-
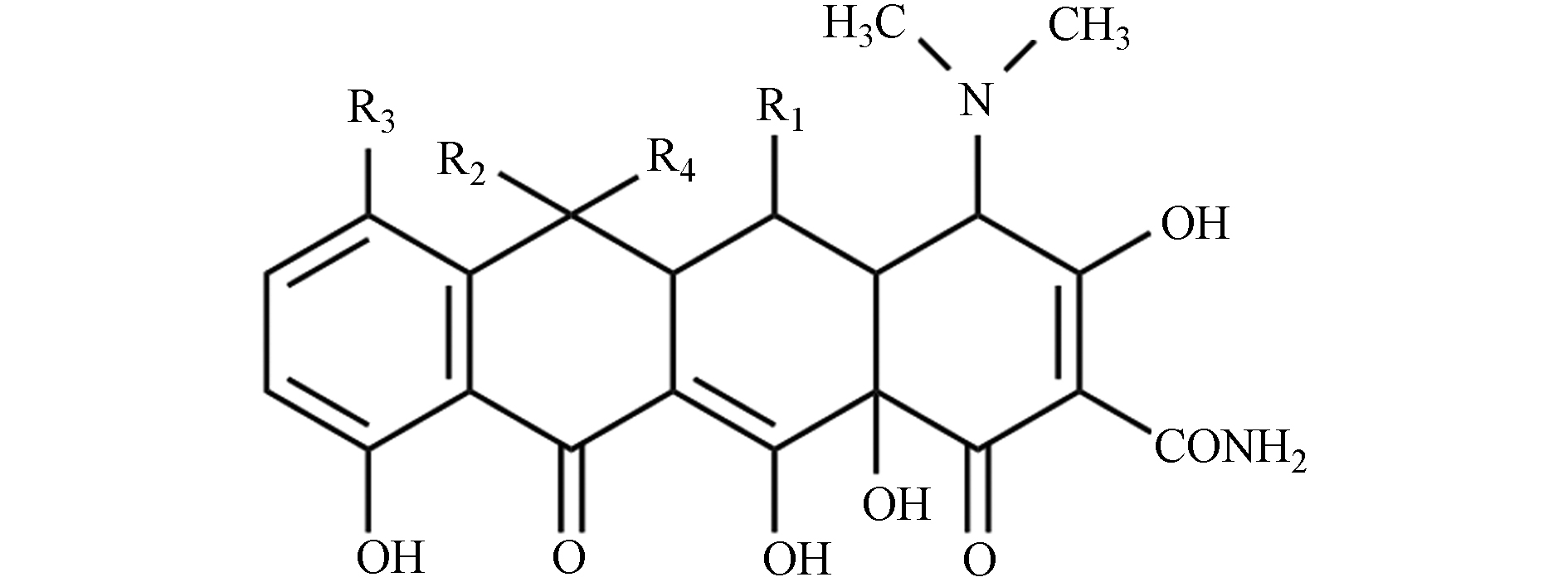
四环素类抗生素(tetracyclines,TCs)是一种天然或半合成的广谱性抗生素,其名称来源于4个碳氢环(如图1),对多种革兰氏阳性和革兰氏阴性细菌等致病菌具有抗菌活性[1]. 其中,四环素(TC)、土霉素(OTC)、金霉素(CTC)和强力霉素(DOX)是目前常用的4种类型[2],其理化性质见表1. 据统计,TCs是中国畜牧业中使用最广泛的兽药抗生素和饲料添加剂[3],每年消耗量达1000 t[4].
研究表明,超过75%的TCs无法被动物体完全代谢或吸收[5],导致其不可避免地排放到水生环境中. 目前已在多个国家的湖泊(ND未检出—680 ng·L−1)、河流(ND—741.85 ng·L−1)甚至饮用水(ND—182 ng·L−1)中频繁检出[3,6],而制药厂的废水虽然经过污水处理厂处理,但在部分处理过的废水中仍能检出,浓度高达88 μg·L-1[7]. 水环境中的TCs污染会直接导致环境微生物生态系统的破坏,可能引起水生环境中微生物(尤为病原微生物)种群抗生素耐药性的变化,诱导TCs抗性基因(TET-ARGs)的产生、传播以及抗性菌产生的风险[8-9]. 研究指出,即使水体中TCs浓度较低,也可能促进具有抗生素耐药性甚至多重耐药性细菌的繁殖,从而危及生命健康[10]. 目前,抗生素耐药性已被世卫组织列为人类健康的全球性威胁[11]. 据估计,如果抗生素耐药性得不到有效缓解,到2050年,每年将会导致约1000万人死亡,造成数万亿美元的经济损失[12]. 基于以上事实,我国“十四五”规划已将抗生素类物质列入环保重点管控新污染物名单. 因此,开发一种有效的方法来降解水环境中的TCs是新污染治理邻域亟待解决的问题.
光催化降解技术是一种处理效率高、环境友好、具有经济效益的降解技术. 其以太阳辐射为能源,利用光催化剂电子-空穴对的强氧化还原特性进行降解,无需加入额外的氧化剂/还原剂便可将TCs降解为二氧化碳和水,可有效缓解抗生素耐药性的传播[13]. 近年来,光催化降解TCs的技术主要围绕单组分光催化剂和复合光催化剂展开. 然而TiO2[14]、WO3[15]、Cu2O[16]等传统的单组分光催化剂仍存在可见光利用不足、光生电荷快速湮灭、目标物质矿化不完全等缺陷,严重制约了其应用. 为提高光催化效率,研究者们主要从扩大光吸收范围、提升光生载流子分离效率、增加活性位点以及增强氧化还原能力等方面对单组分光催化剂进行复合改性以提高其催化性能[17].
本文综述了复合光催化剂降解水体中TCs的最新研究进展. 首先从负载介孔材料、掺杂金属/非金属元素、染料敏化、负载助催化剂、构造异质结结构等方面详细阐述了复合光催化剂在水体中TCs降解领域的发展与应用,然后针对复合光催化剂催化降解TCs的机理及过程进行介绍,最后提出复合光催化剂降解水体中TCs的主要挑战和发展前景.
-
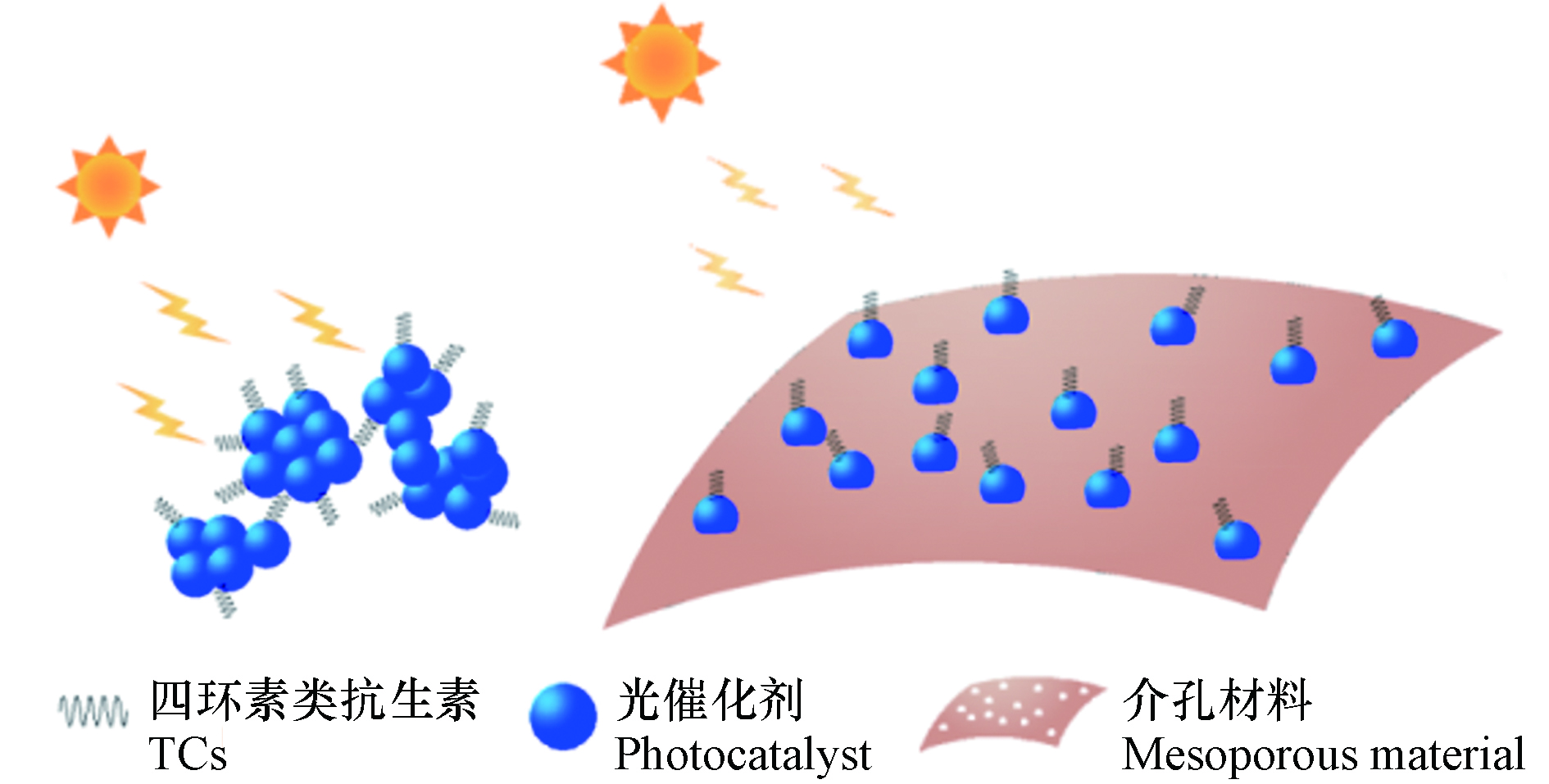
介孔材料是一种孔径介于2—50 nm的多孔材料,如图2所示,介孔材料所具有的较大比表面积和多孔表面,可以使颗粒状的光催化剂均匀分散在其表面或孔隙中,有效解决单一组分的光催化剂因团聚而阻碍活性位点对光的吸收问题[18]. 已有大量文献表明负载介孔材料的光催化剂在光催化降解TCs的应用中具有出色的性能(表2).
目前,常用的介孔材料包括介孔碳基材料、多孔矿物以及金属有机骨架材料(MOFs)[19-25]. 介孔碳基材料主要有石墨相氮化碳(g-C3N4)、石墨烯以及活性炭等[29],它们作为新一代介孔材料,在具有高比表面积、大孔径的同时,还兼具良好的热稳定性、机械稳定性以及化学反应惰性[30],可有效提高TCs的降解效率. Li等[26]采用溶胶-凝胶法将TiO2负载于还原氧化石墨烯(RGO)表面,制取了新型的基于介孔碳基材料的TiO2-RGO-TiO2复合光催化剂. RGO表面的含氧官能团为TiO2纳米颗粒提供了丰富的生长位点;同时,RGO优良的导电性加快了TiO2的光生电子转移,提高了电荷分离效率. 因此与单组分TiO2相比,复合光催化剂的光催化性能得到显著提升,在紫外光和模拟太阳光照射下,TC的降解效率分别提高了22.8%和32.8%.
矿石作为一种成本低廉的材料,还兼具吸附量大的优点,对TCs具有较强的吸附能力[31]. TCs上的羟基(-OH)可通过氢键的方式与矿石结合[32],同时矿石表面的负电荷可通过静电相互作用对TCs产生较强的吸附性. Shi等[27]将TiO2和AgBr负载在具有均匀介孔孔隙的坡缕石(Pal)表面,制备了新型AgBr-TiO2-Pal复合催化剂,在最佳反应条件下,TC去除率为89.0%,矿化率为67.0%,最大吸附量是单一TiO2的4.7倍. Papoulis等[33]通过水热法将TiO2颗粒均匀地沉积和分散在介孔级的海泡石表面,制得了新型的矿石-光催化剂复合材料. 与单一组分的TiO2-P25(平均粒径为25 nm的粉末状TiO2)相比,负载海泡石的复合光催化剂具有更多的活性位点、更高的光催化活性. 上述报道表明,矿石作为廉价原料,不需要复杂、昂贵的处理,便可有效吸附水体中的TCs,是负载光催化剂的良好基质.
近年来,研究者也开展了一系列用于光催化降解TCs的介孔MOFs材料的研究. MOFs是一种由金属离子/团簇和有机连接剂组成的新型多孔晶体材料[34],其特殊的无机-有机结构使得介孔MOFs对TCs分子具有较强吸附能力的同时,还兼具快速的电荷转移速率及较低的电子-空穴对复合效率[35-36]. Deng等[28]通过将介孔级的Material Institut Lavoisier-53(Fe)(MIL-53(Fe))与硫化银(Ag2S)纳米粒子耦合,构造了Ag2S/MIL-53(Fe)复合光催化剂. 与单一的MIL-53(Fe)与Ag2S相比,该复合光催化剂的比表面积提高了2.8倍和18.7倍. 此外,MOFs材料的引入还抑制了电子-空穴对的复合,大大提高了光催化剂的降解效率,在可见光照射下,60 min内即可降解94.0%的TC.
-
元素掺杂可以调控半导体的光学和电学性质及其能带结构,被认为是改善光催化剂光吸收和载流子传输能力的有效策略[37]. 表3对近5年元素掺杂复合光催化剂降解水体中TCs的工作进行了总结.
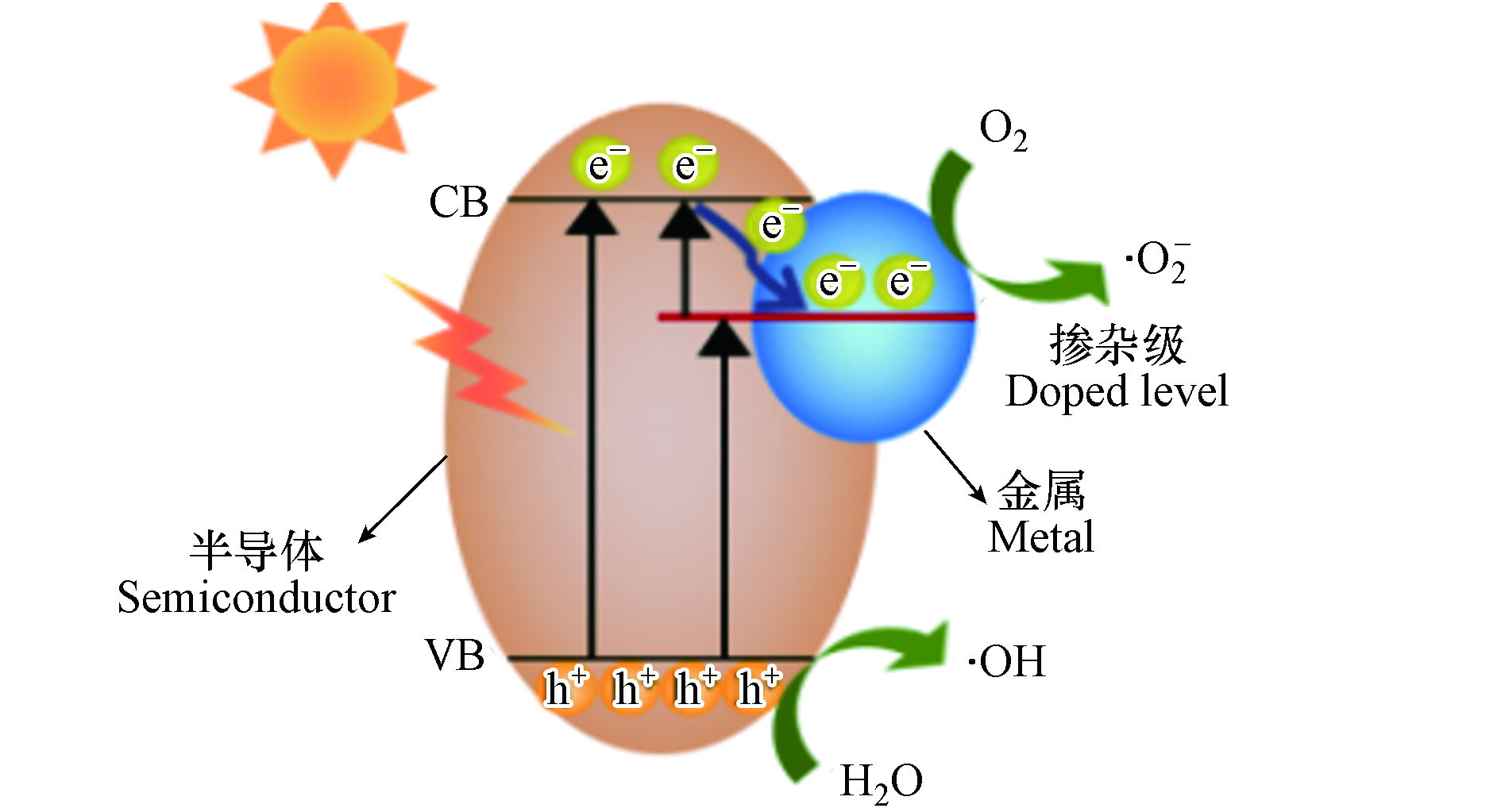
元素掺杂可分为金属掺杂、非金属掺杂与共掺杂的3种情况. 如图3所示,当金属离子掺杂光催化剂时,可在光催化剂中引入中间能级,使光催化剂带隙变窄,光吸收范围扩大. 此外,金属离子强的电子捕获能力,可使光生电子在其周围聚集,提高光生电子-空穴对的分离效率,显著增强光催化活性[47]. 因此,近年来,多种金属(如Fe、Co、Ni、Cu、Zn、Mn、Ag、Au、Er、Ce、Cr、B、Na、K、Ca和Mg等)已被用来掺杂半导体(如TiO2、ZnO、CdSe和g-C3N4等)用于降解水体中的抗生素[38-43, 48-51]. 例如,Qu等[52]合成了一种可见光响应的复合光催化剂—K掺杂的g-C3N4/BiOBr. 光电流和电化学实验结果证明K的引入有效减小了g-C3N4/BiOBr复合材料的禁带宽度、提高了光生载流子的分离效率,实现了对盐酸四环素(tetracycline hydrochloride,TCH)的高效降解. Viet等[53]采用热缩合法制备了贵金属Ag掺杂的g-C3N4光催化剂. 通过对复合材料的结构和表面元素分布表征,证实Ag的掺杂不仅降低了g-C3N4的带隙能,而且增强了其载流子的分离效率,减少了电子-空穴对的复合. 其中,7% Ag/g-C3N4在120 min内对OTC的去除率可达98.7%.
与金属离子相比,非金属离子不易形成复合中心,不会消耗光诱导电荷,可更有效地提高光催化活性[44,54]. 例如,当TiO2通过非金属(N、S、C、B)掺杂后,O的2p轨道便与非金属中能量接近的p轨道进行杂化,导致价带电位上移、禁带宽度减小[55],因而其可见光吸收范围有效扩展、光生载流子增多,光催化性能增强. Panneri等[45]以2%的聚乙烯醇(PVA)为粘结剂,将尿素-硫脲混合物热分解所得的g-C3N4纳米片喷雾造粒成微球,与原始g-C3N4相比,造粒后的热氧化处理导致了C的原位掺杂. 由于C掺杂产生的π键和造粒过程形成的介孔结构有助于减小带隙、增强可见光吸收. 因此,在可见光照射下,该复合催化剂对TC的降解效率可达95.0%. 值得注意的是,金属、非金属元素的共掺杂可产生协同作用,进一步提高可见光的吸收和载流子的分离效率[56,57]. Xu等[46]对磷钼酸水合物和双氰胺混合物进行热处理,得到了金属Mo和非金属P共掺杂的g-C3N4(PM-CN)复合物. 由于共掺杂产生了氰基和氮空位,因此,复合材料具有更强的可见光捕获能力和电荷分离效率. 将其用于TC的降解,结果表明,PM-CN在光催化降解TC方面具有较高的活性,其降解速率为0.01 min−1,是纯CN的3.3倍,优于P和Mo单独掺杂的同类化合物.
-
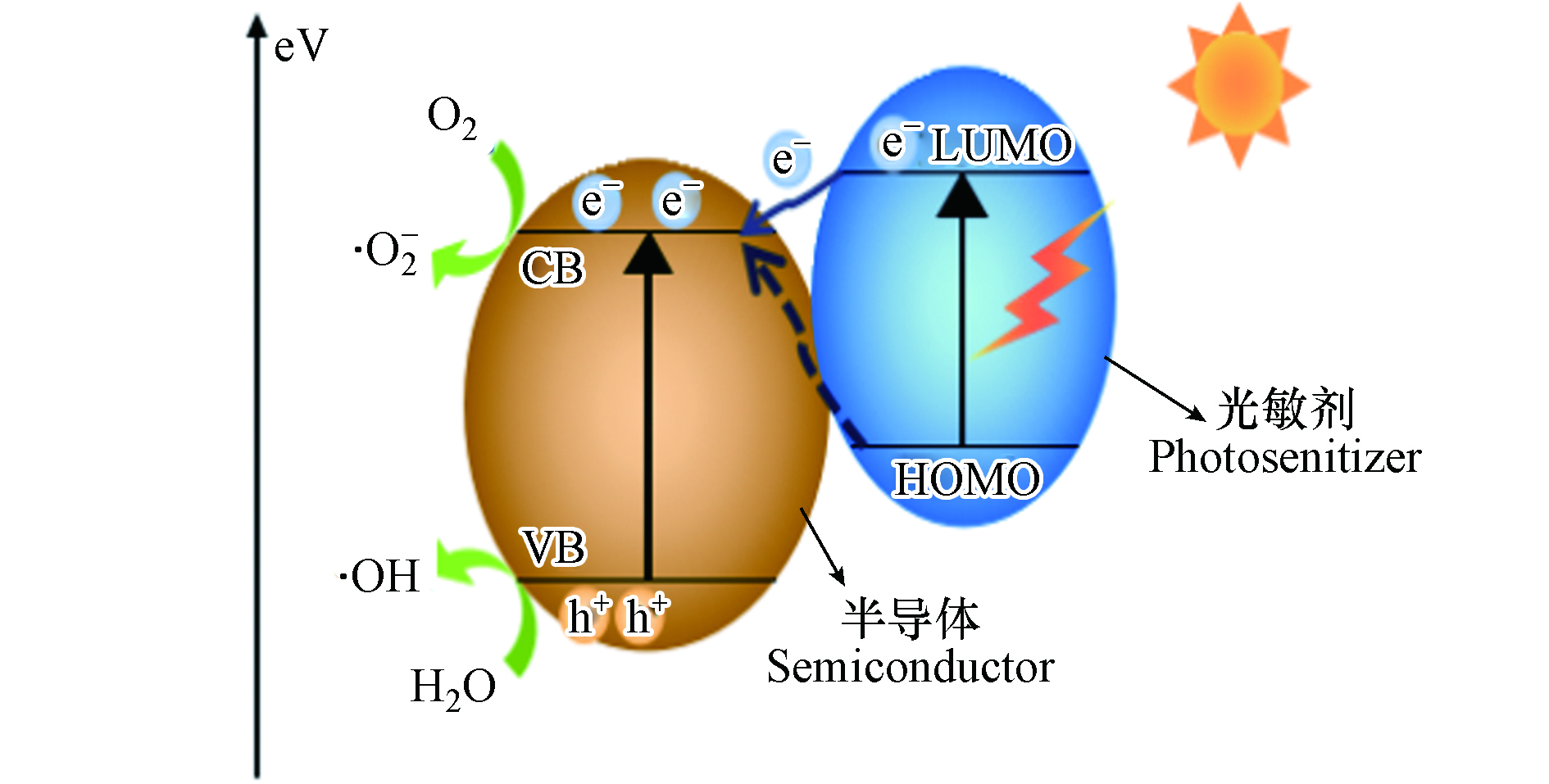
染料敏化是指将染料分子与宽带隙半导体结合,利用染料对可见光的强吸收,将半导体的光谱响应延伸至可见光区,提高半导体对可见光的吸收能力[58]. 如图4所示,光敏化剂在可见光照射下发生电荷分离,电子从染料分子的HOMO能级被激发到LUMO能级并转移到半导体的导带,同时,染料分子也可将电子直接激发至半导体导带[59]. O2等电子捕获剂最终在半导体表面捕获电子,实现电子-空穴对的分离,产生自由基,从而实现对TCs的降解. 表4对近5年来光敏化剂负载光催化剂降解水体中TCs的工作进行了总结.
近年来,低成本、低毒性的有机染料已被广泛应用于扩展宽禁带半导体的光吸收[60]. 例如,Ahadi等[61]采用玫瑰中提取的花青素对ZnS纳米粒子进行表面光敏化改性. 其中,花青素中的羰基和羟基可通过与Zn离子螯合使其固定在ZnS表面,增强界面电子耦合,降低表面态能量,以实现电子快速转移,提高其在可见光下的催化降解性能. 此外,花青素还可抑制ZnS纳米颗粒的过度生长和聚集. 因此,花青素敏化的ZnS纳米粒子在可见光下对TC降解率可达81.0%,而未经敏化的ZnS对TC的降解率不足20.0%.
卟啉是由4个吡啶环通过亚甲基相连而成的平面共轭大环结构的化合物,在400—450 nm及500—700 nm范围内均有很强的吸收[62,66]. Huang等[63]通过溶剂热原位生长法,将二维杆状中-四(4-羧基苯基)卟啉(TCPP)与三维花状BiOCl进行耦合,构建了一种新型的二维/三维有机-无机复合光敏材料TCPP/BiOCl. TCPP的引入极大扩展了BiOCl的可见光吸收范围,促进了光生载流子的分离. 与纯BiOCl相比,TCPP/BiOCl复合材料对TC表现出更好的光催化降解性能,在可见光照射120 min时可降解74.3%的TC,是纯BiOCl的1.5倍. 与卟啉结构类似,酞菁是一种由人工合成的、化学性质非常稳定的大环共轭结构化合物,在可见光区域同样具有较强的光吸收,能与金属离子形成金属酞菁配合物(MPcs),这类配合物在太阳光长波区具有很强的吸收能力以及具有在光激发下产生单线态氧的特性,已成为光催化降解领域的优良光敏化剂[64,67]. Li等[65]通过静电纺丝-溶剂热法制备了由2,9,16,23-四氮杂铜(Ⅱ)酞菁(TNCuPc)敏化的一维CeO2/Bi2MoO6纳米纤维,用于高效降解水体中的TC. 由于TNCuPc的敏化作用,该复合材料在120 min内对TC的降解率可达94.6%.
-
目前,在半导体光催化剂进行表面改性以增强光催化性能的方法中,负载助催化剂可拓宽单一光催化剂的光吸收范围并调控其表面催化性能,是提升光生电荷分离效率的有效方法[68-70]. 表5对近5年来助催化剂负载光催化剂降解水体中TCs的工作进行了总结.
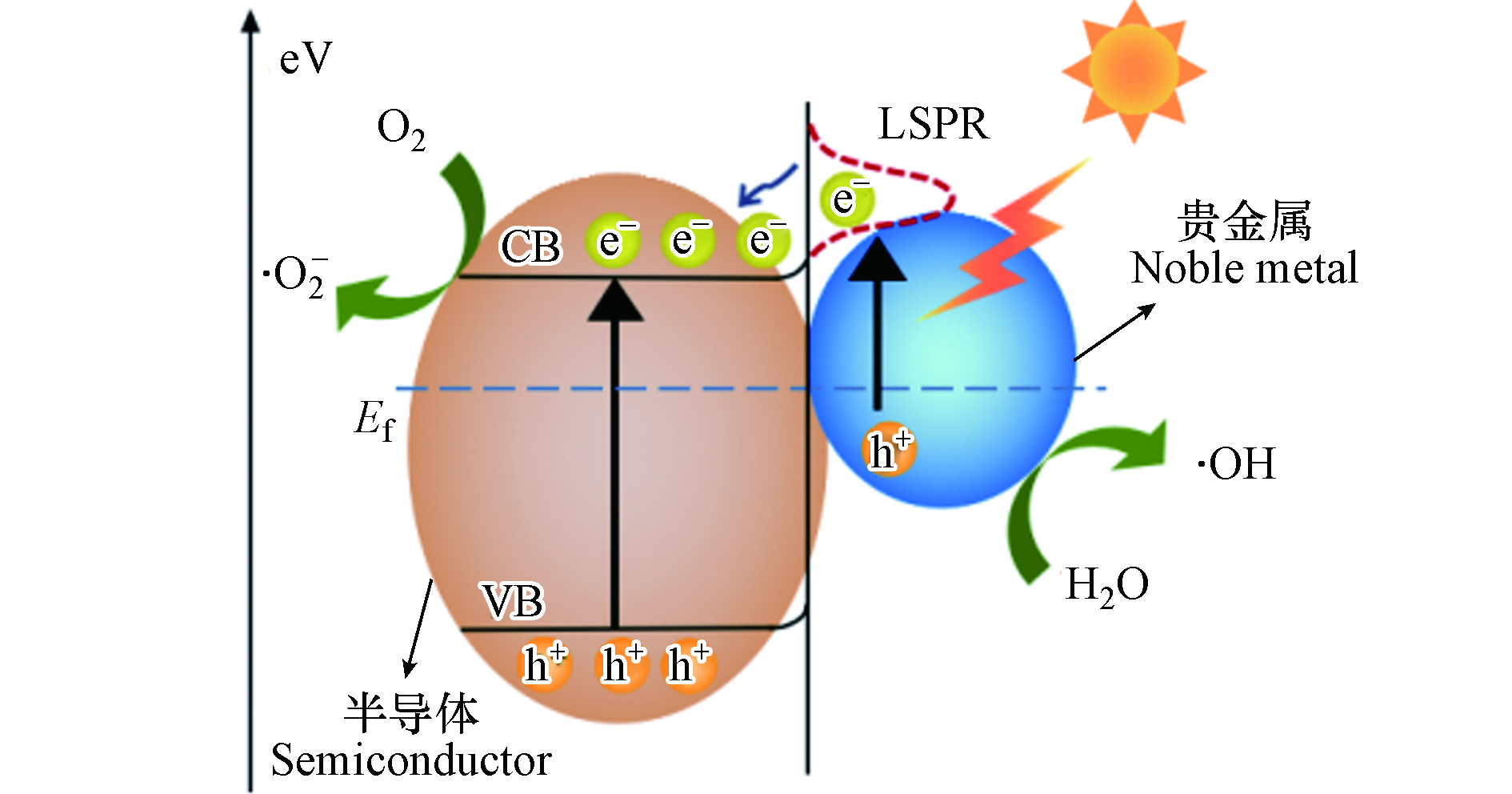
具有良好可见光吸收性能与稳定性的贵金属材料(如Ag、Au、Pt)是负载在单一半导体表面最常见的一类助催化剂. 如图5所示,贵金属的内部和表面存在大量的自由电子[76],当光照射到贵金属表面时,自由电子受到刺激发生振荡,如果入射光的频率与电子振动的频率相匹配,贵金属便会出现局域表面等离子体共振(LSPR)效应,产生LSPR吸收. 因此,当贵金属与半导体接触时,可使半导体一侧表面能带发生弯曲,形成肖特基势垒,成为俘获电子的有效陷阱[77],抑制光生电子-空穴对的复合,提高载流子的分离效率,增强半导体的可见光催化活性[78]. Wu等[71]通过光沉积技术合成了Ag负载的BiVO4复合光催化材料. 与单一BiVO4相比,表面均匀分布小尺寸Ag的BiVO4表现出更优越的光催化性能,在1 h内可降解89.0%以上的TC. 此外,电化学阻抗谱(EIS)结果表明,与纯BiVO4相比,Ag/BiVO4复合光催化剂的光生电子-空穴对分离效率更高,界面电荷迁移速率更快. 这是由于Ag与BiVO4的界面所形成的肖特基势垒可作为电子捕获中心,加快光生电荷的分离,且Ag的LSPR效应显著拓宽了BiVO4的可见光响应范围,提高了光能利用率. Ma等[72]通过水热法制备了以Au NPs为助催化剂的超薄氧化甲酸铋纳米片(Au/BiOCOOH)的可见光响应复合材料. Au NPs的LSPR效应增强了载流子的分离和光吸收能力,从而提高了复合材料的光催化活性. 与单一的BiOCOOH相比,Au/BiOCOOH的光催化活性显著提高,2.0% Au/BiOCOOH对TCH的降解速率常数为5.4×10−3 min−1,是BiOCOOH的13.5倍.
近年来,基于过渡金属、金属氧化物和氢氧化物的新型助催化剂得到了广泛报道. 碳化钨(WC)是一种具有高耐腐蚀性与优良电导率的过渡金属碳化物,同时兼具类贵金属的助催化性能. Zhang等[73]成功合成了一种新型的磷(P)掺杂空心管g-C3N4/WC钨光催化剂,用于TC的降解. 实验结果表明,WC的类铂(Pt)助催化性质加速了电子的转移,显著降低了载流子的重组. He等[74]以纳米氧化钛团簇作为新型非贵金属助催化剂,制备了一种高稳定性、高活性的纳米TiOx簇负载Ag/AgCl新型复合材料. 该复合材料在模拟太阳光照射下5 min内便可降解80.0%以上的TC,光降解活性约为Ag/AgCl的21.7倍. 此外,还有研究者将助催化剂CoS与CeO2结合,用于高效降解TC,60 min内的降解率可达96.5%[75].
-
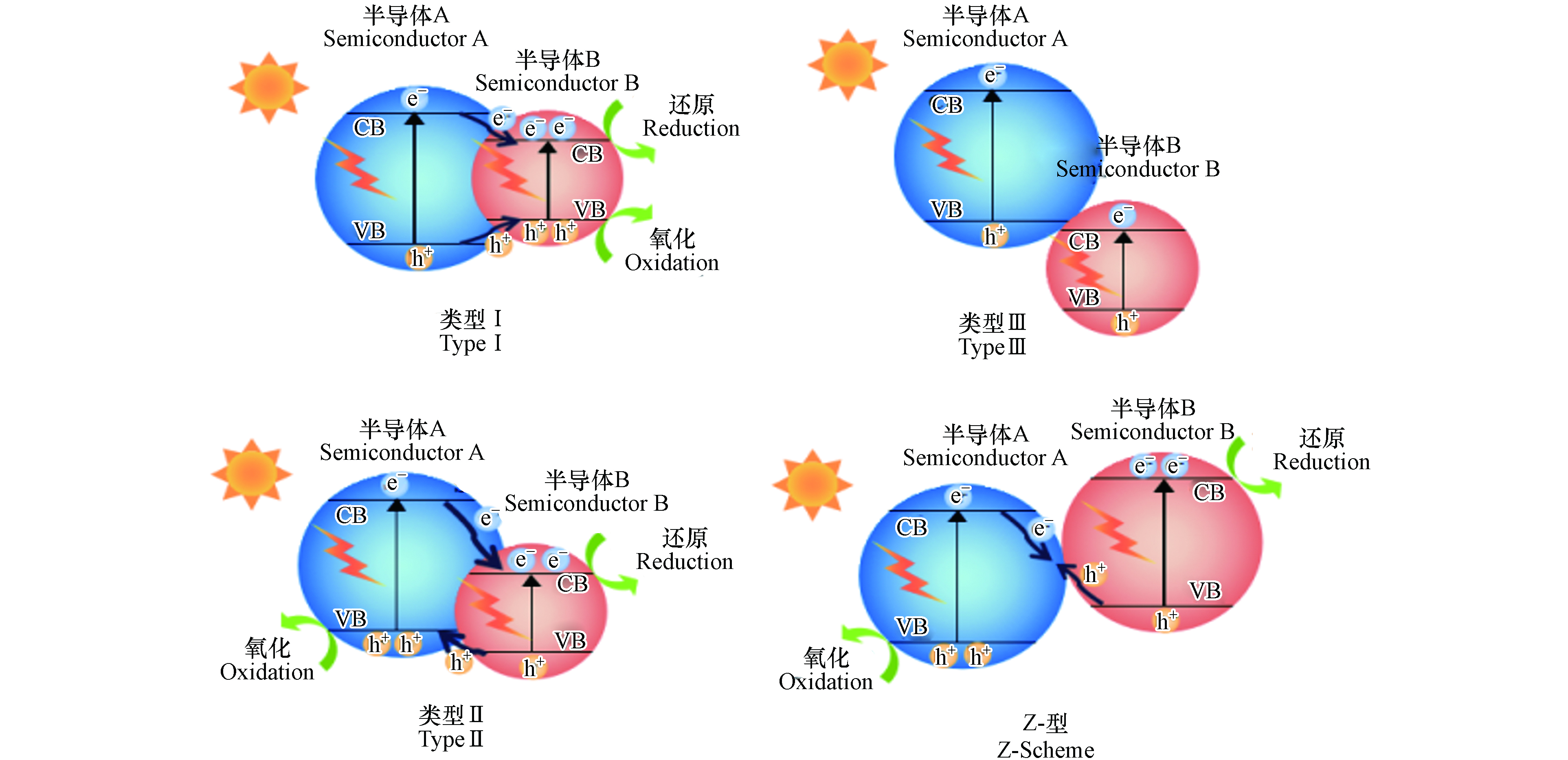
单组分半导体光催化剂往往存在光生载流子复合率高或光吸收范围窄等缺点[79],导致光催化性能较低,难以满足实际应用的需求. 鉴于此,研究者们试图通过将两种或两种以上的半导体材料进行耦合,形成异质结结构,以期克服单组分半导体的缺点,进而提高其光催化性能[80]. 如图6所示,基于半导体的能带匹配情况,半导体异质结包含四种类型,分别是Ⅰ型异质结(跨立型),Ⅱ型异质结(交错型)、Z型异质结和Ⅲ型异质结(断裂型)[81]. Ⅰ型异质结中,半导体A产生的电子和空穴都向半导体B迁移,使得光生载流子聚集在同一半导体内,因此复合率较高,不利于光催化反应的进行.
此外,由于氧化还原反应均发生在氧化还原电位较低的半导体B上,这也将导致光催化剂的催化能力降低. 在Ⅲ型异质结中,由于其带隙不重叠,两个半导体之间的电子-空穴对无法进行转移,因而很难实现载流子的有效分离. 考虑到氧化还原电位和光生电荷转移的影响,目前光催化降解领域最常用的是Ⅱ型异质结和Z型异质结.
-
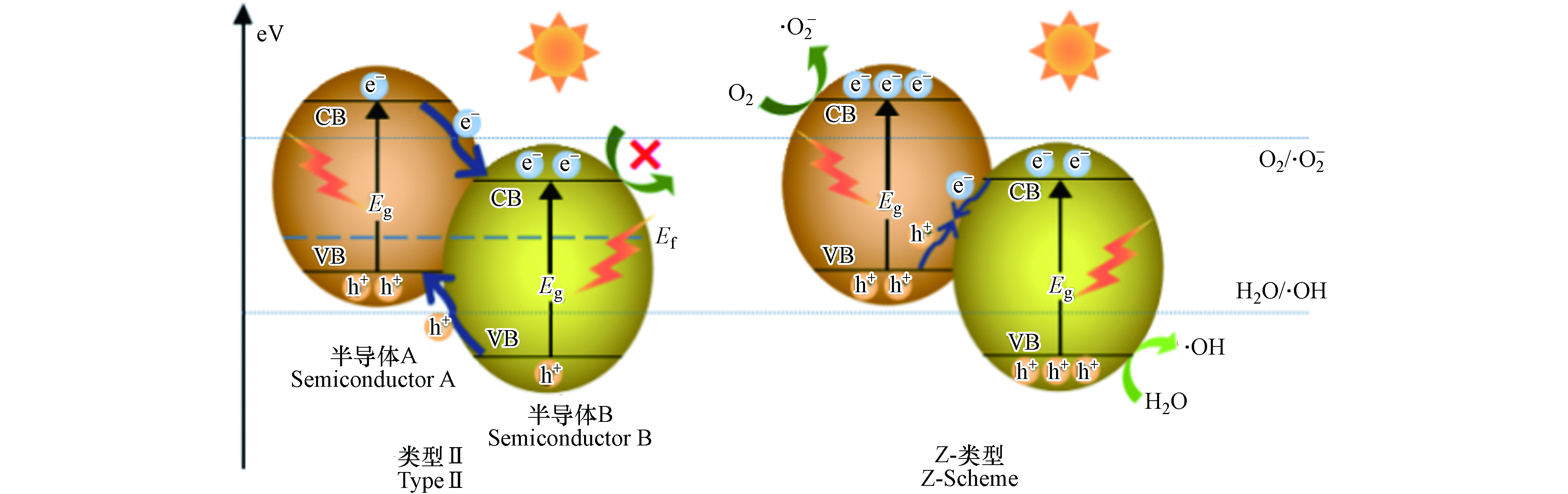
Ⅱ型异质结是能带位置相互交叉的两种材料形成的异质结结构,该结构为载流子的有效分离提供了最佳的能带位置—光生电子从半导体A的导带转移至半导体B的导带,同时,光生空穴则由半导体B的价带转移至半导体A的价带,因此,光生电子-空穴对在空间上彼此分离,显著降低了光生载流子复合率并延长了电子寿命,从而有效提升了光催化效率. Liu等[82]在BiVO4的基础上采用沉淀法制备了新型三元Ag/Ag2CO3/BiVO4复合光催化剂. 与纯BiVO4相比,该Ⅱ型异质结光催化剂在可见光区和紫外区表现出更强、更宽的吸收,对TC具有更高的降解效率,150 min内可降解94.9%的TC.
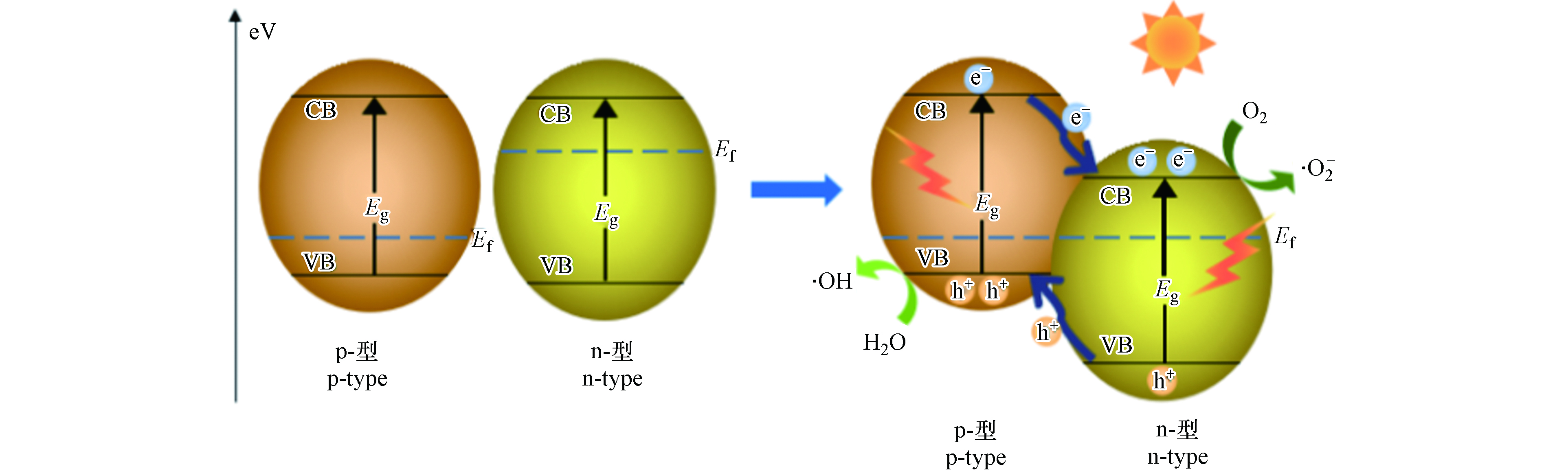
根据材料导电类型的不同,Ⅱ型异质结还可分为同型异质结(p-p结或n-n结)和异型异质结(p-n结). 其中,p-n异质结的性能最为优越,应用领域最为广泛,这是由于p-n异质结不但能够通过敏化作用拓展光吸收范围,而且能够通过内建电场抑制载流子复合,大幅度提高材料的光催化性能[83-85]. 表6是近5年利用p-n型复合光催化剂降解水体中TCs的工作总结.
如图7所示,在光照下,p型和n型材料被激发产生载流子,当p型和n型半导体紧密接触时,由于费米能级(EF)的不同,界面处会形成内建电场,在内建电场作用下,n型半导体中的空穴会向p型半导体移动,而p型半导体中的电子向n型半导体移动,从而有效地分离了电子-空穴对,提高了光催化剂的光催化活性[94]. Zhao等[86]采用沉淀法成功制备了石墨相氮化碳纳米片/铜(I)氧化物(g-C3N4/Cu2O)复合材料,由于该p-n异质结的有效界面电荷转移和Cu2O与g-C3N4纳米片之间的协同作用,光生电子-空穴对的分离效率显著提高. 降解结果表明,该复合材料具有比Cu2O和g-C3N4纳米片更高的光催化活性,30% wt g-C3N4纳米片/Cu2O复合材料在100 min内可降解92.1%的TC,120 min时可去除83.3%的总有机碳(TOC). 在一系列p-n型复合光催化剂中,具有可见光活性的铋系光催化剂表现优越,例如BiOX(X = Cl、Br、I)[87-90]、Bi2O3[91]、Bi2MoO6[92,93]等Bi3+的化合物由于具有利于光生载流子分离与迁移的片层状结构,已被广泛应用于构造p-n异质结光催化材料.
然而与Ⅰ型异质结类似,Ⅱ型异质结的氧化反应和还原反应分别发生在氧化电位较低的半导体A和还原电位较低的半导体B上(图7),因此其氧化还原能力也有所欠缺,为此需要在传统的Ⅱ型异质结的基础上进行改进,以期达到更高的氧化还原效果.
-
与传统的Ⅱ型异质结不同,Z型异质结则率先将A、B半导体中具有较低氧化还原电位的空穴与电子进行复合,同时保留各自具有较高氧化还原电位的电子和空穴[95]. 如图8所示,这种结构不仅有效促进电子-空穴对分离,也保留了催化剂的强氧化和强还原能力. 因此,近年来有关Z型异质结的报道较为多见. 表7对近5年来构造Z型复合光催化剂降解水体中TCs的工作进行了总结.
由于传统Z型光催化体系(离子态Z型)的应用受到穿梭式氧化还原介质的逆向反应、光屏蔽效应以及对pH变化的敏感性等问题的限制,近年来,固态Z型光催化体系在各种光催化应用中受到了广泛的关注并取得了重大进展[96-103]. Gong等[104]采用水热法制备了Z型异质结光催化剂CdTe/TiO2,经可见光照射30 min后可降解78.0%的TCH,具有较好的光催化性能. 这是由于该复合材料良好的可见光吸收和Z型电荷转移机制的协同作用,不仅促进了光生电子-空穴对的分离,还保持了各自相对较高的氧化还原电位. Huang等[105]为了调节CuBi2O4/BiOBr异质结光催化剂对抗生素的吸附和光催化降解性能,研制了一种新型的直接Z型CuBi2O4/BiOBr异质结光催化剂. CuBi2O4与BiOBr的结合有效调节了表面特征和界面相互作用,从而实现直接Z型电荷动力学,提高了光催化活性. 与原始的CuBi2O4和BiOBr相比,CuBi2O4/BiOBr异质结对TCs的吸附能力和光催化性能显著提高,比单一的BiOBr与CuBi2O4分别提高了1.5倍与5.0倍.
在实际构建Z型光催化体系时,半导体之间还存在界面接触差、电荷转移效率低、界面载流子复合不可控等问题. 与块状材料相比,二维(2D)材料具有更高的比表面积、更短的载流子迁移距离以及丰富的活性位点[106]. 因此在Z型光催化剂中,2D/2D Z型异质结由于其较大的界面接触和快速的Z型电荷转移,因而具有更强的氧化还原能力. 例如,Qi等[107]合成的具有较大接触面积的2D-2D NiO/g-C3N4光催化剂,在保留了两种半导体高氧化还原能力的同时,还促进了界面电荷的转移速率,总体可提升约48.0%的降解效率,表现出良好的光催化活性.
-
TCs的光催化降解是在光催化剂存在下进行的光化学反应,其主要过程可分为活性物种的生成(h+、·O2−、·OH、1O2)和TCs的降解. 首先,如方程式(1)—(5)所示,当高于吸收阈值的光照射光催化剂时,价带(VB)上的电子(e−)被激发至导带(CB),同时在VB中产生等量的带正电荷的空穴(h+)[108]. 当VB的标准氧化还原电位高于2.27 eV(E0(H2O/·OH) = 2.27 eV)[109]时,h+被H2O等捕获,生成氧化能力较强的·OH;当CB的标准氧化还原电位低于−0.33 eV (E0(O2/·O2−) =−0.33 eV)时[110],迁移到半导体表面的e−通常被吸附在光催化剂表面的电子受体O2俘获,生成·O2−等一系列自由基,它们能够迁移到催化剂表面,与TCs发生一系列的氧化还原反应,促进TCs的降解[82,111]. 此外·O2−可进一步被h+氧化为单线态氧(1O2),同时,基态氧分子(3O2)也可在光敏化剂的作用下被激发形成1O2. 1O2虽不是自由基,但因解除了自旋限制,其反应活性远高于普通氧,因此与·O2−、·OH一同在TCs高效降解过程中扮演着重要的角色[64,112-113].
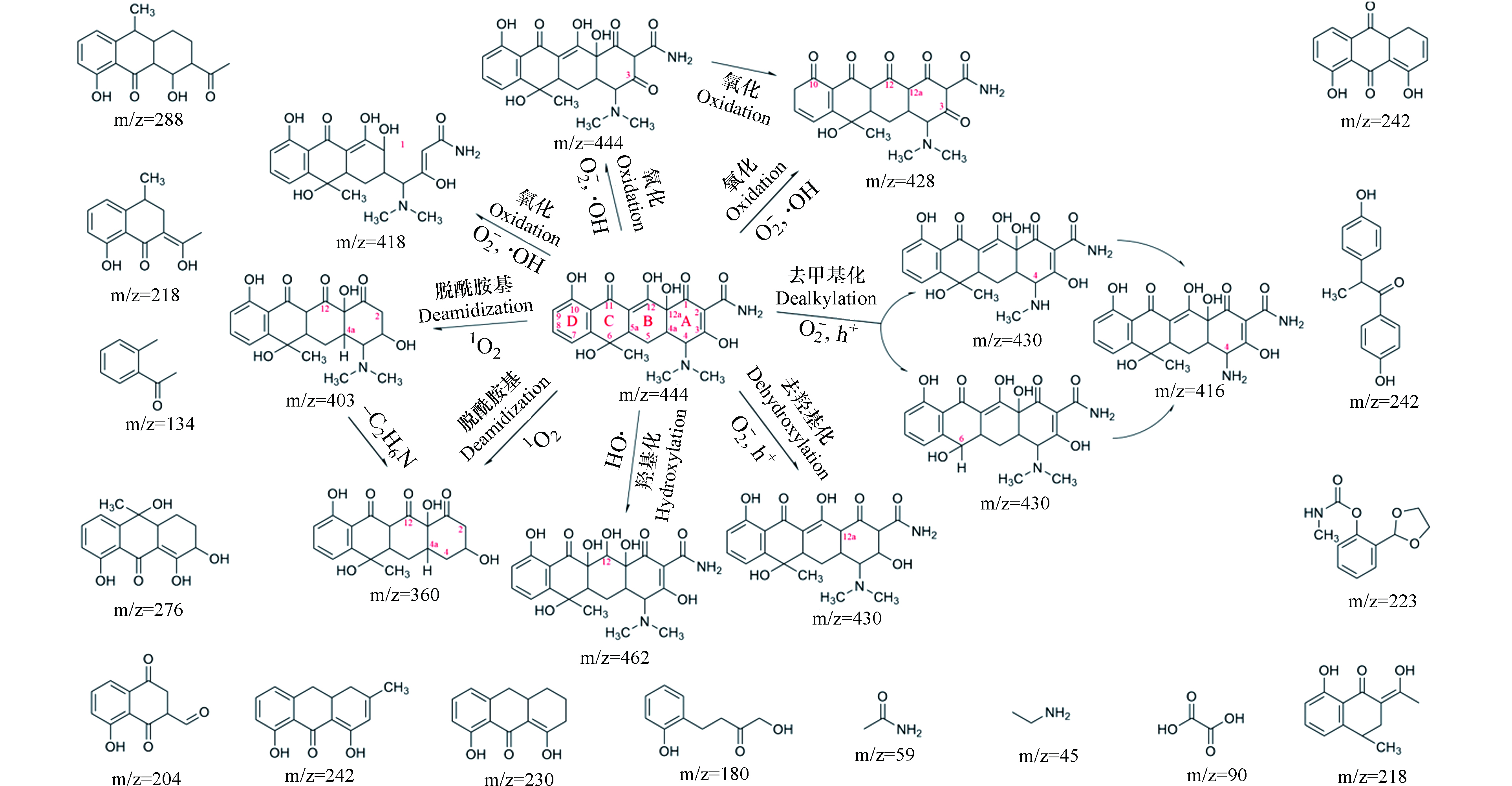
在光催化降解过程中,由于TCs中双键(C=C)、胺基(−NH2)和苯酚基团具有较高的电子密度,容易被活性物种攻击,导致其官能团(氨基、羟基或n-甲基)发生裂解和开环反应,分解为一些小分子有机物,小分子有机物进一步降解,最终生成水和二氧化碳[114]. 以TC为例,如图9所示,与TC在环境中主要的降解产物为差向异构体和脱水四环素不同,TC分子在活性物种的攻击下,可在多个位点发生反应,生成多个中间产物.
当TC分子(m/z = 445)被活性物种·O2−和h+攻击时,其在C-4、C-6、C-12a位点脱去甲基与羟基,形成质荷比为430、416、428与430的中间产物P430、P416、P428、P430[46,115-116];接着,C-12位点可在高亲电性的·OH的进攻下打开C=C双键[117],通过羟基化作用引入羟基,生成中间产物P462[23];同时,·O2−与·OH氧化C-10、C-12位点的羟基,生成中间产物P428,也可氧化C-1使其发生开环反应,生成中间产物P418[118];1O2的两个未充满的轨道具有较强的还原性,因此在1O2的作用下,C-2、C-4位脱落酰胺基团与二甲氨基,生成中间产物P403、P360[64]. 此后,活性物种进一步攻击TC的不同位点,中间体被逐渐分解为多个小分子,最终被矿化为H2O和CO2.
光催化降解TCs的反应途径十分复杂,且目前有关其中间产物及反应过程中毒性变化的报道还较少,相关研究较为缺乏. 此外,经光催化降解后的产物对水生生物的毒性以及未完全矿化的情况下它们在环境中的持久性也未见报道. 因此,针对TCs降解产物的整体风险有待进一步研究.
-
大量研究表明,负载介孔材料和助催化剂、掺杂元素、染料敏化以及构造异质结结构等方法是提高光催化剂降解TCs效率的有效策略. 基于这些方法得到的复合光催化剂,具有丰富的活性位点、较宽的光吸收范围、高效的光生载流子分离效率以及较强的氧化还原能力等特性,能够降解80%以上的TCs. 与单组分光催化剂相比,这些复合光催化体系在整体上提高了TCs的降解效率,可提升达32.7倍.
然而,目前的研究主要集中在复合光催化剂本身上,如提高载流子分离等,从TCs自身特点出发设计、制备光催化剂的研究几乎没有. 因此,所开发的复合光催化剂对TCs往往存在选择性不高、降解不完全的缺陷. 鉴于此,在今后的研究中,首先可通过研究TCs母体、中间体和产物的吸附-解吸行为,针对性地调控光催化剂的表面结构是提高其选择性的可行策略. 同时,还可针对四环素分子本身的特点,如结构、电负性、氧化还原特性、取代基、分子立体空间位阻、几何形状等,通过择形、改变电子/空穴电位、改变表面电负性、调节不同活性氧的比率等,设计和开发相应的光催化剂;其次,可在光催化剂表面修饰对TCs具有高特异性的适配体、分子印迹、微生物以及酶等识别元件、开发适合TCs大小孔隙的MOFs等,以便高效高选择性降解TCs. 同时,现阶段的复合光催化剂仍存在稳定性较差、毒性效应有待进一步探究等问题. 对于降解实际水体中的TCs,复合光催化剂还面临以下挑战:
(1)目前复合光催化剂的应用还处于实验室阶段,所处理的水样中TCs的浓度较高,大部分在20 mg·L−1以上. 而实际水体中TCs含量普遍低于mg·L−1,需要经过富集处理后才能达到较好的降解效果. 因此在提升光催化性能的同时,也需提升复合光催化剂的吸附性能.
(2)现有的复合光催化剂均具有普适性,对水体中其他的有机污染物甚至包括一些常见的溶解性有机质,如乳酸、腐殖酸也同样具有降解效果[119-120]. 因此,需开发对TCs具有高选择性的复合材料以提高其实际应用性能.
(3)如何提高复合光催化剂的稳定性也是光催化降解领域研究的重要问题,即防止催化剂活性组分重新溶解,导致催化效率的降低和使用次数的减少.
(4)在实际应用中,单靠一种技术可能难以处理TCs残留. 建立光电催化、光-Fenton催化等复合体系是高效去除水中TCs残留的有效选择.
(5)虽然复合光催化剂具有高效的TCs降解效率,但是其带来的毒性效应不容小觑. 例如与微米级颗粒相比,纳米级光催化材料粒径较小,具有不同于常规尺寸材料的毒性,对于水生生物的毒性效应可能更大[121]. 因此光催化剂进入水环境后的毒性效应机制有待更深一步的研究.
高效降解环境新污染物四环素的复合光催化剂:从材料设计到降解机制
Composite photocatalysts for efficient degradation of emerging contaminant tetracyclines: From material design to degradation mechanisms
-
摘要: 四环素类抗生素是目前使用量最大,使用频率最高的一类抗生素. 近年来,日益严峻的四环素污染对生态系统和人类健康造成严重危害,我国“十四五”规划已将抗生素类物质纳入环保重点管控的新污染物名单. 因此,如何有效降解四环素已成为环境污染治理领域亟待解决的问题. 光催化降解作为一种低能耗、可重复利用和环境友好型的降解技术,在四环素降解方面具有较大的应用前景. 然而采用单组分光催化剂的光催化降解技术存在着可见光利用不足、光生电荷复合率高等缺陷,导致四环素降解时矿化不完全,有高毒性中间产物生成. 为了克服这些问题,研究者们开发出了兼具广吸收光谱性和强氧化还原性的复合光催化剂. 本文调研和梳理了近五年复合光催化剂降解四环素的研究现状,从复合光催化剂的设计与合成、四环素的降解性能、降解反应机制等方面进行了阐述. 结果显示,在着重于提升光吸收效率、光生载流子传输能力、光生电子-空穴分离效率等方面,通过介孔材料负载、金属/非金属元素掺杂、染料敏化、助催化剂负载、异质结结构构造等调控手段,设计制备出多种复合光催化剂,有效提升了光催化反应活性,显著提高了水体中四环素的光催化降解性能,四环素降解率达到63.7%—100%,矿化率达到34.4%—100%. 最后,针对复合光催化剂现存的问题,包括选择性不高、稳定性较差、毒性效应有待进一步探索等方面进行总结与展望,以期实现其在实际水体中的应用.Abstract: Tetracycline antibiotics (TCs) are the most widely and frequently used antibiotics. In recent years, the increasingly severe tetracycline pollution has caused serious impacts on ecological systems and human health. Antibiotics have been included in the list of emerging contaminants under priority environmental control in the 14th Five-Year Plan of China. Therefore, efficient degradation of tetracycline has become an urgent problem to be solved in the field of environmental pollution control. As a green degradation technology, photocatalysis with low energy consumption, reusablility and environmental friendliness has a great application prospect in the degradation of tetracycline. However, photocatalysis using a single component of photocatalyst has the weakness of insufficient utilization of visible light and high recombination rate of photogenerated charge. In order to overcome these problems, researchers have developed composite photocatalysts with wide absorption spectrum and strong redox capability. In this paper, we surveyed and analyzed the works published in the past 5 years on the photocatalytic degradation of tetracyclines with composite photocatalysts, and described the progress from the aspects of design and synthesis of composite photocatalysts, degradation performance and degradation mechanism of tetracycline. The results showed that focusing on improving the light absorption efficiency of photocatalysts, the separation and transfer capacity of photogenerated carriers, a variety of composite photocatalysts were designed and prepared through a series of strategies including the support of mesoporous materials, metal/non-metal element doping, dye sensitization, cocatalyst loading, and heterojunction constructing etc, which effectively improved the photocatalytic activity and degradation efficiency of tetracycline in water. The degradation rate of tetracycline reached 63.7%—100% and the mineralization rate reached 34.4%—100%. Finally, the existing problems of composite photocatalysts, such as low selectivity, poor stability and unknown toxic effects were summarized and prospected to realize their application in actual water.
-

-
表 1 主要TCs的理化性质
Table 1. Physical and chemical properties of main tetracycline antibiotics
化合物
Compounds缩写
Abbreviation化学物质
登录号
CAS分子式
Molecular formula分子量
Molecular weight极性表面积
Polar surface area油水分配
系数
lgP水溶性/(mg·L−1)
Water solubility四环素 TC 60-54-8 C22H24N2O8 444.4 181.62000 0.48590 231 土霉素 OTC 79-57-2 C22H24N2O9 460.4 201.85000 −0.54330 313 金霉素 CTC 57-62-5 C22H23ClN2O8 478.9 181.62000 1.13930 — 强力霉素 DOX 564-25-0 C22H26N2O9 462.4 181.62000 0.35270 630 P,物质在正辛醇和水中的分配系数比值. P, the ratio of the partition coefficient of a substance in octanol to water. 表 2 介孔材料负载光催化剂降解TCs概况
Table 2. Summary of TCs degradation by photocatalysts supported on mesoporous materials
抗生素
Antibiotic材料
Materials比表面积/
(m2·g−1)
SBET降解条件
Degradation conditions降解效率
Degradation efficiency矿化率
Mineralization efficiency参考
文献
Ref.时间/
min
Time催化剂浓度/
(mg·L−1)
Concentration of
photocatalyst抗生素浓度/(mg·L−1)
Concentration of
antibioticTC 介孔石墨烯/SiO2 341.0 60 50 30 89.0% — [19] TC 介孔SiO2/g-C3N4 — 60 333 20 92.9% — [20] TC 污泥/TiO2 93.0 120 10 5 80.0% — [21] TC 介孔Ag/Bi2Sn2O7/C3N4 66.0 90 1000 20 89.1% — [22] TCH 介孔SiO2-Ag/AgBr 1182.0 150 300 40 92.8% 81.2% [23] TCH 介孔TiO2/非晶TiO2 — 300 500 50 81.1% 53.7% [24] TCH Pt/非晶TiO2/介孔TiO2 34.5 300 500 — 100.0% 75.9% [25] TC 介孔石墨烯/TiO2 55.0 300 10 20 93.4% 68.2% [26] TC AgBr/TiO2/Pal 48.1 120 500 20 90.0% 67.0% [27] TC Ag2S/MIL-53 (Fe) 199.3 60 667 20 94.0% — [28] 表 3 元素掺杂复合光催化剂降解TCs概况
Table 3. Summary of TCs degradation by element-doped composite photocatalysts
抗生素
Antibiotic材料
Materials带隙值/eV
Band gap降解条件
Degradation conditions降解效率
Degradation
efficiency矿化率
Mineralization
efficiency参考文献
Ref.时间/min
Time催化剂浓度/
(mg·L−1)
Concentration of
photocatalyst抗生素浓度/
(mg·L−1)
Concentration of
antibioticTC Cu/WO3 2.69 120 500 50 96.7% — [38] TC La, Cr/SrTiO3 — 90 500 20 83.0% — [39] TC Fe/g-C3N4 2.49 80 500 20 63.7% — [40] TCH Ag/CN/Na2SO4 2.52 60 500 10 70.0% — [41] TCs Pt/g-C3N4/ZnIn2S4 2.48 40 1000 10 83.0%—97.0% — [42] TC Ag/g-C3N4 — 120 20000 20 96.8% 91.5% [43] TC S-CQDs/g-C3N4 2.47 60 1000 20 82.7% 35.2% [44] TC C/g-C3N4 2.54 90 — — 95.0% — [45] TC P/Mo-CN 2.37 120 500 10 69.0% — [46] 表 4 光敏化剂负载光催化剂降解TCs概况
Table 4. Degradation of TCs by sensitized photocatalyst
抗生素
Antibiotic材料
Materials敏化剂
Sensitizer降解条件
Degradation conditions降解效率
Degradation
efficiency矿化率
Mineralization efficiency参考文献
Ref.时间/min
Time催化剂浓度/
(mg·L−1)
Concentration of
photocatalyst抗生素浓度/
(mg·L−1)
Concentration of
antibioticTCH BiOBr0.9I0.1 RhB 8 200 10 67.0% — [60] TC ZnS 花青素 300 2000 40 81.0% — [61] TC g-C3N4 ZnTCPP 120 3000 30 80.3% 63.8% [62] TC BiOCl TCPP 120 400 20 74.3% — [63] TC CoWO4 ZnPcs 170 4 1.45×10−5 mol·L−1 kobs=28.6×10−4 min−1 — [64] TC CeO2/Bi2MoO6 TNCuPc 120 3000 50 94.6% 83.5% [65] kobs,表观一级反应速率常数(min−1). kobs, apparent first order reaction rate constant (min−1). 表 5 助催化剂负载光催化剂降解TCs概况
Table 5. Degradation of TCs by co-catalyst loaded photocatalysts
抗生素
Antibiotic材料
Materials助催化剂
Co-catalyst降解条件
Degradation conditions降解效率
Degradation efficiency矿化率
Mineralization efficiency参考文献
Ref.时间/min
Time催化剂浓度/(mg·L−1)
Concentration of photocatalyst抗生素浓度/
(mg·L−1)
Concentration of
antibioticTC Ag/BCN-CLT Ag 180 1000 30 87.2% — [68] TC Ag/CN Ag 120 200 30 77.0% — [69] TCs Ag/AgBr/BiOIO3 Ag 300 500 10 47.8% — [70] TC Ag-BiVO4 Ag 60 500 20 89.5% 41.7% [71] TC Au/BiOCOOH Au 160 200 20 56.4% — [72] TC P-TCN/WC WC 60 500 20 93.8% 68.2% [73] TC TiOx@Ag/AgCl TiOx 5 5500 40 80.0% 58.8% [74] TC CoS/CeO2 CoS 60 500 40 96.5% — [75] 表 6 p-n异质结光催化剂降解TCs概况
Table 6. Summary of TCs degradation by p-n heterojunction photocatalysts
抗生素
Antibiotic材料
Materials带隙值/eV
Band gap降解条件
Degradation conditions降解效率
Degradation efficiency矿化率
Mineralization efficiency参考文献
Ref.p-Type
p型n-Type
n型时间/min Time 催化剂浓度/
(mg·L−1)
Concentration of
photocatalyst抗生素浓度/
(mg·L−1)
Concentration of
antibioticTC Ag2O/Ta3N5 1.30 2.10 180 2500 10 76.8% 56.4% [83] TC CaFe2O4/ZnFe2O4 1.34 1.63 60 1000 — 78.0% — [84] TCH BN/B-CN 4.10 2.50 60 200 20 88.1% — [85] TC g-C3N4/Cu2O 2.68 2.17 100 1111 30 92.1% 83.3% [86] TC BiOBr/BiOCOOH 2.06 2.68 120 1000 15 83.7% 72.4% [87] TC BiOCl/BiOCOOH 3.50 3.40 60 1000 20 80.4% 89.2% [88] TC BiOI/BiOCOOH 1.80 2.06 125 437.5 15 78.6% — [89] TC BiOI/Bi3O4Cl 1.77 2.61 180 — — 73.5% — [90] TCs Bi2O3/Ti3+-TiO2 2.89 (复合材料) 200 200 10 100.0% 98.0% [91] TCH Ag/Ag6Si2O7/
Bi2MoO61.58 2.66 120 50 20 71.4% 43.7% [92] TCs CuBi2O4/Bi2MoO6 1.77 2.68 60 300 20 72.8%—74.4% 34.4%—48.2% [93] 表 7 Z型异质结光催化剂降解TCs概况
Table 7. Summary of TCs degradation by Z-Scheme heterojunction photocatalysts
抗生素
Antibiotic材料
Materials带隙值/eV
Band gap降解条件
Degradation conditions降解效率
Degradation
efficiency矿化率
Mineralization
efficiency参考
文献
Ref.半导体A
Semicondu-ctor A半导体B
Semicondu-ctor B时间/
min
Time催化剂浓度/(mg·L−1)
Concentration of
photocatalyst抗生素浓度/
(mg·L−1)
Concentration of
antibioticTC Ag6Si2O7/Bi2WO6 1.38 2.70 120 625 20 86.8% 51.2% [96] TC BiVO4/α-Fe2O3 0.48 0.05 120 500 20 75.8% — [97] TC AgI/Zn3V2O8 2.99 3.19 140 333 20 91.0% — [98] TC AgBr/Bi2WO6 2.73 2.80 60 1000 20 87.5% 39.4% [99] TC WO3/g-C3N4 2.64 2.73 120 500 25 82.0% — [100] TCH Ag3PO4/PDIsm 2.43 1.80 8 1000 20 82.8% — [101] TCH Ag3PO4/C3N5 2.48 1.80 60 1000 20 90.5% — [102] DOX Bi7O9I3/g-C3N4 2.24 2.54 120 500 20 80.0% 67.8% [103] TCH CdTe/TiO2 1.78 2.90 30 600 20 78.0% — [104] -
[1] PÉREZ-RODRÍGUEZ M, PELLERANO R G, PEZZA L, et al. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination [J]. Talanta, 2018, 182: 1-21. doi: 10.1016/j.talanta.2018.01.058 [2] JAFARI OZUMCHELOUEI E, HAMIDIAN A H, ZHANG Y, et al. Physicochemical properties of antibiotics: A review with an emphasis on detection in the aquatic environment [J]. Water Environment Research, 2020, 92(2): 177-188. doi: 10.1002/wer.1237 [3] LIU X H, LU S Y, GUO W, et al. Antibiotics in the aquatic environments: A review of lakes, China [J]. Science of the Total Environment, 2018, 627: 1195-1208. doi: 10.1016/j.scitotenv.2018.01.271 [4] ZHANG Q Q, YING G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. [5] LUNDSTRÖM S V, ÖSTMAN M, BENGTSSON-PALME J, et al. Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms [J]. Science of the Total Environment, 2016, 553: 587-595. doi: 10.1016/j.scitotenv.2016.02.103 [6] BEN Y J, HU M, ZHANG X Y, et al. Efficient detection and assessment of human exposure to trace antibiotic residues in drinking water [J]. Water Research, 2020, 175: 115699. doi: 10.1016/j.watres.2020.115699 [7] 雷雨洋, 李方方, 欧阳洁, 等. 浙江地区抗生素残留的环境分布特征及来源分析 [J]. 化学进展, 2021, 33(8): 1414-1425. LEI Y Y, LI F F, OUYANG J, et al. Environmental distribution characteristics and source analysis of antibiotics in Zhejiang area [J]. Progress in Chemistry, 2021, 33(8): 1414-1425(in Chinese).
[8] XU L Y, ZHANG H, XIONG P, et al. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review [J]. Science of the Total Environment, 2021, 753: 141975. doi: 10.1016/j.scitotenv.2020.141975 [9] STACHUROVÁ T, MALACHOVÁ K, SEMERÁD J, et al. Tetracycline induces the formation of biofilm of bacteria from different phases of wastewater treatment [J]. Processes, 2020, 8(8): 989. doi: 10.3390/pr8080989 [10] MARTÍNEZ J L. Antibiotics and antibiotic resistance genes in natural environments [J]. Science, 2008, 321(5887): 365-367. doi: 10.1126/science.1159483 [11] WORLD HEALTH ORGANIZATION. Antimicrobial resistance: Global report on surveillance [R/OL]. Geneva: WHO, 2014. [12] UNITED NATIONS. High-Level meeting on antimicrobial resistance [R/OL]. (2016-09-21)[2021-10-15]. [13] WANG S Z, WANG J L. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater [J]. Chemical Engineering Journal, 2019, 356: 350-358. doi: 10.1016/j.cej.2018.09.062 [14] LIU Y B, GAN X J, ZHOU B X, et al. Photoelectrocatalytic degradation of tetracycline by highly effective TiO2 nanopore arrays electrode [J]. Journal of Hazardous Materials, 2009, 171(1/2/3): 678-683. [15] SZILÁGYI I M, FÓRIZS B, ROSSELER O, et al. WO3 photocatalysts: Influence of structure and composition [J]. Journal of Catalysis, 2012, 294: 119-127. doi: 10.1016/j.jcat.2012.07.013 [16] JING H Y, WEN T, FAN C M, et al. Efficient adsorption/photodegradation of organic pollutants from aqueous systems using Cu2O nanocrystals as a novel integrated photocatalytic adsorbent [J]. Journal of Materials Chemistry A, 2014, 2(35): 14563-14570. doi: 10.1039/C4TA02459A [17] DI T M, XU Q L, HO W, et al. Review on metal sulphide-based Z-scheme photocatalysts [J]. ChemCatChem, 2019, 11(5): 1394-1411. doi: 10.1002/cctc.201802024 [18] DONG H R, ZENG G M, TANG L, et al. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures [J]. Water Research, 2015, 79: 128-146. doi: 10.1016/j.watres.2015.04.038 [19] ALI A, SHOEB M, LI Y, et al. Enhanced photocatalytic degradation of antibiotic drug and dye pollutants by graphene-ordered mesoporous silica (SBA 15)/TiO2 nanocomposite under visible-light irradiation [J]. Journal of Molecular Liquids, 2021, 324: 114696. doi: 10.1016/j.molliq.2020.114696 [20] WANG W, FANG J J, CHEN H. Nano-confined g-C3N4 in mesoporous SiO2 with improved quantum size effect and tunable structure for photocatalytic tetracycline antibiotic degradation [J]. Journal of Alloys and Compounds, 2020, 819: 153064. doi: 10.1016/j.jallcom.2019.153064 [21] ZHU X F, YUAN W Y, LANG M Q, et al. Novel methods of sewage sludge utilization for photocatalytic degradation of tetracycline-containing wastewater [J]. Fuel, 2019, 252: 148-156. doi: 10.1016/j.fuel.2019.04.093 [22] HEIDARI S, HAGHIGHI M, SHABANI M. Sono-photodeposition of Ag over sono-fabricated mesoporous Bi2Sn2O7-two dimensional carbon nitride: Type-II plasmonic nano-heterojunction with simulated sunlight-driven elimination of drug [J]. Chemical Engineering Journal, 2020, 389: 123418. doi: 10.1016/j.cej.2019.123418 [23] LI W, CHU X S, HE S A, et al. A gourd-like hollow mesoporous silica particle-supported Ag/AgBr Schottky junction for highly efficient mineralization of tetracycline hydrochloride [J]. Environmental Science:Nano, 2020, 7(9): 2654-2668. doi: 10.1039/D0EN00746C [24] LYU J Z, SHAO J W, WANG Y H, et al. Construction of a porous core-shell homojunction for the photocatalytic degradation of antibiotics [J]. Chemical Engineering Journal, 2019, 358: 614-620. doi: 10.1016/j.cej.2018.10.085 [25] LYU J Z, ZHOU Z, WANG Y H, et al. Platinum-enhanced amorphous TiO2-filled mesoporous TiO2 crystals for the photocatalytic mineralization of tetracycline hydrochloride [J]. Journal of Hazardous Materials, 2019, 373: 278-284. doi: 10.1016/j.jhazmat.2019.03.096 [26] LI C X, HU R B, LU X F, et al. Efficiency enhancement of photocatalytic degradation of tetracycline using reduced graphene oxide coordinated titania nanoplatelet [J]. Catalysis Today, 2020, 350: 171-183. doi: 10.1016/j.cattod.2019.06.038 [27] SHI Y Y, YAN Z, XU Y T, et al. Visible-light-driven AgBr-TiO2-Palygorskite photocatalyst with excellent photocatalytic activity for tetracycline hydrochloride [J]. Journal of Cleaner Production, 2020, 277: 124021. doi: 10.1016/j.jclepro.2020.124021 [28] DENG L L, YIN D G, KHAING K K, et al. The facile boosting sunlight-driven photocatalytic performance of a metal-organic-framework through coupling with Ag2S nanoparticles [J]. New Journal of Chemistry, 2020, 44(29): 12568-12578. doi: 10.1039/D0NJ02030C [29] YANG H, YU X, LIU J, et al. Preparation of magnetic Fe3O4/activated carbon fiber and a study of the tetracycline adsorption in aquaculture wastewater [J]. Materiali in Tehnologije, 2019, 53(4): 505-510. doi: 10.17222/mit.2018.234 [30] WU Z X, ZHAO D Y. Ordered mesoporous materials as adsorbents [J]. Chemical Communications (Cambridge, England), 2011, 47(12): 3332-3338. doi: 10.1039/c0cc04909c [31] WU H H, XIE H R, HE G P, et al. Effects of the pH and anions on the adsorption of tetracycline on iron-montmorillonite [J]. Applied Clay Science, 2016, 119: 161-169. doi: 10.1016/j.clay.2015.08.001 [32] 张志旭, 吴根义, 许振成. 粘土矿吸附过程中四环素基团变化研究 [J]. 农业资源与环境学报, 2017, 34(2): 115-120. ZHANG Z X, WU G Y, XU Z C. Functional groups’ variation of tetracycline in the process of adsorption in clay minerals [J]. Journal of Agricultural Resources and Environment, 2017, 34(2): 115-120(in Chinese).
[33] PAPOULIS D, PANAGIOTARAS D, TSIGROU P, et al. Halloysite and sepiolite-TiO2 nanocomposites: Synthesis characterization and photocatalytic activity in three aquatic wastes [J]. Materials Science in Semiconductor Processing, 2018, 85: 1-8. doi: 10.1016/j.mssp.2018.05.025 [34] WANG L, JIN P X, DUAN S H, et al. In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction [J]. Science Bulletin, 2019, 64(13): 926-933. doi: 10.1016/j.scib.2019.05.012 [35] YANG H, HU S, ZHAO H, et al. High-performance Fe-doped ZIF-8 adsorbent for capturing tetracycline from aqueous solution [J]. Journal of Hazardous Materials, 2021, 416: 126046. doi: 10.1016/j.jhazmat.2021.126046 [36] NASALEVICH M A, van der VEEN M, KAPTEIJN F, et al. Metal–organic frameworks as heterogeneous photocatalysts: Advantages and challenges [J]. CrystEngComm, 2014, 16(23): 4919-4926. doi: 10.1039/C4CE00032C [37] GUO F, LI M Y, REN H J, et al. Facile bottom-up preparation of Cl-doped porous g-C3N4 nanosheets for enhanced photocatalytic degradation of tetracycline under visible light [J]. Separation and Purification Technology, 2019, 228: 115770. doi: 10.1016/j.seppur.2019.115770 [38] QUYEN V T, KIM J, PARK P M, et al. Enhanced the visible light photocatalytic decomposition of antibiotic pollutant in wastewater by using Cu doped WO3 [J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104737. doi: 10.1016/j.jece.2020.104737 [39] JIANG J Z, JIA Y S, WANG Y B, et al. Insight into efficient photocatalytic elimination of tetracycline over SrTiO3(La, Cr) under visible-light irradiation: The relationship of doping and performance [J]. Applied Surface Science, 2019, 486: 93-101. doi: 10.1016/j.apsusc.2019.04.261 [40] XU Y G, GE F Y, CHEN Z G, et al. One-step synthesis of Fe-doped surface-alkalinized g-C3N4 and their improved visible-light photocatalytic performance [J]. Applied Surface Science, 2019, 469: 739-746. doi: 10.1016/j.apsusc.2018.11.062 [41] WANG K L, LI Y, SUN T, et al. Ultrafine silver nanoparticles deposited on sodium-doped graphitic carbon nitride towards enhanced photocatalytic degradation of dyes and antibiotics under visible light irradiation [J]. Applied Surface Science, 2019, 476: 741-748. doi: 10.1016/j.apsusc.2019.01.168 [42] ZHOU J, DING J, WAN H, et al. Boosting photocatalytic degradation of antibiotic wastewater by synergy effect of heterojunction and phosphorus doping [J]. Journal of Colloid and Interface Science, 2021, 582: 961-968. doi: 10.1016/j.jcis.2020.08.099 [43] MINH TRI N L, KIM J, GIANG B L, et al. Ag-doped graphitic carbon nitride photocatalyst with remarkably enhanced photocatalytic activity towards antibiotic in hospital wastewater under solar light [J]. Journal of Industrial and Engineering Chemistry, 2019, 80: 597-605. doi: 10.1016/j.jiec.2019.08.037 [44] WANG W J, ZENG Z T, ZENG G M, et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light [J]. Chemical Engineering Journal, 2019, 378: 122132. doi: 10.1016/j.cej.2019.122132 [45] PANNERI S, GANGULY P, MOHAN M, et al. Photoregenerable, bifunctional granules of carbon-doped g-C3N4 as adsorptive photocatalyst for the efficient removal of tetracycline antibiotic [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1610-1618. [46] XU Y S, LIANG Y H, ZENG Y A, et al. Co-doping g-C3N4 with P and Mo for efficient photocatalytic tetracycline degradation under visible light [J]. Ceramics International, 2022, 48(17): 24677-24686. doi: 10.1016/j.ceramint.2022.05.114 [47] ZHANG S W, LI J X, WANG X K, et al. in situ ion exchange synthesis of strongly coupled Ag@AgCl/g-C3N4 porous nanosheets as plasmonic photocatalyst for highly efficient visible-light photocatalysis [J]. ACS Applied Materials & Interfaces, 2014, 6(24): 22116-22125. [48] ROZA L, FAUZIA V, RAHMAN M Y A, et al. ZnO nanorods decorated with carbon nanodots and its metal doping as efficient photocatalyst for degradation of methyl blue solution [J]. Optical Materials, 2020, 109: 110360. doi: 10.1016/j.optmat.2020.110360 [49] 张健伟, 苑鹏, 王建桥, 等. Ce掺杂的CNTs-TiO2光催化剂制备及其NO氧化性能 [J]. 环境工程学报, 2020, 14(7): 1852-1861. ZHANG J W, YUAN P, WANG J Q, et al. Preparation of Ce doped CNTs-TiO2 photocatalyst and its NO oxidation performance [J]. Chinese Journal of Environmental Engineering, 2020, 14(7): 1852-1861(in Chinese).
[50] DUTTA D P, RAVAL P. Effect of transition metal ion (Cr3+, Mn2+ and Cu2+) doping on the photocatalytic properties of ZnWO4 nanoparticles [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2018, 357: 193-200. doi: 10.1016/j.jphotochem.2018.02.026 [51] YAN X Q, XUE C, YANG B L, et al. Novel three-dimensionally ordered macroporous Fe3+-doped TiO2 photocatalysts for H2 production and degradation applications [J]. Applied Surface Science, 2017, 394: 248-257. doi: 10.1016/j.apsusc.2016.10.077 [52] QU J N, DU Y, FENG Y B, et al. Visible-light-responsive K-doped g-C3N4/BiOBr hybrid photocatalyst with highly efficient degradation of Rhodamine B and tetracycline [J]. Materials Science in Semiconductor Processing, 2020, 112: 105023. doi: 10.1016/j.mssp.2020.105023 [53] VIET N M, TRUNG D Q, GIANG B L, et al. Noble metal-doped graphitic carbon nitride photocatalyst for enhancement photocatalytic decomposition of antibiotic pollutant in wastewater under visible light [J]. Journal of Water Process Engineering, 2019, 32: 100954. doi: 10.1016/j.jwpe.2019.100954 [54] NI M, LEUNG M K H, LEUNG D Y C, et al. A review and recent developments in photocatalytic water-splitting using TiO2 [J]. Renewable and Sustainable Energy Reviews, 2007, 11(3): 401-425. doi: 10.1016/j.rser.2005.01.009 [55] 洪孝挺, 王正鹏, 陆峰, 等. 可见光响应型非金属掺杂TiO2的研究进展 [J]. 化工进展, 2004, 23(10): 1077-1080. doi: 10.3321/j.issn:1000-6613.2004.10.009 HONG X T, WANG Z P, LU F, et al. Study on visible light-activated non-metal doped titanium dioxide [J]. Chemical Industry and Engineering Progress, 2004, 23(10): 1077-1080(in Chinese). doi: 10.3321/j.issn:1000-6613.2004.10.009
[56] MORI K, YAMASHITA H. Progress in design and architecture of metal nanoparticles for catalytic applications [J]. Physical Chemistry Chemical Physics:PCCP, 2010, 12(43): 14420-14432. doi: 10.1039/c0cp00988a [57] QIAN X F, FUKU K, KUWAHARA Y, et al. Design and functionalization of photocatalytic systems within mesoporous silica [J]. ChemSusChem, 2014, 7(6): 1528-1536. doi: 10.1002/cssc.201400111 [58] HU F, XU S D, LIU B. Photosensitizers with aggregation-induced emission: Materials and biomedical applications [J]. Advanced Materials, 2018, 30(45): 1801350. doi: 10.1002/adma.201801350 [59] KUDO A, MISEKI Y. Heterogeneous photocatalyst materials for water splitting [J]. Chemical Society Reviews, 2009, 38(1): 253-278. doi: 10.1039/B800489G [60] HAN L P, LV Y W, LI B, et al. Enhancing H2 evolution and molecular oxygen activation via dye sensitized BiOBr0.9I0.1 under visible light [J]. Journal of Colloid and Interface Science, 2020, 580: 1-10. doi: 10.1016/j.jcis.2020.07.014 [61] AHADI M, TEHRANI M S, AZAR P A, et al. Novel preparation of sensitized ZnS nanoparticles and its use in photocatalytic degradation of tetracycline [J]. International Journal of Environmental Science and Technology, 2016, 13(12): 2797-2804. doi: 10.1007/s13762-016-1106-0 [62] MA Z Y, ZENG C, HU L L, et al. A high-performance photocatalyst of ZnTCPP sensitized porous graphitic carbon nitride for antibiotic degradation under visible light irradiation [J]. Applied Surface Science, 2019, 484: 489-500. doi: 10.1016/j.apsusc.2019.04.117 [63] HUANG Y, ZHAO P, MIAO H C, et al. Organic-inorganic TCPP/BiOCl hybrids with accelerated interfacial charge separation for boosted photocatalytic performance [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 616: 126367. doi: 10.1016/j.colsurfa.2021.126367 [64] MGIDLANA S, SEN P, NYOKONG T. Photodegradation of tetracycline by asymmetrical zinc(II)phthalocyanines conjugated to cobalt tungstate nanoparticles [J]. Journal of Molecular Structure, 2022, 1261: 132938. doi: 10.1016/j.molstruc.2022.132938 [65] LI K, PANG Y P, LU Q F. in situ growth of copper(ii) phthalocyanine-sensitized electrospun CeO2/Bi2MoO6 nanofibers: A highly efficient photoelectrocatalyst towards degradation of tetracycline [J]. Inorganic Chemistry Frontiers, 2019, 6(11): 3215-3224. doi: 10.1039/C9QI00950G [66] XU C J, SUN W J, DONG Y J, et al. A graphene oxide-molecular Cu porphyrin-integrated BiVO4 photoanode for improved photoelectrochemical water oxidation performance [J]. Journal of Materials Chemistry A, 2020, 8(7): 4062-4072. doi: 10.1039/C9TA13452B [67] GUO X H, ZHOU X J, LI X H, et al. Bismuth oxychloride (BiOCl)/copper phthalocyanine (CuTNPc) heterostructures immobilized on electrospun polyacrylonitrile nanofibers with enhanced activity for floating photocatalysis [J]. Journal of Colloid and Interface Science, 2018, 525: 187-195. doi: 10.1016/j.jcis.2018.04.028 [68] JODEYRI M, HAGHIGHI M, SHABANI M. Plasmon-assisted demolition of antibiotic using sono-photoreduction decoration of Ag on 2D C3N4 nanophotocatalyst enhanced with acid-treated clinoptilolite [J]. Ultrasonics Sonochemistry, 2019, 54: 220-232. doi: 10.1016/j.ultsonch.2019.01.035 [69] ZHANG P, WANG J Q, GONG J, et al. Fabrication of Ag/carbon nitride photocatalysts and their enhanced photocatalytic performance for tetracycline degradation [J]. Functional Materials Letters, 2020, 13(6): 2051033. doi: 10.1142/S1793604720510339 [70] CHEN F, HUANG H W, ZENG C, et al. Achieving enhanced UV and visible light photocatalytic activity for ternary Ag/AgBr/BiOIO3: Decomposition for diverse industrial contaminants with distinct mechanisms and complete mineralization ability [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 7777-7791. [71] WU J D, WANG Y Q, LIU Z X, et al. Preparation of noble metal Ag-modified BiVO4 nanosheets and a study on the degradation performance of tetracyclines [J]. New Journal of Chemistry, 2020, 44(32): 13815-13823. doi: 10.1039/D0NJ03080E [72] MA C, WEI J J, JIANG K N, et al. Self-assembled micro-flowers of ultrathin Au/BiOCOOH nanosheets photocatalytic degradation of tetracycline hydrochloride and reduction of CO2 [J]. Chemosphere, 2021, 283: 131228. doi: 10.1016/j.chemosphere.2021.131228 [73] ZHANG Y, ZHANG M J, TANG L, et al. Platinum like cocatalysts tungsten carbide loaded hollow tubular g-C3N4 achieving effective space separation of carriers to degrade antibiotics [J]. Chemical Engineering Journal, 2020, 391: 123487. doi: 10.1016/j.cej.2019.123487 [74] HE W L, WANG K W, ZHU Z, et al. Ultra-small subnano TiOx clusters as excellent cocatalysts for the photocatalytic degradation of tetracycline on plasmonic Ag/AgCl [J]. Catalysis Science & Technology, 2020, 10(1): 147-153. [75] YU B, MENG F M, ZHOU T, et al. Construction of CoS/CeO2 heterostructure nanocages with enhanced photocatalytic performance under visible light [J]. Journal of the American Ceramic Society, 2020, 103(11): 6136-6148. doi: 10.1111/jace.17340 [76] SUN L, ZHANG R Z, WANG Y, et al. Plasmonic Ag@AgCl nanotubes fabricated from copper nanowires as high-performance visible light photocatalyst [J]. ACS Applied Materials & Interfaces, 2014, 6(17): 14819-14826. [77] WANG W, LAI M, FANG J J, et al. Au and Pt selectively deposited on{0 0 1}-faceted TiO2 toward SPR enhanced photocatalytic Cr(VI) reduction: The influence of excitation wavelength [J]. Applied Surface Science, 2018, 439: 430-438. doi: 10.1016/j.apsusc.2017.12.249 [78] ZHANG J L, LU Y, GE L, et al. Novel AuPd bimetallic alloy decorated 2D BiVO4 nanosheets with enhanced photocatalytic performance under visible light irradiation [J]. Applied Catalysis B:Environmental, 2017, 204: 385-393. doi: 10.1016/j.apcatb.2016.11.057 [79] 刘同同. BiOBr基光催化材料的制备及性能研究[D]. 太原: 太原理工大学, 2020. LIU T T. Preparation and properties of BiOBr based photocatalytic materials[D]. Taiyuan: Taiyuan University of Technology, 2020 (in Chinese).
[80] HE F, LU Z Y, SONG M S, et al. Selective reduction of Cu2+ with simultaneous degradation of tetracycline by the dual channels ion imprinted POPD-CoFe2O4 heterojunction photocatalyst [J]. Chemical Engineering Journal, 2019, 360: 750-761. doi: 10.1016/j.cej.2018.12.034 [81] 施欢贤. 新型铋系可见光复合光催化体系的构建及其杀灭E. coli性能研究[D]. 西安: 西北大学, 2020. SHI H X. Construction of novel bismuth-based visible light composite photocatalytic system and its application for E. coli disinfection[D]. Xi'an: Northwest University, 2020 (in Chinese).
[82] LIU Y, KONG J J, YUAN J L, et al. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation [J]. Chemical Engineering Journal, 2018, 331: 242-254. doi: 10.1016/j.cej.2017.08.114 [83] LI S J, HU S W, XU K B, et al. Construction of fiber-shaped silver oxide/tantalum nitride p-n heterojunctions as highly efficient visible-light-driven photocatalysts [J]. Journal of Colloid and Interface Science, 2017, 504: 561-569. doi: 10.1016/j.jcis.2017.06.018 [84] BEHERA A, KANDI D, MARTHA S, et al. Constructive interfacial charge carrier separation of a p-CaFe2O4@n-ZnFe2O4 heterojunction architect photocatalyst toward photodegradation of antibiotics [J]. Inorganic Chemistry, 2019, 58(24): 16592-16608. doi: 10.1021/acs.inorgchem.9b02610 [85] ACHARYA L, NAYAK S, PATTNAIK S P, et al. Resurrection of boron nitride in p-n type-II boron nitride/B-doped-g-C3N4 nanocomposite during solid-state Z-scheme charge transfer path for the degradation of tetracycline hydrochloride [J]. Journal of Colloid and Interface Science, 2020, 566: 211-223. doi: 10.1016/j.jcis.2020.01.074 [86] MA C C, LEE J, KIM Y, et al. Rational design of α-Fe2O3 nanocubes supported BiVO4 Z-scheme photocatalyst for photocatalytic degradation of antibiotic under visible light [J]. Journal of Colloid and Interface Science, 2021, 581: 514-522. doi: 10.1016/j.jcis.2020.07.127 [87] XIAO T T, TANG Z, YANG Y, et al. in situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics [J]. Applied Catalysis B:Environmental, 2018, 220: 417-428. doi: 10.1016/j.apcatb.2017.08.070 [88] CAI T, ZENG W G, LIU Y T, et al. A promising inorganic-organic Z-scheme photocatalyst Ag3PO4/PDI supermolecule with enhanced photoactivity and photostability for environmental remediation [J]. Applied Catalysis B:Environmental, 2020, 263: 118327. doi: 10.1016/j.apcatb.2019.118327 [89] YIN H F, CAO Y, FAN T L, et al. in situ synthesis of Ag3PO4/C3N5 Z-scheme heterojunctions with enhanced visible-light-responsive photocatalytic performance for antibiotics removal [J]. Science of the Total Environment, 2021, 754: 141926. doi: 10.1016/j.scitotenv.2020.141926 [90] ZHANG Z Z, PAN Z W, GUO Y F, et al. In-situ growth of all-solid Z-scheme heterojunction photocatalyst of Bi7O9I3/g-C3N4 and high efficient degradation of antibiotic under visible light [J]. Applied Catalysis B:Environmental, 2020, 261: 118212. doi: 10.1016/j.apcatb.2019.118212 [91] CHEN Y S, CRITTENDEN J C, HACKNEY S, et al. Preparation of a novel TiO2-based p-n junction nanotube photocatalyst [J]. Environmental Science & Technology, 2005, 39(5): 1201-1208. [92] ZHAO Q, WANG J L, LI Z P, et al. Heterostructured graphitic-carbon-nitride-nanosheets/copper(I) oxide composite as an enhanced visible light photocatalyst for decomposition of tetracycline antibiotics [J]. Separation and Purification Technology, 2020, 250: 117238. doi: 10.1016/j.seppur.2020.117238 [93] HUANG Y C, FAN W J, LONG B, et al. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions [J]. Applied Catalysis B:Environmental, 2016, 185: 68-76. doi: 10.1016/j.apcatb.2015.11.043 [94] LI S J, CHEN J L, HU S W, et al. A novel 3D Z-scheme heterojunction photocatalyst: Ag6Si2O7 anchored on flower-like Bi2WO6 and its excellent photocatalytic performance for the degradation of toxic pharmaceutical antibiotics [J]. Inorganic Chemistry Frontiers, 2020, 7(2): 529-541. doi: 10.1039/C9QI01201J [95] LI S J, XUE B, WANG C C, et al. Facile fabrication of flower-like BiOI/BiOCOOH p-n heterojunctions for highly efficient visible-light-driven photocatalytic removal of harmful antibiotics [J]. Nanomaterials (Basel, Switzerland), 2019, 9(11): 1571. doi: 10.3390/nano9111571 [96] HUANG L Y, YANG L, LI Y P, et al. P-n BiOI/Bi3O4Cl hybrid junction with enhanced photocatalytic performance in removing methyl orange, bisphenol A, tetracycline and Escherichia coli [J]. Applied Surface Science, 2020, 527: 146748. doi: 10.1016/j.apsusc.2020.146748 [97] TANG T, YIN Z L, CHEN J R, et al. Novel p-n heterojunction Bi2O3/Ti3+-TiO2 photocatalyst enables the complete removal of tetracyclines under visible light [J]. Chemical Engineering Journal, 2021, 417: 128058. doi: 10.1016/j.cej.2020.128058 [98] LI S J, XUE B, CHEN J L, et al. Constructing a plasmonic p-n heterojunction photocatalyst of 3D Ag/Ag6Si2O7/Bi2MoO6 for efficiently removing broad-spectrum antibiotics [J]. Separation and Purification Technology, 2021, 254: 117579. doi: 10.1016/j.seppur.2020.117579 [99] SHI W L, LI M Y, HUANG X L, et al. Construction of CuBi2O4/Bi2MoO6 p-n heterojunction with nanosheets-on-microrods structure for improved photocatalytic activity towards broad-spectrum antibiotics degradation [J]. Chemical Engineering Journal, 2020, 394: 125009. doi: 10.1016/j.cej.2020.125009 [100] YE L Q, SU Y R, JIN X L, et al. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms [J]. Environmental Science:Nano, 2014, 1(2): 90-112. doi: 10.1039/c3en00098b [101] YAN M, HUA Y Q, ZHU F F, et al. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 p-n junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics [J]. Applied Catalysis B:Environmental, 2017, 202: 518-527. doi: 10.1016/j.apcatb.2016.09.039 [102] SHEN X P, WU S K, ZHAO H, et al. Synthesis of single-crystalline Bi2O3 nanowires by atmospheric pressure chemical vapor deposition approach [J]. Physica E:Low-Dimensional Systems and Nanostructures, 2007, 39(1): 133-136. doi: 10.1016/j.physe.2007.02.001 [103] KOTHE T, PLUMERÉ N, BADURA A, et al. Combination of A Photosystem 1-based photocathode and a Photosystem 2-based photoanode to a Z-scheme mimic for biophotovoltaic applications [J]. Angewandte Chemie International Edition, 2013, 52(52): 14233-14236. doi: 10.1002/anie.201303671 [104] GONG Y Y, WU Y J, XU Y, et al. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water [J]. Chemical Engineering Journal, 2018, 350: 257-267. doi: 10.1016/j.cej.2018.05.186 [105] HUANG S S, WANG G D, LIU J Q, et al. A novel CuBi2O4/BiOBr direct Z-scheme photocatalyst for efficient antibiotics removal: Synergy of adsorption and photocatalysis on degradation kinetics and mechanism insight [J]. ChemCatChem, 2020, 12(17): 4431-4445. doi: 10.1002/cctc.202000634 [106] LIU X L, ZHANG Q Z, MA D L. Advances in 2D/2D Z-scheme heterojunctions for photocatalytic applications [J]. Solar RRL, 2021, 5(2): 2000397. doi: 10.1002/solr.202000397 [107] QI K Z, ZADA A, YANG Y, et al. Design of 2D-2D NiO/g-C3N4 heterojunction photocatalysts for degradation of an emerging pollutant [J]. Research on Chemical Intermediates, 2020, 46(12): 5281-5295. doi: 10.1007/s11164-020-04262-0 [108] ANDREOZZI R, CAPRIO V, INSOLA A, et al. Advanced oxidation processes (AOP) for water purification and recovery [J]. Catalysis Today, 1999, 53(1): 51-59. doi: 10.1016/S0920-5861(99)00102-9 [109] KUMAR A, SHARMA G, KUMARI A, et al. Construction of dual Z-scheme g-C3N4/Bi4Ti3O12/Bi4O5I2 heterojunction for visible and solar powered coupled photocatalytic antibiotic degradation and hydrogen production: Boosting via I−/I3− and Bi3+/Bi5+ redox mediators [J]. Applied Catalysis B:Environmental, 2021, 284: 119808. doi: 10.1016/j.apcatb.2020.119808 [110] YOU J K, XIAO M, WANG Z L, et al. Non-noble metal-based cocatalysts for photocatalytic CO2 reduction [J]. Journal of CO2 Utilization, 2022, 55: 101817. doi: 10.1016/j.jcou.2021.101817 [111] 袁金华. 光催化降解和光降解氯代乙酸的研究[D]. 兰州: 兰州大学, 2008. YUAN J H. Studies on photocatalytic degradation and photolytic degradation of chloroacetic acids[D]. Lanzhou: Lanzhou University, 2008 (in Chinese).
[112] DENG Y, ZHAO R Z. Advanced oxidation processes (AOPs) in wastewater treatment [J]. Current Pollution Reports, 2015, 1(3): 167-176. doi: 10.1007/s40726-015-0015-z [113] KISELEV V M, KISLYAKOV I M, BURCHINOV A N. Generation of singlet oxygen on the surface of metal oxides [J]. Optics and Spectroscopy, 2016, 120(4): 520-528. doi: 10.1134/S0030400X16040123 [114] ZHU X D, WANG Y J, SUN R J, et al. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2 [J]. Chemosphere, 2013, 92(8): 925-932. doi: 10.1016/j.chemosphere.2013.02.066 [115] CHEN X Y, XUE X L, GONG X W. A novel Z-scheme porous g-C3N4 nanosheet/Ag3PO4 photocatalyst decorated with N-doped CDs for high efficiency removal of antibiotics [J]. Dalton Transactions (Cambridge, England:2003), 2020, 49(16): 5205-5218. doi: 10.1039/D0DT00408A [116] CHEN Q H, WU S N, XIN Y J. Synthesis of Au-CuS-TiO2 nanobelts photocatalyst for efficient photocatalytic degradation of antibiotic oxytetracycline [J]. Chemical Engineering Journal, 2016, 302: 377-387. doi: 10.1016/j.cej.2016.05.076 [117] 黄成群, 徐彦芹, 曹渊. 介孔材料负载光催化剂在废水处理中的应用 [J]. 工业水处理, 2010, 30(9): 20-24. doi: 10.3969/j.issn.1005-829X.2010.09.005 HUANG C Q, XU Y Q, CAO Y. Application of photocatalyst supported by mesoporous material to wastewater treatment [J]. Industrial Water Treatment, 2010, 30(9): 20-24(in Chinese). doi: 10.3969/j.issn.1005-829X.2010.09.005
[118] ZHANG S Q, ZHANG Z F, LI B, et al. Hierarchical Ag3PO4@ZnIn2S4 nanoscoparium: An innovative Z-scheme photocatalyst for highly efficient and predictable tetracycline degradation [J]. Journal of Colloid and Interface Science, 2021, 586: 708-718. doi: 10.1016/j.jcis.2020.10.140 [119] 曹驰程, 王友权, 章奇, 等. 典型湖泊天然有机质与四环素的相互作用 [J]. 湖泊科学, 2018, 30(4): 1004-1011. doi: 10.18307/2018.0413 CAO C C, WANG Y Q, ZHANG Q, et al. Interactions of tetracycline and dissolved organic matter from freshwater lakes [J]. Journal of Lake Sciences, 2018, 30(4): 1004-1011(in Chinese). doi: 10.18307/2018.0413
[120] ZHU R C, DIAZ A J, SHEN Y, et al. Mechanism of humic acid fouling in a photocatalytic membrane system [J]. Journal of Membrane Science, 2018, 563: 531-540. doi: 10.1016/j.memsci.2018.06.017 [121] CHEN J Y, DONG X, XIN Y Y, et al. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure [J]. Aquatic Toxicology, 2011, 101(3/4): 493-499. -



 下载:
下载: