(1): 44-52. [11] MASKOS Z, KHACHATRYAN L, DELLINGER B. Role of the filters in the formation and stabilization of semiquinone radicals collected from cigarette smoke[J]. Energy & Fuels, 2013, 27(9): 10.1021/ef4010253. [12] EDWARDS K C, KAPUR S, FANG T, et al. Residential wood burning and vehicle emissions as major sources of environmentally persistent free radicals in Fairbanks, Alaska[J]. Environmental Science & Technology, 2024, 58(32): 14293-14305. [13] WANG L Y, ZHAO W D, LUO P R, et al. Environmentally persistent free radicals in PM 2.5 from a typical Chinese industrial city during COVID-19 lockdown: The unexpected contamination level variation[J]. Journal of Environmental Sciences, 2024, 135: 424-432. doi: 10.1016/j.jes.2022.08.024 [14] JIA S M, WANG D Q, LIU L Y, et al. Size-resolved environmentally persistent free radicals in cold region atmosphere: Implications for inhalation exposure risk[J]. Journal of Hazardous Materials, 2023, 443: 130263. doi: 10.1016/j.jhazmat.2022.130263 [15] DELLINGER B, PRYOR W A, CUETO R, et al. Role of free radicals in the toxicity of airborne fine particulate matter[J]. Chemical Research in Toxicology, 2001, 14(10): 1371-1377. doi: 10.1021/tx010050x [16] REED J R, CAWLEY G F, ARDOIN T G, et al. Environmentally persistent free radicals inhibit cytochrome P450 activity in rat liver microsomes[J]. Toxicology and Applied Pharmacology, 2014, 277(2): 200-209. doi: 10.1016/j.taap.2014.03.021 [17] MAHNE S, CHUANG G C, PANKEY E, et al. Environmentally persistent free radicals decrease cardiac function and increase pulmonary artery pressure[J]. American Journal of Physiology. Heart and Circulatory Physiology, 2012, 303(9): H1135-H1142. doi: 10.1152/ajpheart.00545.2012 [18] LIAO S H, PAN B, LI H, et al. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings[J]. Environmental Science & Technology, 2014, 48(15): 8581-8587 [19] ZHANG D M, WANG J, CHEN H, et al. Fast hydroxyl radical generation at the air-water interface of aerosols mediated by water-soluble PM2.5 under ultraviolet A radiation[J]. Journal of the American Chemical Society, 2023, 145(11): 6462-6470. doi: 10.1021/jacs.3c00300 [20] YAMAMOTO A, SLY P D, KHACHATRYAN L, et al. Environmentally persistent free radicals modify oxidative stress related gene expression in well-differentiated human nasal epithelium[C]//C68. Topics In Airway And Alveolar Epithelial Cell Biology. American Thoracic Society, 2023, 207: A5682. [21] WANG S Y, GALLIMORE P J, LIU-KANG C, et al. Dynamic wood smoke aerosol toxicity during oxidative atmospheric aging[J]. Environmental Science & Technology, 2023, 57(3): 1246-1256. [22] LI H, CHEN Q C, WANG C, et al. Pollution characteristics of environmental persistent free radicals (EPFRs) and their contribution to oxidation potential in road dust in a large city in northwest China[J]. Journal of Hazardous Materials, 2023, 442: 130087. doi: 10.1016/j.jhazmat.2022.130087 [23] EVANS C S, DELLINGER B. Mechanisms of dioxin formation from the high-temperature pyrolysis of 2-chlorophenol[J]. Environmental Science & Technology, 2003, 37(7): 1325-1330. [24] FARQUAR G R, ALDERMAN S L, POLIAKOFF E D, et al. X-ray spectroscopic studies of the high temperature reduction of Cu(II)O by 2-chlorophenol on a simulated fly ash surface[J]. Environmental Science & Technology, 2003, 37(5): 931-935. [25] KHACHATRYAN L, BURCAT A, DELLINGER B. An elementary reaction-kinetic model for the gas-phase formation of 1, 3, 6, 8- and 1, 3, 7, 9-tetrachlorinated dibenzo-p-dioxins from 2, 4, 6–trichlorophenol[J]. Combustion and Flame, 2003, 132(3): 406-421. doi: 10.1016/S0010-2180(02)00486-8 [26] LOMNICKI S, DELLINGER B. Formation of PCDD/F from the pyrolysis of 2-chlorophenol on the surface of dispersed copp er oxide particles[J]. Proceedings of the Combustion Institute, 2002, 29(2): 2463-2468. doi: 10.1016/S1540-7489(02)80300-5 [27] ALDERMAN S L, DELLINGER B. FTIR investigation of 2-chlorophenol chemisorption on a silica surface from 200 to 500 degrees C[J]. The Journal of Physical Chemistry. A, 2005, 109(34): 7725-7731. doi: 10.1021/jp051071t [28] EVANS C S, DELLINGER B. Mechanisms of dioxin formation from the high-temperature oxidation of 2-bromophenol[J]. Environmental Science & Technology, 2005, 39(7): 2128-2134. [29] KHACHATRYAN L, ADOUNKPE J, MASKOS Z, et al. Formation of cyclopentadienyl radical from the gas-phase pyrolysis of hydroquinone, catechol, and phenol[J]. Environmental Science & Technology, 2006, 40(16): 5071-5076. [30] LOMNICKI S, TRUONG H, VEJERANO E, et al. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter[J]. Environmental Science & Technology, 2008, 42(13): 4982-4988. [31] YANG L L, LIU G R, ZHENG M H, et al. Molecular mechanism of dioxin formation from chlorophenol based on electron paramagnetic resonance spectroscopy[J]. Environmental Science & Technology, 2017, 51(9): 4999-5007. [32] LIU X Y, YANG L L, LIU G R, et al. Formation of environmentally persistent free radicals during thermochemical processes and their correlations with unintentional persistent organic pollutants[J]. Environmental Science & Technology, 2021, 55(10): 6529-6541. [33] LIU S T, LIU G R, YANG L L, et al. Metal-catalyzed formation of organic pollutants intermediated by organic free radicals[J]. Environmental Science & Technology, 2022, 56(20): 14550-14561. [34] TUPPURAINEN K, HALONEN I, RUOKOJÄRVI P, et al. Formation of PCDDs and PCDFs in municipal waste incineration and its inhibition mechanisms: A review[J]. Chemosphere, 1998, 36(7): 1493-1511. doi: 10.1016/S0045-6535(97)10048-0 [35] YANG Y P, LIU G R, ZHENG M H, et al. Discovery of significant atmospheric emission of halogenated polycyclic aromatic hydrocarbons from secondary zinc smelting[J]. Ecotoxicology and Environmental Safety, 2022, 238: 113594. doi: 10.1016/j.ecoenv.2022.113594 [36] NI Y W, ZHANG H J, FAN S, et al. Emissions of PCDD/Fs from municipal solid waste incinerators in China[J]. Chemosphere, 2009, 75(9): 1153-1158. doi: 10.1016/j.chemosphere.2009.02.051 [37] JIN R, ZHAN J Y, LIU G R, et al. Variations and factors that influence the formation of polychlorinated naphthalenes in cement kilns co-processing solid waste[J]. Journal of Hazardous Materials, 2016, 315: 117-125. doi: 10.1016/j.jhazmat.2016.05.003 [38] LIU G R, ZHENG M H, LIU W B, et al. Atmospheric emission of PCDD/Fs, PCBs, hexachlorobenzene, and pentachlorobenzene from the coking industry[J]. Environmental Science & Technology, 2009, 43(24): 9196-9201. [39] LIU G R, ZHENG M H, BA T, et al. A preliminary investigation on emission of polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls from coke plants in China[J]. Chemosphere, 2009, 75(5): 692-695. doi: 10.1016/j.chemosphere.2009.01.006 [40] LI S M, LIU G R, ZHENG M H, et al. Unintentional production of persistent chlorinated and brominated organic pollutants during iron ore sintering processes[J]. Journal of Hazardous Materials, 2017, 331: 63-70. doi: 10.1016/j.jhazmat.2017.02.027 [41] SIDHU S, KASTI N, EDWARDS P, et al. Hazardous air pollutants formation from reactions of raw meal organics in cement kilns[J]. Chemosphere, 2001, 42(5/6/7): 499-506. [42] WANG X Y, LIU H J, XUE Y G, et al. Formation of environmentally persistent free radicals and their risks for human health: A review[J]. Environmental Chemistry Letters, 2024, 22(3): 1327-1343. doi: 10.1007/s10311-024-01701-x [43] YANG J Z, ZHANG B T, TIAN L, et al. Free radical formation via BDE-209 thermolysis in the precalciner of a cement kiln: Simulation and DFT study[J]. Science of the Total Environment, 2023, 905: 167145. doi: 10.1016/j.scitotenv.2023.167145 [44] WANG Y, HUANG J B, LI S J, et al. A mechanistic and kinetic investigation on the oxidative thermal decomposition of decabromodiphenyl ether[J]. Environmental Pollution, 2023, 333: 121991. doi: 10.1016/j.envpol.2023.121991 [45] XU Y L, LU X F, SU G J, et al. Scientific and regulatory challenges of environmentally persistent free radicals: From formation theory to risk prevention strategies[J]. Journal of Hazardous Materials, 2023, 456: 131674. doi: 10.1016/j.jhazmat.2023.131674 [46] YUAN Z H, HUANG Q J, WANG Z Q, et al. Medium-Low Temperature Conditions Induce the Formation of Environmentally Persistent Free Radicals in Microplastics with Conjugated Aromatic-Ring Structures during Sewage Sludge Pyrolysis[J]. Environmental Science & Technology, 2022, 56(22): 16209-16220. [47] KHACHATRYAN L, BAREKATI-GOUDARZI M, ASATRYAN R, et al. Metal-Free Biomass-Derived Environmentally Persistent Free Radicals (Bio-EPFRs) from Lignin Pyrolysis[J]. ACS Omega, 2022, 7(34): 30241-30249. doi: 10.1021/acsomega.2c03381 [48] VEJERANO E, LOMNICKI S, DELLINGER B. Formation and stabilization of combustion-generated environmentally persistent free radicals on an Fe(III)2O3/silica surface[J]. Environmental Science & Technology, 2011, 45(2): 589-594. [49] KHACHATRYAN L, ADOUNKPE J, DELLINGER B. Formation of phenoxy and cyclopentadienyl radicals from the gas-phase pyrolysis of phenol[J]. The Journal of Physical Chemistry. A, 2008, 112(3): 481-487. doi: 10.1021/jp073999m [50] PRATALI MAFFEI L, PELUCCHI M, FARAVELLI T, et al. Theoretical study of sensitive reactions in phenol decomposition[J]. Reaction Chemistry & Engineering, 2020, 5(3): 452-472. [51] WANG W, ZHANG R Y, LIU Z H, et al. Periodic DFT calculation for the formation of EPFRs from phenol on gamma-Al2O3 (110): Site-dependent mechanism and the role of ambient water[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108386. doi: 10.1016/j.jece.2022.108386 [52] HUANG M J, HAN Y, XIANG W, et al. In Situ-Formed Phenoxyl Radical on the CuO Surface Triggers Efficient Persulfate Activation for Phenol Degradation[J]. Environmental Science & Technology, 2021, 55(22): 15361-15370. [53] THIBODEAUX C A, POLIAKOFF E D, KIZILKAYA O, et al. Probing environmentally significant surface radicals: Crystallographic and temperature dependent adsorption of phenol on ZnO[J]. Chemical Physics Letters, 2015, 638: 56-60. doi: 10.1016/j.cplett.2015.08.026 [54] YANG L L, LIU G R, ZHENG M H, et al. Pivotal roles of metal oxides in the formation of environmentally persistent free radicals[J]. Environmental Science & Technology, 2017, 51(21): 12329-12336. [55] ADOUNKPE J, KHACHATRYAN L, DELLINGER B. Radicals from the gas-phase pyrolysis of hydroquinone: 1. temperature dependence of the total radical yield[J]. Energy & Fuels, 2008, 22(5): 2986-2990. [56] KHACHATRYAN L, REZK M Y, NDE D, et al. New features of laboratory-generated EPFRs from 1, 2-dichlorobenzene (DCB) and 2-monochlorophenol (MCP)[J]. ACS Omega, 2024, 9(8): 9226-9235. doi: 10.1021/acsomega.3c08271 [57] LIU X Y, LIU G R, LIU S T, et al. Free radical mechanism of toxic organic compound formations from o-chlorophenol[J]. Journal of Hazardous Materials, 2023, 443: 130367. doi: 10.1016/j.jhazmat.2022.130367 [58] KIRURI L W, KHACHATRYAN L, DELLINGER B, et al. Effect of copper oxide concentration on the formation and persistency of environmentally persistent free radicals (EPFRs) in particulates[J]. Environmental Science & Technology, 2014, 48(4): 2212-2217. [59] ROSS M M, CHEDEKEL M R, RISBY T H, et al. Electron paramagnetic resonance spectrometry of diesel particulate matter[J]. Environment International, 1982, 7(5): 325-329. doi: 10.1016/0160-4120(82)90124-6 [60] ARANGIO A M, TONG H J, SOCORRO J, et al. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles[J]. Atmospheric Chemistry and Physics, 2016, 16(20): 13105-13119. doi: 10.5194/acp-16-13105-2016 [61] CHEN Q C, WANG M M, WANG Y Q, et al. Rapid determination of environmentally persistent free radicals (EPFRs) in atmospheric particles with a quartz sheet-based approach using electron paramagnetic resonance (EPR) spectroscopy[J]. Atmospheric Environment, 2018, 184: 140-145 doi: 10.1016/j.atmosenv.2018.04.046 [62] GUAN F Y, WEN J L, LIU J Y, et al. Simple Colorimetric Assay for Quantification of Persistent Free Radicals in Biochars[J]. Environmental Science & Technology Letters, 2023, 10(1): 46-51. [63] ZHONG L J, ZHU B, SU W Y, et al. Molecular characterization of diverse quinone analogs for discrimination of aerosol-bound persistent pyrolytic and photolytic radicals[J]. Science Bulletin, 2024, 69(5): 612-620. doi: 10.1016/j.scib.2023.12.011 [64] XU Y, YANG L L, WANG X P, et al. Risk evaluation of environmentally persistent free radicals in airborne particulate matter and influence of amospheric factors[J]. Ecotoxicology and Environmental Safety, 2020, 196: 110571 doi: 10.1016/j.ecoenv.2020.110571 [65] YU X Q, CHANG J M, LIU X, et al. Theoretical study on the formation mechanism of polychlorinated dibenzothiophenes/thianthrenes from 2-chlorothiophenol molecules[J]. Journal of Environmental Sciences, 2018, 66: 318-327. doi: 10.1016/j.jes.2017.05.007 [66] BORROWMAN C K, ZHOU S M, BURROW T E, et al. Formation of environmentally persistent free radicals from the heterogeneous reaction of ozone and polycyclic aromatic compounds[J]. Physical Chemistry Chemical Physics, 2016, 18(1): 205-212. doi: 10.1039/C5CP05606C [67] LOMNICKI S, DELLINGER B. A detailed mechanism of the surface-mediated formation of PCDD/F from the oxidation of 2-chlorophenol on a CuO/silica surface[J]. The Journal of Physical Chemistry A, 2003, 107(22): 4387-4395 doi: 10.1021/jp026045z [68] KHACHATRYAN L, LOMNICKI S, DELLINGER B. An expanded reaction kinetic model of the CuO surface-mediated formation of PCDD/F from pyrolysis of 2-chlorophenol[J]. Chemosphere, 2007, 68(9): 1741-1750 doi: 10.1016/j.chemosphere.2007.03.042 [69] EVANS C S, DELLINGER B. Surface-mediated formation of polybrominated dibenzo-p-dioxins and dibenzofurans from the high-temperature pyrolysis of 2-bromophenol on a CuO/silica surface[J]. Environmental Science & Technology, 2005, 39(13): 4857-4863. [70] KIM D H, MULHOLLAND J A, RYU J Y. Formation of polychlorinated naphthalenes from chlorophenols[J]. Proceedings of the Combustion Institute, 2005, 30(1): 1245-1253. doi: 10.1016/j.proci.2004.08.013 [71] KIM D H, MULHOLLAND J A, RYU J Y. Chlorinated naphthalene formation from the oxidation of dichlorophenols[J]. Chemosphere, 2007, 67(9): S135-S143. doi: 10.1016/j.chemosphere.2006.05.095 [72] XU F, SHI X L, ZHANG Q Z. Quantum chemical and kinetic study on polychlorinated naphthalene formation from 3-chlorophenol precursor[J]. International Journal of Molecular Sciences, 2015, 16(9): 20620-20640. doi: 10.3390/ijms160920620 [73] XU F, YU W N, GAO R, et al. Dioxin formations from the radical/radical cross-condensation of phenoxy radicals with 2-chlorophenoxy radicals and 2, 4, 6-trichlorophenoxy radicals[J]. Environmental Science & Technology, 2010, 44(17): 6745-6751. [74] XU F, ZHANG R M, LI Y F, et al. Homogeneous gas-phase formation of polychlorinated naphthalene from dimerization of 4-chlorophenoxy radicals and cross-condensation of phenoxy radical with 4-chlorophenoxy radical: Mechanism and kinetics study[J]. Chemical Physics Letters, 2015, 638: 153-160. doi: 10.1016/j.cplett.2015.08.052 [75] LIU S T, LIU G R, YANG L L, et al. Critical influences of metal compounds on the formation and stabilization of environmentally persistent free radicals[J]. Chemical Engineering Journal, 2022, 427: 131666. doi: 10.1016/j.cej.2021.131666 [76] LIN B C, YANG L L, ZHENG M H, et al. Synergetic promoting/inhibiting mechanisms of copper/calcium compounds in the formation of persistent organic pollutants and environmentally persistent free radicals from anthracene[J]. Chemical Engineering Journal, 2022, 441: 136102. doi: 10.1016/j.cej.2022.136102 [77] LIU W B, ZHENG M H, ZHANG B, et al. Inhibition of PCDD/Fs formation from dioxin precursors by calcium oxide[J]. Chemosphere, 2005, 60(6): 785-790. doi: 10.1016/j.chemosphere.2005.04.020 [78] LIN X Q, ZHAN M X, YAN M, et al. Suppression of dioxins in waste incinerator emissions by recirculating SO2[J]. Chemosphere, 2015, 133: 75-81. doi: 10.1016/j.chemosphere.2015.03.080 [79] WANG Y F, QIAN L X, YU Z W, et al. Inhibition behavior of PCDD/Fs congeners by addition of N-containing compound in the iron ore sintering[J]. Aerosol and Air Quality Research, 2020, 20(11): 2568-2579. doi: 10.4209/aaqr.2019.12.0660 [80] WANG P J, XIE F, YAN F, et al. Inhibitory effect and mechanism of an N-S-based inhibitor (CH4N2S) on PCDD/fs in flue gas and fly ash in a full-scale municipal solid waste incinerator[J]. ACS ES& T Engineering, 2023, 3(10): 1557-1567. [81] FELD-COOK E E, BOVENKAMP-LANGLOIS L, LOMNICKI S M. Effect of particulate matter mineral composition on environmentally persistent free radical (EPFR) formation[J]. Environmental Science & Technology, 2017, 51(18): 10396-10402. [82] WANG X, LIN B C, WANG J, et al. Insight into the inhibition mechanisms of Ca-, N-, S-containing compounds on persistent organic pollutant formation from the thermal reaction of anthracene on copper chloride[J]. Chemical Engineering Journal, 2024, 497: 154824. doi: 10.1016/j.cej.2024.154824 -



 下载:
下载: