-
纳米银(AgNPs)由于优良的物理、化学和生物特性,被广泛应用到航空、医学、农业、工业等领域,是目前使用量最多的纳米材料. 其产量巨大,据估计目前全球AgNPs年产量在500—1000 t之间[1]. 随着AgNPs产品的大量使用,其在生产、运输、使用及后期处理中不可避免地会流入环境中,导致环境中AgNPs含量不断增加[2]. 现有分析技术还很难检测AgNPs在环境中的真实浓度,Giese通过建模估算了AgNPs流入到不同环境中的范围值(表1)[1],发现AgNPs含量在环境中不断增加. 土壤和水体是AgNPs汇集的主要场所[3-4]. Sun等预计欧盟污水处理厂污泥中AgNPs含量在(0.008—0.01)mg·kg−1之间,自然和城市土壤中AgNPs每年增加量为(0.91—1.8)ng·kg−1[5]. Deycard等报道污水中可能检测出的AgNPs含量在(6.9±2.0)mg·kg−1[6]. 环境中的AgNPs会通过食物链进入生物体产生毒害作用,已经在许多动物体中广泛研究,包括软体、甲壳、鱼类、鸟类和哺乳类动物等. 同时如本文中所讨论的AgNPs理化性质、环境、涂层等因素都会影响其毒性作用.
目前国内外文献关于AgNPs毒性的研究进展主要从生物种类(如微生物、植物、动物等)、生物个体(如小鼠、斑马鱼、水蚤、昆虫等)或者特定环境(如水、土壤等)等角度总结,其中涉及到对无脊椎和脊椎动物的毒性[7-9]. 而无脊椎动物种类数占动物总种类数的95%,是生态系统的重要组成. 由此,AgNPs的扩大生产和广泛应用更易对无脊椎动物构成严重危害. 相较于脊椎动物,蚯蚓、秀丽隐杆线虫、贻贝、水蚤等无脊椎动物具有生理特征代表性、便于获取和培养、繁殖周期短、饲养成本低、无伦理限制等特点[10-14],已被广泛应用于AgNPs生态毒性测试. 研究性文献中因实验对象、暴露时间、毒性指标、AgNPs的理化性质、暴露浓度等差异,对这些研究结果很难进行比较分析,AgNPs的毒性机制也仍不清晰.
本文主要从累积效应、急性毒性、生长发育毒性、组织病症、生殖毒性、遗传毒性和回避行为等方面总结了AgNPs对无脊椎动物的影响. 除此之外,AgNPs的理化特性、表面涂层、暴露途径、环境[15-18]等因素都可以影响AgNPs对无脊椎动物的毒性. 从无脊椎动物所获得的AgNPs毒性效应,可用于推测AgNPs对生态系统的影响以及对脊椎等其他动物体可能产生的毒性. 但迄今还未见AgNPs对无脊椎动物毒性效应的研究综述. 本文通过阅读大量国内外文献,较为系统地总结了AgNPs对无脊椎动物的影响,并对未来研究方向及重点做了进一步展望.
-
生物累积是纳米材料致毒的前期行为,是对生物产生危害的一种机制[19]. AgNPs在环境和生物之间可以进行迁移转化,使其在无脊椎动物体内的累积作用研究尤为必要[20-21]. 由于研究所用的受试生物、培养环境、粒径、表面涂层、暴露时间以及浓度不同,很难通过生物累积量来判断各类无脊椎动物对AgNPs生物累积能力高低. 但可以看出,不同无脊椎动物对AgNPs都具有一定程度的生物累积能力. 通常,消化系统是无脊椎动物积累AgNPs最高的部位. Li等研究显示AgNPs暴露组威廉环毛蚓体内的Ag主要分布在消化系统,且消化系统中的Ag含量占整个威廉环毛蚓中总Ag的68.9%, AgNPs在消化系统中的高积累可能是土壤中释放的高浓度AgNPs被肠道上皮细胞吸收所造成,肠粘膜中的一些关键受体(如Na+、K+、ATP酶)、与肠道及其微生物群落相关的特定氧化还原和溶质条件可能会促进AgNPs的摄取;此外,Ag在消化系统中的高积累可能还与无脊椎动物的解毒策略有关,AgNPs在肠细胞中可以与金属硫蛋白(MT)结合,产生的Ag-MT复合物被隔离在溶酶体中,阻碍了AgNPs向机体其他部位的转移[22].
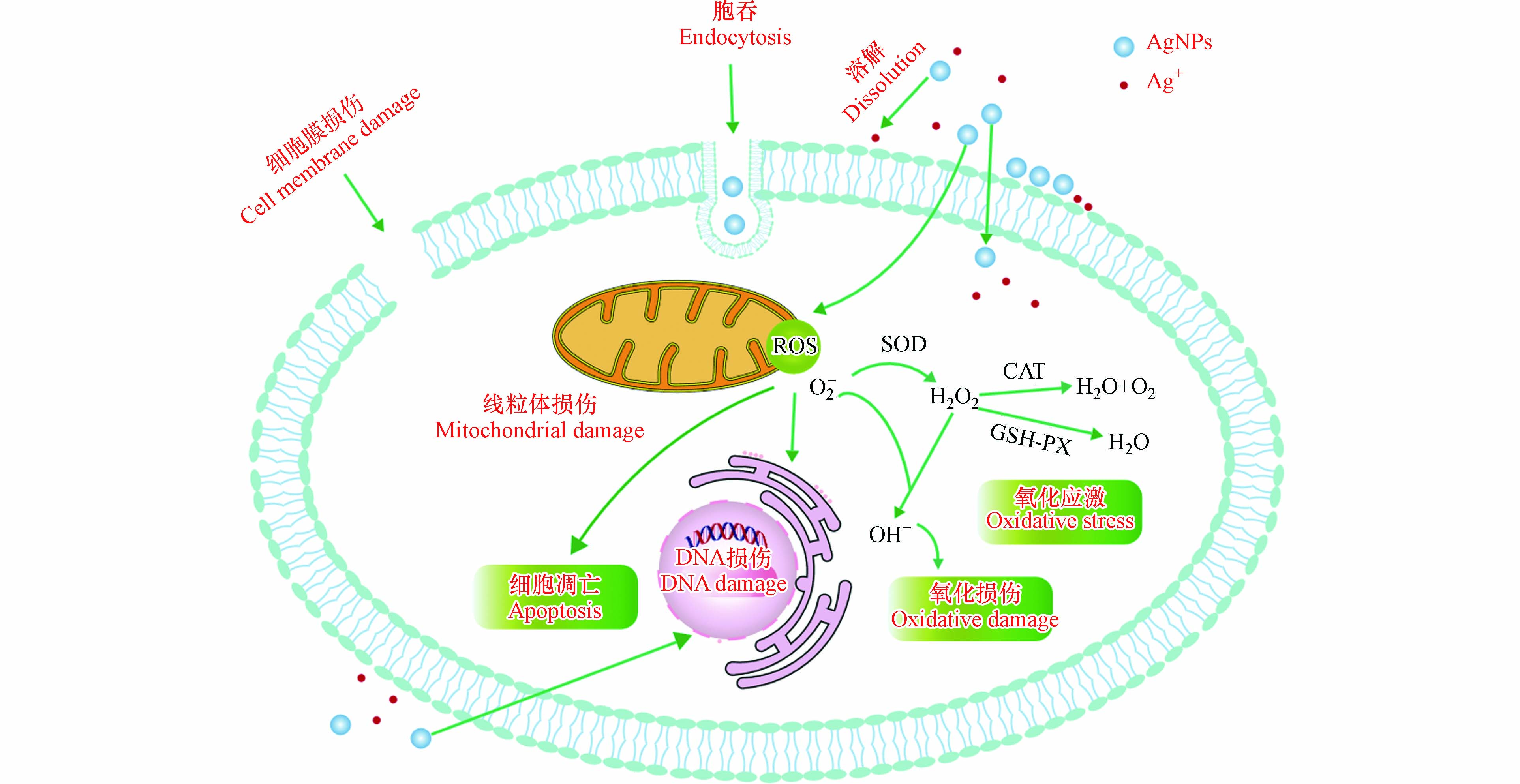
Cozzari 等研究表明,沙蚕从水溶性银中累积Ag的效率高于AgNPs [23]. 同样,Bao等研究了AgNPs及硝酸银对淡水蜗牛不同组织的银负荷,结果发现,蜗牛从硝酸银中累积的银含量高于AgNPs [24]. 造成这种现象的原因,可能是由于摄取途径的不同,硝酸银主要通过质子耦合通道、质子泵、吞噬和胞饮作用等非特异性摄取途径大量积累,AgNPs则主要是通过内吞途径来积累[25](图1),并且由于AgNPs容易聚集并形成生物电晕,也降低了其生物累积量[26]. 但是Cong等研究了硝酸银和AgNPs对沙蚕的生物累积,发现沙蚕对于两种形式的银负荷没有差异[27]. 这表明不同的无脊椎动物,对于两种形式银的累积差异,至今仍没有统一的定论. 表2 集中列出了AgNPS对无脊椎动物的生物累积。
-
(1)急性毒性和生长发育毒性
AgNPs对无脊椎动物的急性毒性结果,可以用来直接快速、直观的判断AgNPs毒性的强弱. 急性毒性测试指标主要为半数有效浓度(50% effect concentration,EC50)和半数致死浓度(50% lethal concentration,LC50)(表3). 有研究表明,AgNPs对无脊椎动物的急性毒性在很大程度上是因为AgNPs颗粒的溶解[38]. Ivask等将大型溞的EC50标准化为溶解的Ag+,发现AgNPs的溶解校正EC50值与AgNO3相似,这就表明AgNPs的急性毒性是由释放的Ag+引起的,但10 nm的AgNPs急性毒性却高于Ag+,原因可能是由于10 nm及更小的AgNPs颗粒与细胞相互作用后在细胞外表面或细胞内部溶解,从而更具生物利用度 [39].
急性毒性试验只是初步了解AgNPs对无脊椎动物的毒性高低. 在自然环境中,无脊椎动物会长期暴露在含有AgNPs的环境中. 有研究报道,在长期接触下,AgNPs会影响无脊椎动物的生长发育[40]. Zhao等发现,AgNPs可以显著抑制大型溞的体长,造成抑制的原因可能是因为食物质量的下降,水中AgNPs和藻类在大型溞肠道结合,导致大型溞对食物吸收率下降,从而影响大型溞的生长发育[41]. Mehennaoui等研究了不同粒径AgNPs对淡水钩虾生长发育的影响,结果发现10 nm AgNPs比60 nm AgNPs更具有抑制效果,可能因为小粒径的AgNPs在动物内部溶解,并释放出更有抑制效果的Ag+,研究指出对于生长发育的抑制,可能是因为淡水钩虾甲壳上有关吸附的感官被破坏;另一种假设是生物体运动量的减少,更加有利于防御机制的能量再分配[30]. 但AgNPs并非只对无脊椎动物的生长发育产生抑制效果,Mackevica等发现,大型溞在低浓度(10 μg·L−1)AgNPs暴露下,反而促进了其生长发育[42].
(2)组织病症
组织病症相较于死亡率和体重的变化对低水平AgNPs暴露更敏感[22]. 有研究发现,蜗牛经AgNPs食物相暴露14 d后,其胃肠道、肾脏、消化腺以及足出现不同程度组织学形态变化. 蜗牛胃肠道出现明显的黏膜损伤与细胞死亡脱落,肾脏组织细胞出现不规则排列、部分细胞脱落,消化小管排列疏松、细胞质内出现较多圆形空泡、部分基底破损、组织细胞部分糜烂脱落,足组织胶原蛋白原纤维排列疏松、细胞间隙增宽并出现较多嗜碱性颗粒团块[17]. AgNPs对消化腺的损害,会使蜗牛消化食物的能力下降,从而对蜗牛的生长发育产生影响[55]. Chen等也发现,400 mg·L−1AgNPs对家蚕组织结构的损害,家蚕的基底膜被破坏,杯状细胞扩张,柱状细胞变形,此外还观察到异常的细胞结构和许多片状结构的出现,这表明AgNPs会对组织产生不良影响,并可能对主要靶器官,例如对家蚕的消化器官产生有害影响[56].
(3)生殖毒性
AgNPs会诱导无脊椎动物生殖细胞的凋亡,从而抑制其繁殖. Luo等发现秀丽隐杆线虫捕食暴露于1、5、10 μg·mL−1AgNPs的大肠杆菌后,其性腺细胞凋亡的数量显著增加,性腺减数分裂区的生殖细胞死亡率显著增加[57]. 但也有研究发现,在低浓度AgNPs暴露下,大型溞的繁殖得到了促进,推测可能是因为AgNPs具有优良的灭菌性能;而随着AgNPs浓度的提高,大型溞的产溞数显著减少,则是因为高浓度时AgNPs毒性较大,对大型溞的机体造成了不可逆的损伤,从而对大型溞的繁殖产生了不良影响[28];另一种推测是因为AgNPs抑制了大型溞的摄食,导致其能量储备减少,进一步影响了大型溞的繁殖[58]. 氧化应激是AgNPs诱导生殖毒性的主要机制,Lim等对秀丽隐杆线虫中氧化应激及其信号通路进行研究,发现AgNPs会导致野生型(N2)秀丽隐杆线虫中活性氧自由基(reactive oxygen species,ROS)增加,低氧诱导结合蛋白(HIF-1)、谷胱甘肽S转移酶(glutathione s-transferase,GST)的激活以及繁殖潜力下降,其中PMK-1 p38丝裂原活化蛋白激酶(MAPK)在AgNPs诱导的氧化应激中起重要作用[59].
(4)遗传毒性
遗传毒性是化学毒性测试和风险评估中重要的毒性终点之一[60]. AgNPs的遗传毒性机制,目前认为主要是以下两种:第一种是通过诱导ROS增加,干扰DNA复制、转录、抑制相关蛋白质,还会导致嘧啶和嘌呤衍生的氧化损伤;第二种是AgNPs直接进入细胞作用于细胞器(例如,纺锤体、着丝粒等)[61],抑制复制、转录,导致染色体丢失,断裂[62],见图1. Choi等研究了AgNPs对赤子爱胜蚓的遗传毒性,发现AgNPs会引起赤子爱胜蚓DNA损伤, AgNPs暴露可以使细胞内ROS增加,引起细胞氧化应激,最终导致DNA损伤[63]. 同样,Alaraby等通过彗星实验发现,AgNPs对果蝇产生遗传毒性是因为氧化应激所引起的DNA损伤[64]. Botelho等研究发现,不同浓度和不同时间AgNPs暴露增加了片脚甲壳动物Parhyale hawaiensis的微核、核芽和核异常的比例[65]. AgNPs不仅会对亲代产生毒性效应,对其子代也会造成损害. 目前已有研究观察到AgNPs对土壤线虫繁殖的多代效应,Wamucho等发现秀丽隐杆线虫多代暴露于AgNPs会诱导未暴露子代的表观遗传变化[66]. 同样,Pakrashi发现,AgNPs不仅对大型溞母体产生毒害,而且使母体中AgNPs约1%—2%的总累积Ag转移到新生水蚤中,这就表明AgNPs可能会对水蚤的繁殖产生更持久的不利影响[67].
(5)回避行为
回避行为是动物遇到不利环境的逃避反应,它是一个生态相关的测量结点,用来快速判断污染物的毒性强弱. 回避试验装置简单,操作便捷,反应快速,周期短,是一种很好地检测AgNPs毒性的方法. 目前,人们已经做过蚯蚓、秀丽隐杆线虫、土鳖虫等无脊椎动物的回避试验,通过测定一定浓度AgNPs对无脊椎动物个体行为的影响程度来判定其毒性大小[68]. Brami等研究了蚯蚓对AgNPs的回避试验,发现蚯蚓对AgNPs回避行为比繁殖等传统终点更为敏感,在最低浓度(12.5 mg·kg−1)时就可以观察到回避现象,但目前还不清楚回避行为是由AgNPs释放的Ag+触发,还是AgNPs触发[10]. 在现有文献中主要用以下3种机制来解释土壤无脊椎动物的回避行为:第一种是污染物使土壤中微生物群发生变化,导致土壤无脊椎动物适口性的改变(如:AgNPs以及Ag+会改变微生物群落结构);第二种是土壤无脊椎动物具有化学和机械感受器,金属颗粒的神经毒性作用或生物化学感觉器敏感性可以使它们主动避开有害环境或移动到更有利的环境;第三种是由于污染物进入到土壤无脊椎动物体内,引起土壤无脊椎动物的不适,从而引起回避行为[69-71].
-
目前,关于AgNPs对无脊椎动物的毒性机制仍不是十分清晰. 根据现有研究总结其可能的机制如图1所示. AgNPs暴露后,释放Ag+,离子与颗粒通过内吞等方式进入细胞,在细胞内产生大量ROS,ROS与细胞膜表面蛋白结合,破坏细胞膜的完整性,从而导致细胞膜损伤[72]. 线粒体是AgNPs诱导氧化应激的主要靶细胞器之一,线粒体中高水平的ROS导致线粒体损伤,最终导致细胞凋亡[73]. 细胞内ROS的增加,会导致氧化应激并破坏细胞成分,造成氧化型DNA损伤,链断裂,核酸修饰,脂质过氧化,蛋白质变性,抗氧化酶的激活以及抗氧化分子的消耗;此外,AgNPs诱导的ROS通过激活细胞信号传递,触发炎症反应[72-73].
Bao等发现,蜗牛暴露于AgNPs后,足部和消化道在Ag浓度很低的情况下也会诱发显著的氧化应激,其中过氧化氢酶(catalase,CAT)和过氧化物酶(peroxidase,POD)活性较对照组酶活显著增加,这些氧化应激可能是由于AgNPs与生物体细胞的接触触发的[24]. Pan等研究发现,AgNPs对原生动物嗜热四膜虫的毒性是由于高活性氧水平引起的,从而导致了脂质过氧化和线粒体功能障碍,为了对抗氧化胁迫,嗜热四膜虫激活了抗氧化系统,增加了谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-PX)和其他抗氧化剂的活性[74]. Chen研究了家蚕中AgNPs的毒性机制,表明ROS的增加诱导了肌醇加氧酶(MIOX)基因表达下调,影响线粒体形态并导致氧化损伤,且AgNPs也会抑制超氧化物歧化酶(superoxide dismutase,SOD)活性,引起持续的氧化应激,从而诱导细胞凋亡[56]. Ahamde等研究了AgNPs在果蝇中的毒性机制,发现经AgNPs暴露后,细胞内产生大量ROS,导致膜损伤、氧化应激以及线粒体损伤,最终导致细胞凋亡;此外,还发现氧化应激导致细胞周期检查点蛋白p53和细胞信号蛋白p38上调,这两种蛋白参与DNA损伤和凋亡等多种过程,是评估遗传毒性的分子标记,说明氧化应激诱导了DNA损伤[75].
-
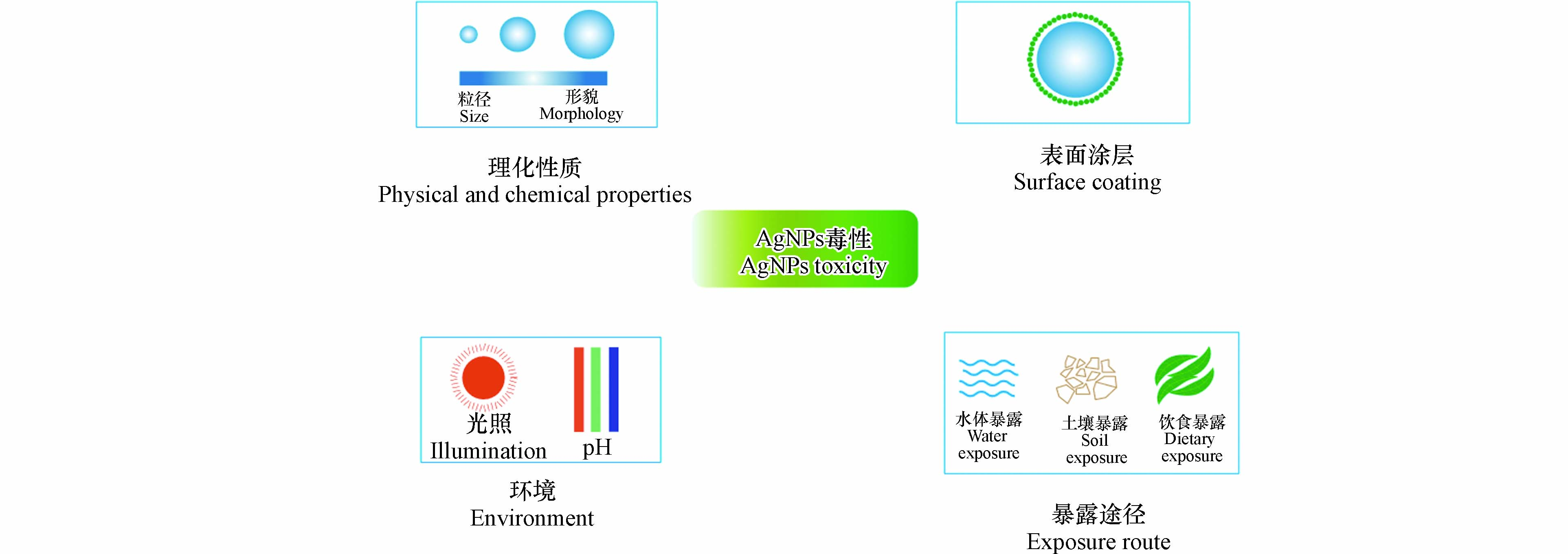
AgNPs的理化性质、表面涂层、环境、暴露途径等因素都可以影响AgNPs对无脊椎动物的毒性(图2). 表4集中汇总了部分AgNPs对无脊椎动物毒性的影响因素.
-
AgNPs的理化性质,包括AgNPs的尺寸、形貌、晶型、表面特性等[84]. AgNPs的理化性质对无脊椎动物的毒性有很大影响. 目前普遍认为AgNPs粒径越小其毒性越强,AgNPs粒径在20—80 nm的毒性效应主要由其溶解释放的Ag+引起,而粒径较小的AgNPs颗粒(10 nm)更容易被生物体利用,毒性效应更大[39]. Hou等使用40 nm和110 nm AgNPs对大型溞做了毒性研究,结果发现,40 nm AgNPs比110 nm AgNPs的LC50值更低,这可能是由于40 nm AgNPs的溶解速率比110 nm AgNPs快,小粒径AgNPs颗粒比表面积更高,生成焓更高[44]. Moon等使用AgNPs颗粒、纳米银线、纳米银板分别对秀丽隐杆线虫进行毒性测试,结果发现AgNPs颗粒和纳米银板对秀丽隐杆线虫的生长和繁殖具有抑制现象,而纳米银线的影响却微乎其微,其中AgNPs颗粒毒性最大,从侧面也说明AgNPs颗粒在毒性水平上起着重要作用,AgNPs颗粒毒性大于纳米银线及纳米银板可能是因为球形更容易在消化道中溶解[79].
-
为了提高AgNPs的性能,可以使用不同的表面涂层. 目前比较常用的表面涂层有PVP、CIT、聚乙烯醇(polyvinyl alcohol,PVA)[85-87]等. 使用PEG可以降低AgNPs颗粒表面能,从而增加其胶体稳定性[88];CIT具有低分子量,并通过静电斥力保护AgNPs;亲水性的PVP具有空间稳定性[89]. 不同表面涂层AgNPs对无脊椎动物毒性大小也不一样. Hou等通过大型溞的急性毒性实验,发现CIT-AgNPs的LC50比PVP-AgNPs的LC50更低,说明CIT包覆的AgNPs毒性更大,这可能是因为PVP涂层的稳定性使Ag能够被完整的封装,减少了Ag+的释放,因而AgNPs毒性降低[44].
-
由于生态系统中物理化学等变量的变化,生物体对AgNPs的可变敏感性正受到越来越多的关注[43]. 例如环境中不同的光照、盐度、pH、温度、暴露介质等,都可能影响AgNPs对无脊椎动物的毒性. Lish等研究了AgNPs在不同环境条件下对卤虫无节幼体的急性毒性效应,发现环境条件可以改变AgNPs的稳定性,使其稳定性下降,导致更多的Ag+释放,从而导致其毒性的改变[83]. Conine等则是研究了不同湖泊水中AgNPs对水蚤的毒性效应,发现颗粒物C:N和C:P比率、总溶解有机碳(dissolved organic carbon,DOC)、总溶解磷(total dissolved phosphate,TDP),都会影响AgNPs的浓度,从而对水蚤产生毒性影响,C:N是影响水蚤存活率的最大因素[43]. 在土壤暴露中,通过活性污泥的加入,可以使部分AgNPs转变成AgS,而AgS具有高的化学稳定性,从而降低了AgNPs的毒性;并且由于活性污泥中含有丰富的食物,导致蚯蚓的体重增加了34%—176%[90].
-
AgNPs不同的暴露途径对无脊椎动物的毒性也不同. Wang研究了水体暴露和饮食暴露两种途径下AgNPs对大型溞生长、繁殖、生物累积等指标的影响. 发现在生长和繁殖实验中,水体暴露下的AgNPs会刺激大型溞的繁殖和蜕皮,在高浓度下会显著抑制大型溞的繁殖和蜕皮,而饮食暴露下的AgNPs在所有浓度下都会显著抑制大型溞的繁殖和生长;在生物累积实验中,水体暴露下AgNPs在最低浓度时,在大型溞体内累积量达到最高,之后随AgNPs暴露浓度增加而累积量减少;饮食暴露下,大型溞体内Ag+ 量随着AgNPs暴露浓度增加而增加[28]. 同样,Qing研究了土壤相暴露和食物相暴露下AgNPs对蜗牛毒性的影响,发现食物相中AgNPs的毒性大于土壤相中AgNPs的毒性;在相同AgNPs浓度下,食物相导致蜗牛消化腺中脂质过氧化物(lipid hydroperoxide,LPO)含量,CAT和SOD活性极显著增加,分别是土壤相的1.5、1.7、2.5倍[17].
-
由于AgNPs材料的优良特性,使其被广泛应用到农、工、医等各种领域,而AgNPs的毒性问题,一直是人们关心的重点. 到目前为止,国内外已有很多关于AgNPs毒性的文献,相关实验结果并不一致甚至自相矛盾. 本文结合文献中的相关研究结果,总结了AgNPs对无脊椎动物的毒性效应及其影响因素. AgNPs可以在环境和生物链中迁移转化,最终累积到无脊椎动物体内,对无脊椎动物造成毒性损害,影响无脊椎动物的生长发育、破坏无脊椎动物的组织结构以及损害其系统功能. AgNPs引起细胞氧化应激,会造成无脊椎动物DNA损伤、线粒体损坏、染色体畸变以及细胞凋亡. 此外,AgNPs的理化性质、表面涂层、环境、暴露途径等因素,都会影响AgNPs对无脊椎动物的毒性大小. 最后基于目前的研究提出一些展望,为AgNPs对无脊椎动物毒性的研究提供新的思路.
(1) AgNPs与AgNPs溶解Ag+之间的毒性没有研究清楚,是AgNPs溶解Ag+所引起的毒性,还是AgNPs与Ag+共同引起的毒性,这方面在无脊椎动物毒性机制中需要更深层的研究.
(2) 生物制备AgNPs具有反应条件温和,绿色环保,成本低等优点,已经成为近些年研究的热点. 随着生物合成法技术的成熟与完善,环境中生物AgNPs日益增多. 无脊椎动物是生物类群和生态系统的重要组成部分,后续应加强生物AgNPs对无脊椎动物的毒性效应研究.
(3) 随着AgNPs被释放到环境中,会与环境中其他污染物相互作用. 可能会影响AgNPs在环境中的理化性质和生物学活性. 因此有必要加强AgNPs与其他污染物的联合效应及其共存特性研究.
(4) 尽管目前关于AgNPs对无脊椎动物毒性的研究在逐渐增多,但大多数研究只考虑了对无脊椎动物的急性毒性试验. 因此,需要更多的研究来确定无脊椎动物体内低浓度AgNPs颗粒的长期影响,隔代影响,以便进行适当的安全评估和识别生物标志物.
(5) 目前AgNPs的试验生物以水生无脊椎动物为主,如:水蚤、大型溞、贻贝等,后续应加深对陆生无脊椎动物的影响研究,为AgNPs在土壤中的评价提供依据.
(6) 需要建立评价AgNPs对无脊椎动物单一和复合污染毒性的标准方法,以完善AgNPs的生态毒性安全评价系统,进而便于政府规范AgNPs的生产和使用.
纳米银对无脊椎动物毒性效应的研究进展
Research advances on silver nanoparticles toxic effects in invertebrates
-
摘要: 纳米银(AgNPs)因优良的抗菌特性,已成为全球使用量最多的纳米材料. 随着AgNPs使用量的增多,其不可避免流入环境中,对生态系统造成危害. 无脊椎动物是动物类群重要的组成部分,本文主要从累积效应、急性毒性、生长发育毒性、组织病症、生殖毒性、遗传毒性和回避行为等方面总结了AgNPs对无脊椎动物的影响和潜在毒性机制,介绍了AgNPs对无脊椎动物毒性的影响因素,分析了AgNPs关于无脊椎动物毒性研究的不足并对研究趋势进行了展望. 本文旨在为AgNPs对无脊椎动物的毒性研究以及AgNPs的安全生产和合理使用提供参考.Abstract: Silver nanoparticles (AgNPs) have become the most frequently used nanomaterials in the world due to their excellent antimicrobial properties. With the increasing use of AgNPs, they are inevitably released into the environment and may produce negative effects on the ecosystem. Invertebrates are an important part of animal taxa. This paper summarized the effects of AgNPs on invertebrates including bioaccumulation, acute toxicity, growth and development toxicity, histopathology, reproductive toxicity, genotoxicity and avoidance behavior, as well as their potential toxicity mechanism. The factors influencing AgNPs toxicity in invertebrates were introduced. Finally, the deficiencies of AgNPs in invertebrate toxicity and the research trend were discussed. This review aimed to provide references for toxicity studies of AgNPs on invertebrates and the safe production and reasonable use of AgNPs.
-
Key words:
- silver nanoparticles /
- invertebrate /
- toxic effect /
- influencing factor.
-

-
环境
Environment浓度单位
Concentration unit2017年 2030年 2050年 污水处理出水 ng·L−1 1.224—103.788 2.120—211.239 6.496—472.948 污水处理污泥 μg·kg−1 32.423—2730.256 62.254—4553.974 187.964—11669.229 地表水 ng·L−1 0.035—2.789 0.063—6.258 0.134—12.896 海水 ng·L−1 0.000—0.000 0.000—0.000 0.000—0.000 沉积物(淡水) μg·kg−1 0.025—33.671 0.043—50.715 0.118—160.683 农业土壤 ng·kg−1 0.236—67.727 0.526—100.645 2.223—219.630 天然土壤 ng·kg−1 0.584—167.456 1.301—48.845 5.497—543.034 城市土壤 ng·kg−1 0.934—267.929 2.082—398.152 8.795—868.855 污泥处理土壤 ng·kg−1 20.085—1661.164 27.211—2122.734 88.785—5249.632 空气 ng·m−3 0.001—0.495 0.002—0.734 0.005—1.608 注:此表格数据来源Giese文章. Note: This table was obtained by collating data from Giese’s paper 表 2 AgNPs对无脊椎动物的生物累积
Table 2. Bioaccumulation of AgNPs in invertebrates
受试生物
Tested organism培养环境
Cultivation environment粒径/nm
Size涂层
Coating暴露时长
Exposure duration暴露浓度
Exposure concentration生物累积量
Bioaccumulation文献
Refs.大型溞
(Daphnia magna)M4 9.15±3.45 CIT 24 h 120 μg·L−1 126.7 μg·g−1 [21] 大型溞
(Daphnia magna)M7 21.73±0.94 CIT 14 d 12 μg·L−1 2.77 μg·g−1 [28] 大型溞
(Daphnia magna)M7 20—30 CIT 21 d 19.23 μg·L−1 (1.37±0.69 )ng·D.magna−1 [16] 粉正蚓
(Lumbricus rubellus)土壤 38.2±4.5 PVP 28 d 250 mg·kg−1 50 mg·kg−1 [29] 威廉环毛蚓
(Pheretima guillemi )土壤 22.6±7.8 PVP 28 d 7.2 mg·kg−1 (0.9 ±0.1) mg·kg−1 [22] 淡水钩虾
(Gammarus fossarum)Volvic 40 CIT 15 d 5 μg·L−1 (2.09 ± 0.19) μg·L−1 [30] 白玉蜗牛
(Achatina fulica)土壤 57.6±2.5 无 14 d 20—50 mg·kg−1 3—245.7 mg·kg−1 [17] 海蠕虫
(Capitella teleta)沉积物 13.9±3.17 CIT 14 d 100 μg·g−1 (215± 92) μg·g−1 [31] 蛤蜊
(Scrobicularia plana)池塘水 40—50 乳酸 10 d 10 μg·L−1 (228 ± 64) ng·g−1 [32] 秀丽隐杆线虫
(Caenorhabditis elegans)土壤 30—50 PVP 5 d 10 μg·L−1 1.15 μg·g−1 [33] 石牡蛎
(Saccostrea glomerata)人工海水 20±5 吐温20 7 d 125 μg·L−1 (1.42 ± 0.03) μg·g−1 [34] 沙蚕
(Nereis diversicolor)沉积物 <100 PVP 10 d 50 μg·g−1 (8.56 ± 6.63) μg·g−1 [35] 杜氏阔沙蚕
(Platynereis dumerilii)人工海水 13.1±3.7 腐殖酸 24 h 200 μg·L−1 (28 ± 5) μg·g−1 [36] 贻贝
(Mytilus galloprovincialis)SW 5.08±2.03 PVP/PEI 21 d 10 μg·L−1 0.73 μg·g−1 [37] 培养环境:M4-Elendt M4培养基,M7-Elendt M7 培养基,Volvic-矿泉水,SW-自然过滤海水. 涂层:CIT-柠檬酸盐,PVP-聚乙烯比咯烷酮,PEI-聚乙烯亚胺. 表中单个暴露浓度仅是用于该生物累积量所对应的暴露浓度.
Cultivation environment: M4-elendt M4 medium, M7-elendt M7 medium, Volvic-mineral water, SW-naturally filtered seawater. Coating: CIT-citrate, PVP-polyvinyl pyrrolidone, PEI-polyethyleneimine. The single exposure concentration in the table is only used for the exposure concentration corresponding to the bioaccumulation.表 3 AgNPs对无脊椎动物急性毒性数据
Table 3. Acute toxicity data of AgNPs to invertebrates
受试生物
Tested organism培养环境
Cultivation environment粒径/nm
Size涂层
Coating暴露时长
Exposure durationEC50/LC50 文献
Refs.大型溞
(Daphnia magna)AFW 47.7±8 CIT 48 h EC50 = 0.141 mg·L−1 [39] 水蚤
(Daphnia)实验湖水 30—50 PVP 24 h LC50 = (34—292) μg·L−1 [43] 大型溞
(Daphnia magna)BBM 45.2±0.2 PVP 24 h LC50 = 24.97 μg·L−1 [44] 大型溞
(Daphnia magna)M4 8.6±3 CIT 48 h EC50 = (110±9.3) μg·L−1 [45] 大型溞
(Daphnia magna)ASTM 3—8 烷烃 24 h EC50 = 13.64 μg·L−1 [46] 盔形溞
(Daphnia galeata)MHW 56.6±10.1 PVP 48 h EC50 = 35.51
(CI:34.06—37.02) μg·L−1[47] 隆线蚤
(Daphnia carinata)ASTM 30 络氨酸 48 h LC50 = 35.48
(CI: 29.02—42.19) μg·L−1[48] 大型溞
(Daphnia magna)M7 21.73±0.94 CIT 48 h LC50 = 34.27 μg·L−1 [28] 大型溞
(Daphnia magna)M7 50±0.5 CIT 48 h LC50 = 0.078 mg·L−1 [49] 硬壳蛤
(Mercenaria mercenaria)ASW 21.5±0.1 CIT 24 h LC50=1050
(CI: 900—1360) μg·L−1[50] 夹杂带丝蚓
(Lumbriculus variegatum)水 15.16±3.06 吐温20 96 h LC50 = 0.51
(CI: 0.45—0.56) mg·L−1[51] 盐卤虫
(Artemia salina)ASW <100 PVP 72 h EC50 = (10.7±1.3) mg·L−1 [52] 埃及伊蚊
(Aedes aegypti)水 22.3—34.4 生物合成 48 h LC50 = 44.77 mg·L−1 [53] 埃及伊蚊
(Aedes aegypti)水 25.9—28.9 生物合成 24 h LC50 = 37.87
(CI: 4.91—158.15) mg·L−1[54] 培养环境:AFW-OECD 202人工淡水,BBM-Bold’s Basal Medium 培养基,M4-Elendt M4培养基,ASTM-美国材料与试验协会培养基,MHW-中等硬度水,M7-Elendt M7 培养基, ASW-人造海水. 涂层:CIT-柠檬酸盐,PVP-聚乙烯比咯烷酮. CI:为95%置信区间,未标注CI的则在原文献中没有标出置信区间.
Cultivation environment: AFW-OECD 202 artificial fresh water, BBM-bold's basal medium, M4-elendt M4 medium, ASTM-american society for testing and materials medium, MHW-medium hard water, M7-elendt M7 medium, ASW-artificial seawater. Coating: CIT-citrate, PVP- polyvinyl pyrrolidone. CI: 95% Confidence intervals. Unlabeled CI were not marked with confidence intervals in the original literature.表 4 AgNPs对无脊椎动物毒性的主要影响因素
Table 4. Main influencing factors of AgNPs toxicity to invertebrates
受试生物
Tested organism影响因素
Influencing factor结果
Result文献
Refs.黄粉虫
(Tenebrio molitor)涂层 CIT涂层摄取和消除效率低于石蜡和PVP涂层 [76] 粉正蚓
(Lumbricus rubellus)涂层 毒性:BSA>PVP>Chit [29] 淡水钩虾
(Gammarus fossarum)涂层 毒性:CIT>PEG [77] 端足虫
(Hyalella-azteca)涂层 毒性:CIT>PVP [78] 大型溞
(Daphnia magna)涂层、粒径 毒性:40 nm>110 nm;CIT>PVP [44] 大型溞
(Daphnia magna)粒径 毒性:10 nm>20 nm>40 nm>60 nm>80 nm [39] 铜锈环棱螺
(Bellamya aeruginosa)粒径 毒性:40 nm、80 nm>20 nm [24] 秀丽隐杆线虫
(Caenorhabditis elegans)形貌 毒性:纳米银颗粒>纳米银板>纳米银线 [79] 盔形溞
(Daphnia galeata)形貌 毒性:纳米银板>纳米银颗粒>纳米银线 [47] 大型溞
(Daphnia magna)环境 富磷食物、藻类可以降低纳米银毒性 [80] 水蚤
(Daphnia)环境 纳米银毒性随天然有机物的增加而下降 [81] 土壤线蚓
(Enchytraeus crypticus)环境 乙酰半胱氨酸可以降低纳米银毒性 [82] 盐卤虫
(Artemia salina)环境 温度升高、盐度下降提高纳米银毒性 [83] 贻贝
(Mytilus galloprovincialis)环境 银积累量,秋季高于春季 [37] 大型蚤
(Daphnia magna)途径 毒性:饮食途径>水体途径 [28] 白玉蜗牛
(Achatina fulica)途径 毒性:饮食途径>土壤途径 [17] 涂层:CIT-柠檬酸盐,PVP-聚乙烯比咯烷酮,BSA-牛血清白蛋白,Chit-壳聚糖,PEG-聚乙二醇.
Coating: CIT-citrate, PVP- polyvinyl pyrrolidone, BSA-bovine albumin, Chit-chitosan, PEG-polyethylene glycol. -
[1] GIESE B, KLAESSIG F, PARK B, et al. Risks, release and concentrations of engineered nanomaterial in the environment [J]. Scientific Reports, 2018, 8: 1565. doi: 10.1038/s41598-018-19275-4 [2] 杨亚宁. 环境中离子强度对纳米银物化特性及其毒理学效应的影响[D]. 合肥: 中国科学技术大学, 2019. YANG Y N. Effects of ionic strength on physicochemical and toxicological properties of silver nanoparticles in the environment[D]. Hefei: University of Science and Technology of China, 2019(in Chinese).
[3] NOWACK B. Evaluation of environmental exposure models for engineered nanomaterials in a regulatory context [J]. NanoImpact, 2017, 8: 38-47. doi: 10.1016/j.impact.2017.06.005 [4] DOOLETTE C L, MCLAUGHLIN M J, KIRBY J K, et al. Bioavailability of silver and silver sulfide nanoparticles to lettuce (Lactuca sativa): Effect of agricultural amendments on plant uptake [J]. Journal of Hazardous Materials, 2015, 300: 788-795. doi: 10.1016/j.jhazmat.2015.08.012 [5] SUN T Y, GOTTSCHALK F, HUNGERBÜHLER K, et al. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials [J]. Environmental Pollution, 2014, 185: 69-76. doi: 10.1016/j.envpol.2013.10.004 [6] DEYCARD V N, SCHÄFER J, PETIT J C J, et al. Inputs, dynamics and potential impacts of silver (Ag) from urban wastewater to a highly turbid estuary (SW France) [J]. Chemosphere, 2017, 167: 501-511. doi: 10.1016/j.chemosphere.2016.09.154 [7] COURTOIS P, RORAT A, LEMIERE S, et al. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals [J]. Environmental Pollution, 2019, 253: 578-598. doi: 10.1016/j.envpol.2019.07.053 [8] TORTELLA G R, RUBILAR O, DURÁN N, et al. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment [J]. Journal of Hazardous Materials, 2020, 390: 121974. doi: 10.1016/j.jhazmat.2019.121974 [9] DU J, TANG J H, XU S D, et al. A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms [J]. Regulatory Toxicology and Pharmacology, 2018, 98: 231-239. doi: 10.1016/j.yrtph.2018.08.003 [10] BRAMI C, GLOVER A R, BUTT K R, et al. Effects of silver nanoparticles on survival, biomass change and avoidance behaviour of the endogeic earthworm Allolobophora chlorotica [J]. Ecotoxicology and Environmental Safety, 2017, 141: 64-69. doi: 10.1016/j.ecoenv.2017.03.015 [11] 李文华. 纳米银致秀丽线虫神经毒性效应研究[D]. 南京: 东南大学, 2020. LI W H. Study on neurotoxic effects of silver nanoparticles in Caenorhabditis elegans[D]. Nanjing: Southeast University, 2020(in Chinese).
[12] SMÉKALOVÁ M, PANÁČEK A, JANČULA D, et al. Culture medium mediated aggregation and re-crystallization of silver nanoparticles reduce their toxicity [J]. Applied Materials Today, 2018, 12: 198-206. doi: 10.1016/j.apmt.2018.05.004 [13] AUCLAIR J, PEYROT C, WILKINSON K J, et al. The geometry of the toxicity of silver nanoparticles to freshwater mussels [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2021, 239: 108841. [14] 王晓科, 石清清, 邓代莉, 等. 基于土壤模式生物的纳米材料毒理研究进展 [J]. 生态毒理学报, 2018, 13(3): 31-41. WANG X K, SHI Q Q, DENG D L, et al. Review on toxicology of nanomaterials based on soil model organisms [J]. Asian Journal of Ecotoxicology, 2018, 13(3): 31-41(in Chinese).
[15] 彭宇旭. 枸杞多糖减少纳米银引起秀丽隐杆线虫损伤的实验研究[D]. 武汉: 华中师范大学, 2020. PENG Y X. Lycium barbarum polysaccharides reduce the damage of nanosilver to c. elegans[D]. Wuhan: Central China Normal University, 2020(in Chinese).
[16] SAKKA Y, SKJOLDING L M, MACKEVICA A, et al. Behavior and chronic toxicity of two differently stabilized silver nanoparticles to Daphnia magna [J]. Aquatic Toxicology, 2016, 177: 526-535. doi: 10.1016/j.aquatox.2016.06.025 [17] 卿婷. 白玉蜗牛对土壤和食物相纳米银的生物富集与毒性响应规律[D]. 湘潭: 湘潭大学, 2021. QING T. The bioaccumulation and toxicity response of silver nanoparticles in the terrestrial snail Achatina fulica under soil and food exposures[D]. Xiangtan: Xiangtan University, 2021(in Chinese).
[18] DUROUDIER N, CARDOSO C, MEHENNAOUI K, et al. Changes in protein expression in mussels Mytilus galloprovincialis dietarily exposed to PVP/PEI coated silver nanoparticles at different seasons [J]. Aquatic Toxicology, 2019, 210: 56-68. doi: 10.1016/j.aquatox.2019.02.010 [19] 秦捷, 隋铭皓, 袁博杰, 等. 纳米银在水环境中的行为及毒性效应 [J]. 四川环境, 2017, 36(6): 155-160. doi: 10.3969/j.issn.1001-3644.2017.06.026 QIN J, SUI M H, YUAN B J, et al. The behavior and effects of silver nanoparticles in the aquatic environment [J]. Sichuan Environment, 2017, 36(6): 155-160(in Chinese). doi: 10.3969/j.issn.1001-3644.2017.06.026
[20] 衣俊, 黄俊, 程金平. 纳米银在水环境中的环境行为和毒性效应研究进展 [J]. 生态毒理学报, 2015, 10(1): 101-109. YI J, HUANG J, CHENG J P. Review of environmental behavior and toxicity of silver nanoparticles in the aquatic environment [J]. Asian Journal of Ecotoxicology, 2015, 10(1): 101-109(in Chinese).
[21] 胡奕. 纳米银的大型蚤毒性效应与生物累积[D]. 杭州: 浙江大学, 2017. HU Y. Toxicity and bioaccumulation of AgNPs to Daphnia magna[D]. Hangzhou: Zhejiang University, 2017(in Chinese).
[22] LI M, RUAN L Y, DANG F, et al. Metabolic response of earthworms (Pheretima guillemi) to silver nanoparticles in sludge-amended soil [J]. Environmental Pollution, 2022, 300: 118954. doi: 10.1016/j.envpol.2022.118954 [23] COZZARI M, ELIA A C, PACINI N, et al. Bioaccumulation and oxidative stress responses measured in the estuarine ragworm (Nereis diversicolor) exposed to dissolved, nano- and bulk-sized silver [J]. Environmental Pollution, 2015, 198: 32-40. doi: 10.1016/j.envpol.2014.12.015 [24] BAO S P, HUANG J L, LIU X W, et al. Tissue distribution of Ag and oxidative stress responses in the freshwater snail Bellamya aeruginosa exposed to sediment-associated Ag nanoparticles [J]. Science of the Total Environment, 2018, 644: 736-746. doi: 10.1016/j.scitotenv.2018.07.011 [25] KHAN F R, MISRA S K, BURY N R, et al. Inhibition of potential uptake pathways for silver nanoparticles in the estuarine snail Peringia ulvae [J]. Nanotoxicology, 2015, 9(4): 493-501. doi: 10.3109/17435390.2014.948519 [26] WESTMEIER D, CHEN C Y, STAUBER R H, et al. The bio-Corona and its impact on nanomaterial toxicity [J]. European Journal of Nanomedicine, 2015, 7(3): 153-168. [27] CONG Y, BANTA G T, SELCK H, et al. Toxicity and bioaccumulation of sediment-associated silver nanoparticles in the estuarine polychaete, Nereis (Hediste) diversicolor [J]. Aquatic Toxicology, 2014, 156: 106-115. doi: 10.1016/j.aquatox.2014.08.001 [28] 王娜. 不同暴露途径下纳米银对大型溞的毒性效应[D]. 徐州: 中国矿业大学, 2021. WANG N. Toxic effects of AgNPs on Daphnia magna under different exposure routes[D]. Xuzhou: China University of Mining and Technology, 2021(in Chinese).
[29] MAKAMA S, PIELLA J, UNDAS A, et al. Properties of silver nanoparticles influencing their uptake in and toxicity to the earthworm Lumbricus rubellus following exposure in soil [J]. Environmental Pollution, 2016, 218: 870-878. doi: 10.1016/j.envpol.2016.08.016 [30] MEHENNAOUI K, CAMBIER S, MINGUEZ L, et al. Sub-chronic effects of AgNPs and AuNPs on Gammarus fossarum (Crustacea Amphipoda): From molecular to behavioural responses [J]. Ecotoxicology and Environmental Safety, 2021, 210: 111775. doi: 10.1016/j.ecoenv.2020.111775 [31] RAMSKOV T, FORBES V E, GILLILAND D, et al. Accumulation and effects of sediment-associated silver nanoparticles to sediment-dwelling invertebrates [J]. Aquatic Toxicology, 2015, 166: 96-105. doi: 10.1016/j.aquatox.2015.07.002 [32] BUFFET P E, PAN J F, POIRIER L, et al. Biochemical and behavioural responses of the endobenthic bivalve Scrobicularia plana to silver nanoparticles in seawater and microalgal food [J]. Ecotoxicology and Environmental Safety, 2013, 89: 117-124. doi: 10.1016/j.ecoenv.2012.11.019 [33] CHAN C Y S, CHIU J M. Chronic effects of coated silver nanoparticles on marine invertebrate larvae: A proof of concept study [J]. PLoS One, 2015, 10(7): e0132457. doi: 10.1371/journal.pone.0132457 [34] CARRAZCO-QUEVEDO A, RÖMER I, SALAMANCA M J, et al. Bioaccumulation and toxic effects of nanoparticulate and ionic silver in Saccostrea glomerata (rock oyster) [J]. Ecotoxicology and Environmental Safety, 2019, 179: 127-134. doi: 10.1016/j.ecoenv.2019.04.032 [35] CONG Y, BANTA G T, SELCK H, et al. Toxic effects and bioaccumulation of nano-, micron- and ionic-Ag in the polychaete, Nereis diversicolor [J]. Aquatic Toxicology (Amsterdam, Netherlands), 2011, 105(3/4): 403-411. [36] GARCÍA-ALONSO J, RODRIGUEZ-SANCHEZ N, MISRA S K, et al. Toxicity and accumulation of silver nanoparticles during development of the marine polychaete Platynereis dumerilii [J]. Science of the Total Environment, 2014, 476/477: 688-695. doi: 10.1016/j.scitotenv.2014.01.039 [37] DUROUDIER N, KATSUMITI A, MIKOLACZYK M, et al. Cell and tissue level responses in mussels Mytilus galloprovincialis dietarily exposed to PVP/PEI coated Ag nanoparticles at two seasons [J]. Science of the Total Environment, 2021, 750: 141303. doi: 10.1016/j.scitotenv.2020.141303 [38] JO H J, CHOI J W, LEE S H, et al. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods [J]. Journal of Hazardous Materials, 2012, 227/228: 301-308. doi: 10.1016/j.jhazmat.2012.05.066 [39] IVASK A, KURVET I, KASEMETS K, et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro [J]. PLoS One, 2014, 9(7): e102108. doi: 10.1371/journal.pone.0102108 [40] MEYER J N, LORD C A, YANG X Y, et al. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans [J]. Aquatic Toxicology, 2010, 100(2): 140-150. doi: 10.1016/j.aquatox.2010.07.016 [41] ZHAO C M, WANG W X. Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna [J]. Nanotoxicology, 2012, 6(4): 361-370. doi: 10.3109/17435390.2011.579632 [42] MACKEVICA A, SKJOLDING L M, GERGS A, et al. Chronic toxicity of silver nanoparticles to Daphnia magna under different feeding conditions [J]. Aquatic Toxicology, 2015, 161: 10-16. doi: 10.1016/j.aquatox.2015.01.023 [43] CONINE A L, REARICK D C, XENOPOULOS M A, et al. Variable silver nanoparticle toxicity to Daphnia in boreal lakes [J]. Aquatic Toxicology, 2017, 192: 1-6. doi: 10.1016/j.aquatox.2017.09.004 [44] HOU J, ZHOU Y, WANG C J, et al. Toxic effects and molecular mechanism of different types of silver nanoparticles to the aquatic crustacean Daphnia magna [J]. Environmental Science & Technology, 2017, 51(21): 12868-12878. [45] HU Y, CHEN X J, YANG K, et al. Distinct toxicity of silver nanoparticles and silver nitrate to Daphnia magna in M4 medium and surface water [J]. Science of the Total Environment, 2018, 618: 838-846. doi: 10.1016/j.scitotenv.2017.08.222 [46] RIBEIRO F, GALLEGO-URREA J A, JURKSCHAT K, et al. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio [J]. Science of the Total Environment, 2014, 466/467: 232-241. doi: 10.1016/j.scitotenv.2013.06.101 [47] CUI R X, CHAE Y, AN Y J. Dimension-dependent toxicity of silver nanomaterials on the cladocerans Daphnia magna and Daphnia galeata [J]. Chemosphere, 2017, 185: 205-212. doi: 10.1016/j.chemosphere.2017.07.011 [48] LEKAMGE S, MIRANDA A F, ABRAHAM A, et al. The toxicity of silver nanoparticles (AgNPs) to three freshwater invertebrates with different life strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis [J]. Frontiers in Environmental Science, 2018, 6: 152. doi: 10.3389/fenvs.2018.00152 [49] 陆梦甜. 脂质体包封银(AgNPs、Ag+)对大型溞的毒性效应研究[D]. 徐州: 中国矿业大学, 2019. LU M T. Toxic effects of liposome-encapsulated silver (AgNPs, Ag+) on Daphnia magna[D]. Xuzhou: China University of Mining and Technology, 2019(in Chinese).
[50] JASSIM A Y, WANG J J, CHUNG K W, et al. Comparative assessment of the fate and toxicity of chemically and biologically synthesized silver nanoparticles to juvenile clams [J]. Colloids and Surfaces B:Biointerfaces, 2022, 209: 112173. doi: 10.1016/j.colsurfb.2021.112173 [51] LITTLE S, JOHNSTON H J, STONE V, et al. Acute waterborne and chronic sediment toxicity of silver and titanium dioxide nanomaterials towards the oligochaete, Lumbriculus variegatus [J]. NanoImpact, 2021, 21: 100291. doi: 10.1016/j.impact.2020.100291 [52] AN H J, SARKHEIL M, PARK H S, et al. Comparative toxicity of silver nanoparticles (AgNPs) and silver nanowires (AgNWs) on saltwater microcrustacean, Artemia salina [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2019, 218: 62-69. [53] NASIR S, WALTERS K F A, PEREIRA R M, et al. Larvicidal activity of acetone extract and green synthesized silver nanoparticles from Allium sativum L. (Amaryllidaceae) against the dengue vector Aedes aegypti L. (Diptera: Culicidae) [J]. Journal of Asia-Pacific Entomology, 2022, 25(3): 101937. doi: 10.1016/j.aspen.2022.101937 [54] ELUMALAI D, HEMAVATHI M, DEENADHAYALAN N, et al. A novel approach for synthesis of silver nanoparticles using Pila virens shell and its mosquito larvicidal activity [J]. Toxicology Reports, 2021, 8: 1248-1254. doi: 10.1016/j.toxrep.2021.06.018 [55] DUMMEE V, TANHAN P, KRUATRACHUE M, et al. Histopathological changes in snail, Pomacea canaliculata, exposed to sub-lethal copper sulfate concentrations [J]. Ecotoxicology and Environmental Safety, 2015, 122: 290-295. doi: 10.1016/j.ecoenv.2015.08.010 [56] CHEN L, MENG X, GU J, et al. Silver nanoparticle toxicity in silkworms: Omics technologies for a mechanistic understanding [J]. Ecotoxicology and Environmental Safety, 2019, 172: 388-395. doi: 10.1016/j.ecoenv.2019.01.055 [57] LUO X, ZHANG Y J, FU X L, et al. Effects of environmental factor fulvic acid on AgNPs food chain delivery and bioavailability [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2022, 258: 109369. [58] VÖLKER C, BOEDICKER C, DAUBENTHALER J, et al. Comparative toxicity assessment of nanosilver on three Daphnia species in acute, chronic and multi-generation experiments [J]. PLoS One, 2013, 8(10): e75026. doi: 10.1371/journal.pone.0075026 [59] LIM D, ROH J Y, EOM H J, et al. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans [J]. Environmental Toxicology and Chemistry, 2012, 31(3): 585-592. doi: 10.1002/etc.1706 [60] NAIR P M G, PARK S Y, LEE S W, et al. Differential expression of ribosomal protein gene, gonadotrophin releasing hormone gene and Balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius [J]. Aquatic Toxicology, 2011, 101(1): 31-37. doi: 10.1016/j.aquatox.2010.08.013 [61] 李婷竹. 不同特性纳米银制备及遗传毒性定量研究[D]. 南京: 东南大学, 2017. LI T Z. Study on preparation and genotoxicity of silver nanoparticles with different characteristics[D]. Nanjing: Southeast University, 2017(in Chinese).
[62] CYPRIYANA P J J, S S, ANGALENE J L A, et al. Overview on toxicity of nanoparticles, it's mechanism, models used in toxicity studies and disposal methods - A review [J]. Biocatalysis and Agricultural Biotechnology, 2021, 36: 102117. doi: 10.1016/j.bcab.2021.102117 [63] CHOI J S, PARK J W. Molecular characterization and toxicological effects of citrate-coated silver nanoparticles in a terrestrial invertebrate, the earthworm (Eisenia fetida) [J]. Molecular & Cellular Toxicology, 2015, 11(4): 423-431. [64] ALARABY M, ROMERO S, HERNÁNDEZ A, et al. Toxic and genotoxic effects of silver nanoparticles in Drosophila [J]. Environmental and Molecular Mutagenesis, 2019, 60(3): 277-285. doi: 10.1002/em.22262 [65] BOTELHO M T, de ARRUDA ROCHA CAMPOS PASSOS M J, TREVIZANI T H, et al. Genotoxic effects of silver nanoparticles on a tropical marine amphipod via feeding exposure [J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2022, 881: 503527. doi: 10.1016/j.mrgentox.2022.503527 [66] WAMUCHO A, HEFFLEY A, TSYUSKO O V. Epigenetic effects induced by silver nanoparticles in Caenorhabditis elegans after multigenerational exposure [J]. Science of the Total Environment, 2020, 725: 138523. doi: 10.1016/j.scitotenv.2020.138523 [67] PAKRASHI S, TAN C, WANG W X. Bioaccumulation-based silver nanoparticle toxicity in Daphnia magna and maternal impacts [J]. Environmental Toxicology and Chemistry, 2017, 36(12): 3359-3366. doi: 10.1002/etc.3917 [68] 卜春红, 高大文. 蚯蚓回避反应在生态毒理研究中的应用进展[J]. 农业环境科学学报, 2006, 25(S2): 799-804. BU C H, GAO D W. Application progress of earthworm avoidance response test in ecotoxicity research[J]. Journal of Agro-Environment Science, 2006, 25(Sup 2): 799-804(in Chinese).
[69] GONZÁLEZ-ALCARAZ M N, MALHEIRO C, CARDOSO D N, et al. Soil moisture influences the avoidance behavior of invertebrate species in anthropogenic metal(loid)-contaminated soils [J]. Environmental Pollution, 2019, 248: 546-554. doi: 10.1016/j.envpol.2019.01.105 [70] SAHA S, CHUKWUKA A V, MUKHERJEE D, et al. Behavioral and physiological toxicity thresholds of a freshwater vertebrate (Heteropneustes fossilis) and invertebrate (Branchiura sowerbyi), exposed to zinc oxide nanoparticles (nZnO): A General Unified Threshold model of Survival (GUTS) [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2022, 262: 109450. [71] ZIDAR P, KOS M, ILIČ E, et al. Avoidance behaviour of isopods (Porcellio scaber) exposed to food or soil contaminated with Ag- and CeO2- nanoparticles [J]. Applied Soil Ecology, 2019, 141: 69-78. doi: 10.1016/j.apsoil.2019.05.011 [72] 王秀娟, 薛玉英, 唐萌. 纳米银的体内毒性及毒作用机制研究进展 [J]. 生态毒理学报, 2018, 13(1): 50-60. doi: 10.7524/AJE.1673-5897.20170424002 WANG X J, XUE Y Y, TANG M. Research progress on internal toxicity and the toxic mechanism of silver nanoparticles [J]. Asian Journal of Ecotoxicology, 2018, 13(1): 50-60(in Chinese). doi: 10.7524/AJE.1673-5897.20170424002
[73] MANKE A, WANG L Y, ROJANASAKUL Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity [J]. BioMed Research International, 2013, 2013: 942916. [74] PAN Y B, LIN S J, ZHANG W J. Epigenetic effects of silver nanoparticles and ionic silver in Tetrahymena thermophila [J]. Science of the Total Environment, 2021, 768: 144659. doi: 10.1016/j.scitotenv.2020.144659 [75] AHAMED M, POSGAI R, GOREY T J, et al. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster [J]. Toxicology and Applied Pharmacology, 2010, 242(3): 263-269. doi: 10.1016/j.taap.2009.10.016 [76] KHODAPARAST Z, van GESTEL C A M, PAPADIAMANTIS A G, et al. Toxicokinetics of silver nanoparticles in the mealworm Tenebrio molitor exposed via soil or food [J]. Science of the Total Environment, 2021, 777: 146071. doi: 10.1016/j.scitotenv.2021.146071 [77] MEHENNAOUI K, CAMBIER S, SERCHI T, et al. Do the pristine physico-chemical properties of silver and gold nanoparticles influence uptake and molecular effects on Gammarus fossarum (Crustacea Amphipoda)? [J]. Science of the Total Environment, 2018, 643: 1200-1215. doi: 10.1016/j.scitotenv.2018.06.208 [78] KUSI J, MAIER K J. Evaluation of silver nanoparticle acute and chronic effects on freshwater amphipod (Hyalella azteca) [J]. Aquatic Toxicology, 2022, 242: 106016. doi: 10.1016/j.aquatox.2021.106016 [79] MOON J, KWAK J I, AN Y J. The effects of silver nanomaterial shape and size on toxicity to Caenorhabditis elegans in soil media [J]. Chemosphere, 2019, 215: 50-56. doi: 10.1016/j.chemosphere.2018.09.177 [80] CONINE A L, FROST P C. Variable toxicity of silver nanoparticles to Daphnia magna: Effects of algal particles and animal nutrition [J]. Ecotoxicology (London, England), 2017, 26(1): 118-126. doi: 10.1007/s10646-016-1747-2 [81] GAO J, POWERS K, WANG Y, et al. Influence of Suwannee River humic acid on particle properties and toxicity of silver nanoparticles [J]. Chemosphere, 2012, 89(1): 96-101. doi: 10.1016/j.chemosphere.2012.04.024 [82] MENDONÇA M C P, RODRIGUES N P, SCOTT-FORDSMAND J J, et al. The toxicity of silver nanomaterials (NM 300K) is reduced when combined with N-Acetylcysteine: Hazard assessment on Enchytraeus crypticus [J]. Environmental Pollution, 2020, 256: 113484. doi: 10.1016/j.envpol.2019.113484 [83] ASADI DOKHT LISH R, JOHARI S A, SARKHEIL M, et al. On how environmental and experimental conditions affect the results of aquatic nanotoxicology on brine shrimp (Artemia salina): A case of silver nanoparticles toxicity [J]. Environmental Pollution, 2019, 255: 113358. doi: 10.1016/j.envpol.2019.113358 [84] 许志珍, 赵鹏, 张元宝, 等. 人工纳米材料对典型生物的毒性效应研究进展 [J]. 安全与环境学报, 2017, 17(2): 786-792. XU Z Z, ZHAO P, ZHANG Y B, et al. Research progress review in the toxic effects of the engineering nanomaterials on the typical organisms [J]. Journal of Safety and Environment, 2017, 17(2): 786-792(in Chinese).
[85] LIU H Q, WANG X X, WU Y Z, et al. Toxicity responses of different organs of zebrafish (Danio rerio) to silver nanoparticles with different particle sizes and surface coatings [J]. Environmental Pollution, 2019, 246: 414-422. doi: 10.1016/j.envpol.2018.12.034 [86] GHETAS H A, ABDEL-RAZEK N, SHAKWEER M S, et al. Antimicrobial activity of chemically and biologically synthesized silver nanoparticles against some fish pathogens [J]. Saudi Journal of Biological Sciences, 2022, 29(3): 1298-1305. doi: 10.1016/j.sjbs.2021.11.015 [87] 刘艳娥. 分散剂在纳米银制备中的影响 [J]. 山东化工, 2015, 44(3): 90-91,97. doi: 10.3969/j.issn.1008-021X.2015.03.030 LIU Y E. The impact of the dispersant in the preparation of silver nanoparticles [J]. Shandong Chemical Industry, 2015, 44(3): 90-91,97(in Chinese). doi: 10.3969/j.issn.1008-021X.2015.03.030
[88] JU-NAM Y, LEAD J R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications [J]. Science of the Total Environment, 2008, 400(1/2/3): 396-414. [89] BURKOWSKA-BUT A, SIONKOWSKI G, WALCZAK M. Influence of stabilizers on the antimicrobial properties of silver nanoparticles introduced into natural water [J]. Journal of Environmental Sciences, 2014, 26(3): 542-549. doi: 10.1016/S1001-0742(13)60451-9 [90] COURTOIS P, RORAT A, LEMIERE S, et al. Medium-term effects of Ag supplied directly or via sewage sludge to an agricultural soil on Eisenia fetida earthworm and soil microbial communities [J]. Chemosphere, 2021, 269: 128761. doi: 10.1016/j.chemosphere.2020.128761 -



 下载:
下载: