-
作为一种广谱抗菌药物,磺胺甲恶唑(SMX)常被用于治疗人类与动物因细菌感染所引起的疾病,是最早广泛使用的抗生素之一[1]。然而,使用后的SMX仅有10%—50%在体内被代谢,其它则以母体形式直接排出体外[2],导致沉积物、活性污泥、土壤及污水中常被检出[3]。虽然地表水和污水厂出水中SMX的含量在ng·L−1—μg·L−1级别[4],但它们可加速耐药菌和耐药基因的形成;且由于持续输入环境及生物降解性差,SMX可在环境中发生累积[5]。因此,去除污水中SMX残留对于人类健康与生态环境的保护具有重要意义。
目前在传统方法处理污水中SMX残留方面的研究报道较多,但费用高、过程复杂、效率低等问题导致难以广泛推广和应用[6]。高级氧化技术(如臭氧、芬顿、UV/H2O2、UV/过硫酸盐、过渡金属/过硫酸盐)在去除水中SMX残留展现出良好的性能。其中,基于羟基自由基(·OH)的高级氧化技术被证实可有效去除水中SMX残留,通过羟基化反应使其降解速率常数高达(5.8 ±0.2)×109 L·mol−1·s-1 [7-8]。然而,因仪器灵敏度低、产物生成量少及反应速率过快等原因,实验方法难以阐明·OH与SMX的反应机理。通过获取不稳定中间体和过渡态的结构与能量信息,理论计算方法可有效解释实验现象,从而弥补了纯实验方法的不足。Yang等[9]通过理论计算研究了水相中·OH引发SMX降解的机制,但反应速率常数低于实验数据达1个数量级,并且在计算过程中未考虑溶质扩散过程对反应速率的影响。
为了进一步阐明SMX与·OH的反应机制,本研究采用量子化学计算分析SMX的反应活性位点并明确加成反应与抽氢反应的过渡态,根据自由能垒和反应热大小判定反应的可行性,通过基于过渡态理论计算获得反应速率常数以确定反应快慢,采用ECOSAR软件预测加成产物的生态毒性,研究成果可为基于·OH的高级氧化技术去除水中SMX残留的实验研究提供理论支撑。
-
本研究中所有量子化学计算均由Gaussian 16程序[10]完成。反应物、过渡态及产物的结构优化、谐振频率及内禀反应坐标(IRC)的计算均采用M06-2X/6-311+G**方法,其中过渡态有且仅有一个虚频,IRC中过渡态两端与反应物和产物相连;考虑溶剂对结构的影响,采用了基于SCRF参数化的溶剂化模型SMD进行计算。单点能采用更高级别的b2plypd3/def2tzvp方法计算;在ZPE校正因子取0.970的前提下,使用Shermo软件[11]获得不同物质在298.15 K时自由能(G气)。采用M052X/6-31G*方法在气相和水相下优化并计算溶解自由能(G溶解),最终水相中物质的自由能为G气+G溶解+1.89 kcal·mol−1,其中1.89 kcal·mol−1为298.15 K下气体压力由1 atm转变成1 mol·L−1水溶液时自由能变化值。所有分子的结构绘制均采用CYLview软件[12]。
-
为了描述水相中不同路径下·OH与SMX的反应快慢,本研究利用传统过渡态理论(TST)计算水相条件下的二级反应速率常数(ka),计算公式如下:
其中,σ为反应路径简并度,г为Wigner方法计算的隧穿效应因子,kB为玻尔兹曼常数,h为普朗克常数,ΔG‡为298.15 K、浓度为1 mol·L−1下的自由能垒(kcal·mol−1),即G过渡态-G反应物。
当反应速率常数ka受扩散限制影响时,为了获得表观反应速率常数k,使用Collins-Kimball理论对ka进行校正,具体计算公式:
式中,kd是不可逆双分子扩散控制反应的扩散限制速率常数,可通过Smoluchowski方程获得,计算公式如下:
式中,RAB为反应物距离,NA为阿伏伽德罗常数,DAB为反应物A与B的相互扩散系数,由DA与DB通过Stokes-Einstein方法计算得出,具体公式如下:
式中,kB、T、η和α分别为玻尔兹曼常数、温度、溶剂黏度和溶质半径。
-
为了预测反应物和加成产物的生态毒性,本研究采用美国环保局的ECOSAR程序评估其对藻类、水蚤和鱼类3种水生生物的急性毒性和慢性毒性,采用表1标准判别毒性大小。
-
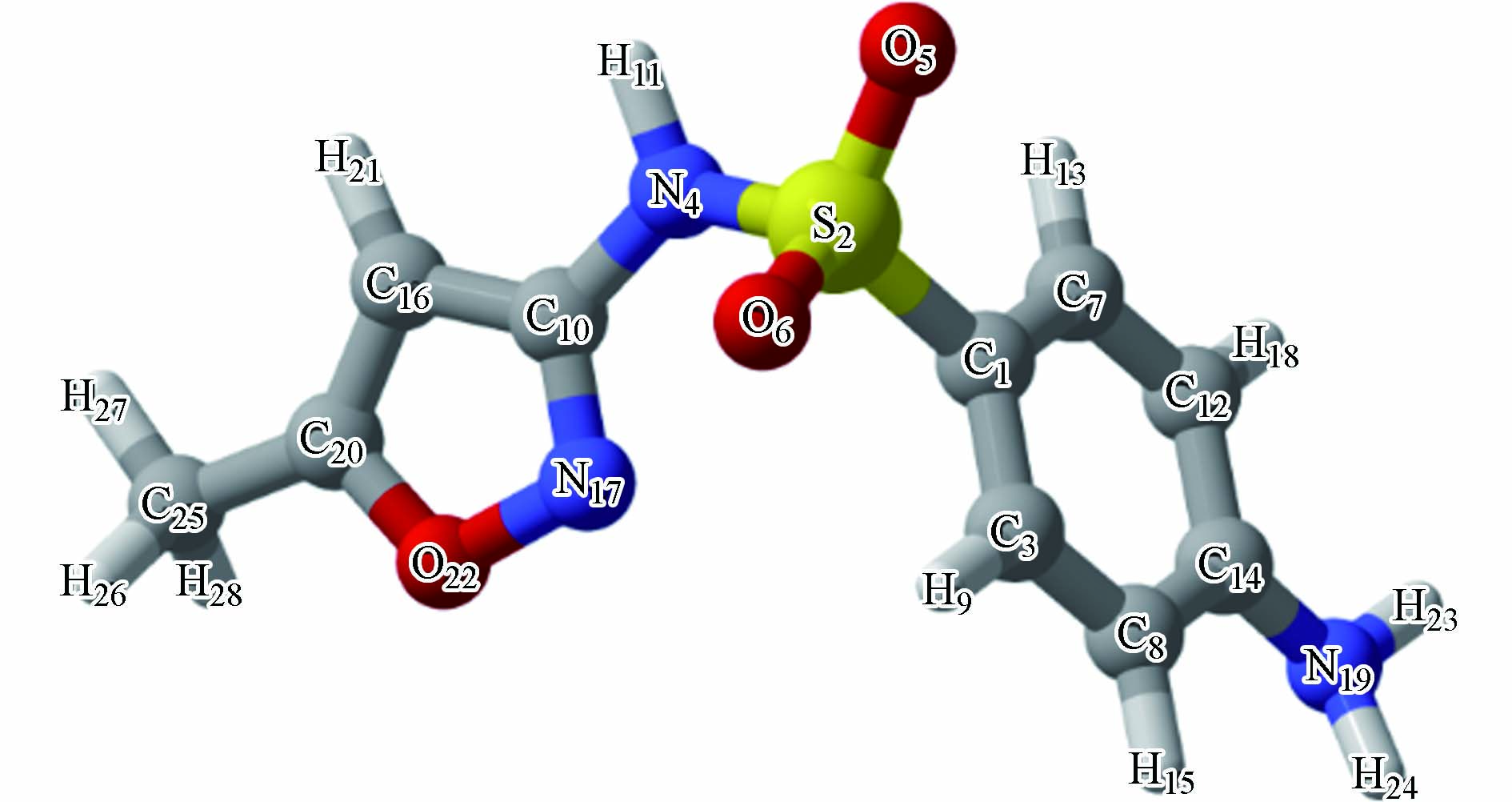
为了确定·OH与SMX反应的过渡态,本研究采用M06-2X/6-311+G**方法对SMX进行了结构优化,优化构型如图1所示,其中键角N4-S2-C1为104.8 o,二面角C10-N4-S2-C1为73.0 o,结果表明了苯环与杂环处在不同平面上。
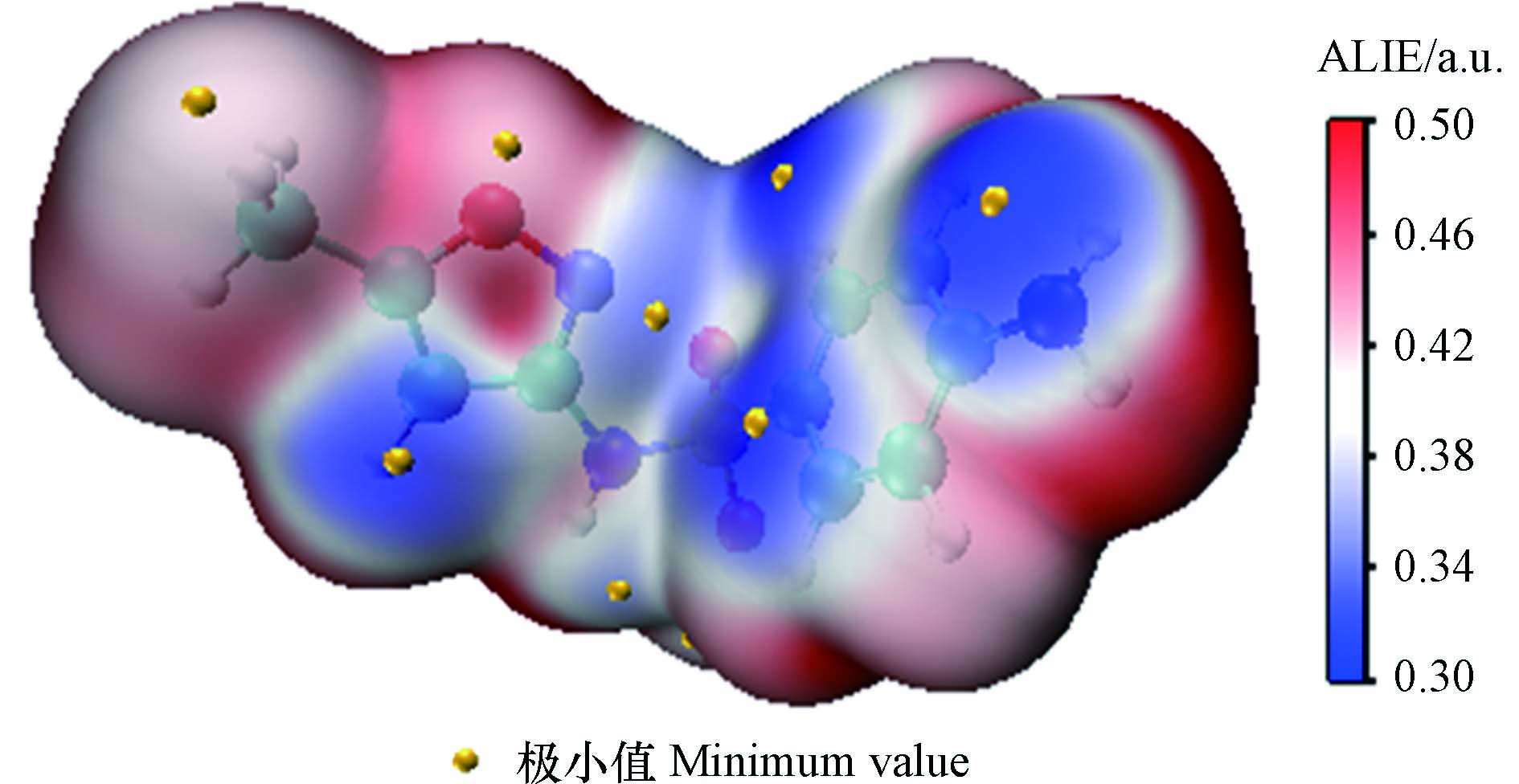
从头算动力学模拟与过渡态理论方法在预测反应活性位点时,常存在耗时长、计算量大或准确度不高等问题;密度泛函活性理论的发展为反应活性位点的预测提供了新的途径,具有计算量小且预测准确的特点,其中分子局部离子化能(ALIE)常用于有机化合物反应活性位点的预测[15]。ALIE可描述局部位置上电子的电离能,其值越低,代表此处电子束缚性越弱、活性越高,越易发生亲电反应或自由基反应[16]。本研究采用Multifwn 3.8[17]和VMD软件[18],以电子密度值为0.0005 a.u.绘制等值面图(见图2),黄色亮点为极小值点,是电子束缚最弱、最易发生亲电反应的区域。由图2可以看出,除N原子和O原子外,蓝色区域主要分布在C8、C12及C16等位点,表明这些位点ALIE值较低,更易发生反应,而N17与C10区域呈现白色或浅蓝色,说明它们的反应活性相对较低。
-
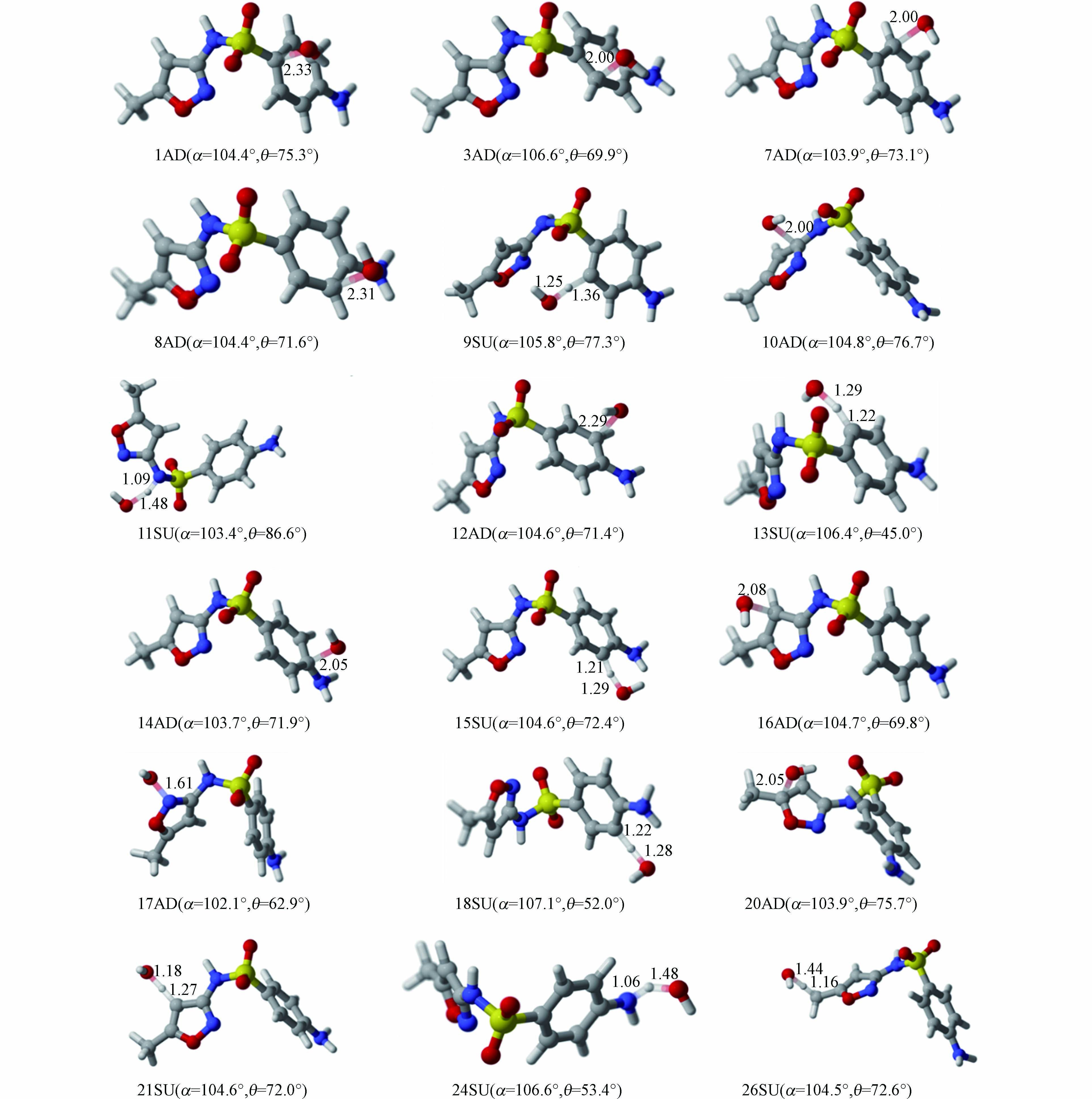
通常情况下,·OH与有机化合物之间主要发生抽氢和双键加成两种反应。因SMX分子呈现不对称性、两个环处在不同平面、甲基氢和氨基氢具有化学等价性等原因,本研究主要考察了10种双键加成反应路径(1AD、3AD、7AD、8AD、10AD、12AD、14AD、16AD、17AD和20AD)和8种抽氢反应路径(9SU、11SU、13SU、15SU、18SU、21SU、24SU和26SU)。过渡态的具体结构及相关参数如图3所示。
由图3可以看出,·OH与SMX的加成反应中所有过渡态的碳氧间距在0.161—0.233 nm之间,最大和最小距离发生在C1和N17上;而在抽氢反应过程中,羟基氧与氢的间距在0.118 —0.148 nm之间,其值远小于加成反应的碳氧间距。同时,部分反应对SMX的构象产生较大影响,以键角N4-S2-C1和二面角C10-N4-S2-C1为例,其变化范围分别在102.1o—107.1o和45.0o—86.6o,键角变化最大值和最小值分别为18SU和17AD两条反应路径,相应的二面角分别为11SU和13SU,这可能是由于·OH与SMX形成氢键使优化前后结构存在较大差异。
同时,在C1位点发生的加成反应与C1-S2键的断裂存在协同效应,具体过程如图4所示。当·OH的逐渐靠近时,C1原子则偏离原有位置;加成后,·OH与磺酰基的电负性使磺酰基氧向左偏离平衡位置,C—S键的键长由0.175 nm拉伸至0.185 nm进而发生断裂。并且,整个IRC过程未出现任何中间体,说明该加成反应和C—S键的断裂协同进行。
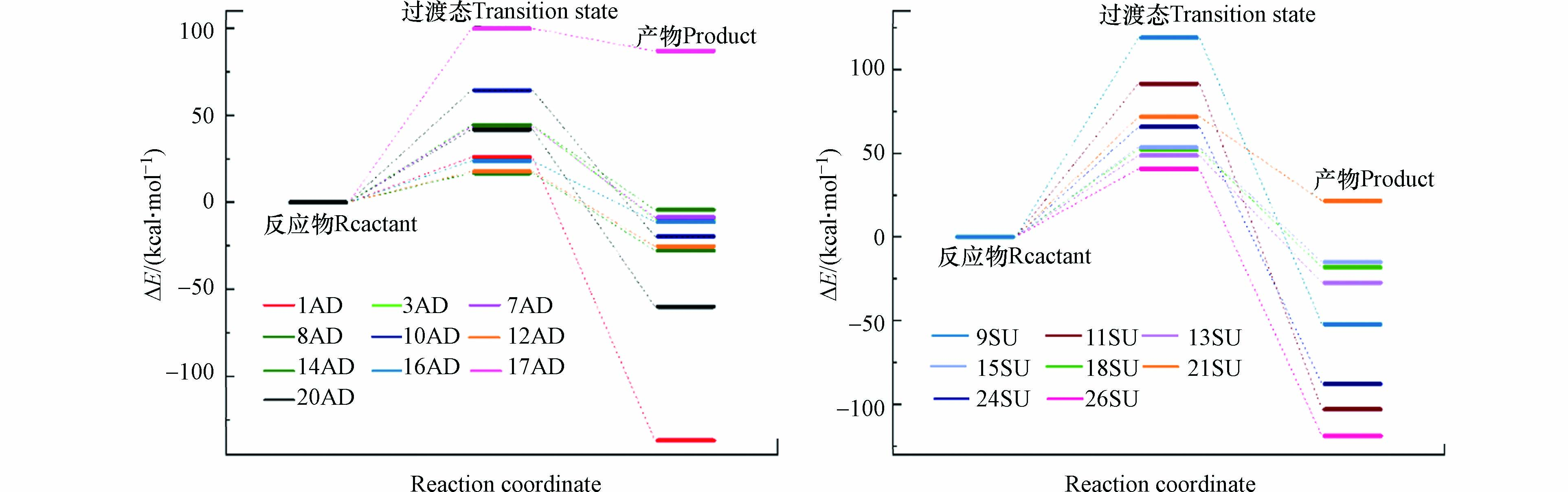
为了进一步确定反应过程中的能量变化,本研究计算了反应物、过渡态及产物的自由能,反应势能面如图5所示。在加成反应过程中,反应能垒在4.0—24.0 kcal·mol−1之间变化,其中最大值和最小值分别发生在C8和C17位点;反应焓变在-25.0—11.0 kcal·mol−1之间,除17AD外其他反应均为放热反应,且20AD放热量最大。从热力学角度来说,放热反应有利于反应的发生,部分实验结果也表明了苯环或恶唑环的羟基化是·OH与SMX反应的第一步,然后才形成其他产物[19-22]。
在抽氢反应过程中,·OH与SMX的反应能垒在9.71—28.6 kcal·mol−1之间变化,其中最大值和最小值分别发生在H9和H26位点上,反应焓变在-24.0—5.77 kcal·mol−1之间变化,除21SU外其他反应过程均为放热反应,且H26位点的反应放热量最大。从热力学角度来看,除21SU外的其它抽氢反应均可发生,且加成反应的自由能垒低于抽氢反应。能垒计算结果与ALIE的预测结果基本一致,这表明ALIE在预测反应活性位点的可靠性。
-
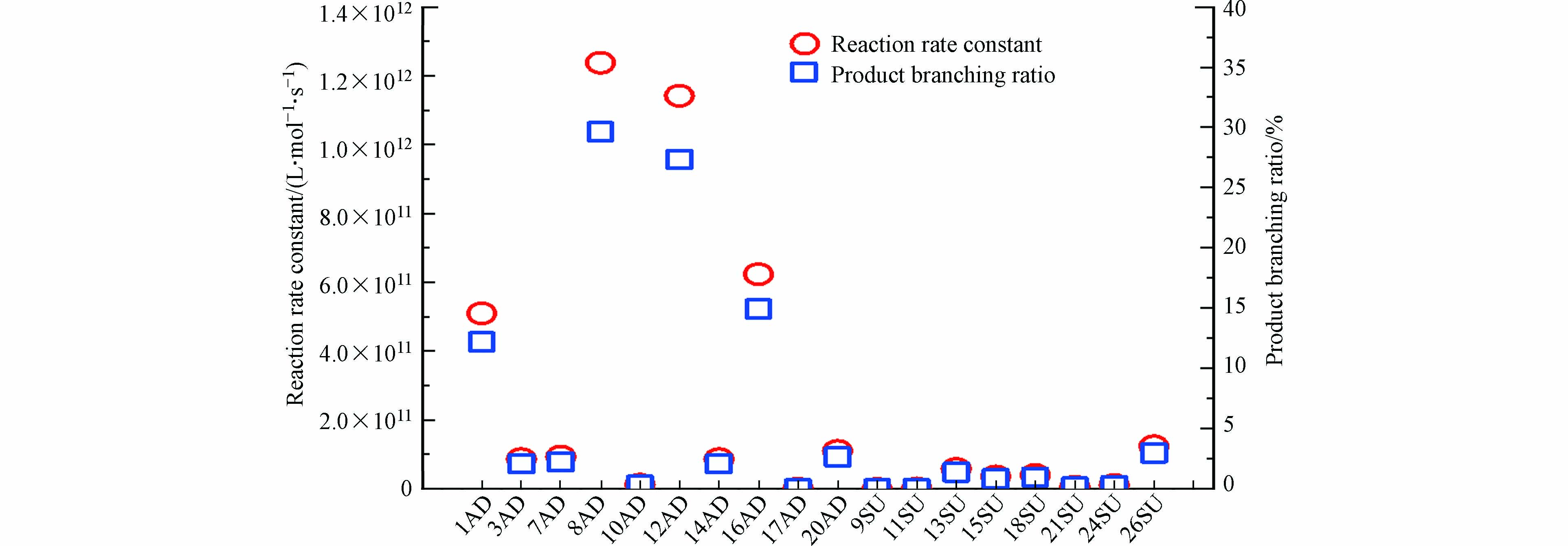
为了进一步研究·OH引发反应的快慢,本研究在298.15K、反应物浓度为1 mol·L−1下计算反应速率常数和反应产物分支比,结果如图6所示。由图6可以看出,加成反应与抽氢反应的总反应速率常数为4.18×1012 L·mol−1·s−1,其中反应速率常数最大为8AD反应(1.24×1012 L·mol−1·s−1),反应速率常数最小为17AD加成反应(3.88×108 L·mol−1·s−1)。加成反应的速率常数之和为3.90×1012 L·mol−1·s−1,而抽氢反应远低于加成反应,仅为2.75×1011 L·mol−1·s−1,表明在整个反应过程中加成反应占主导地位。此外,加成反应和抽氢反应的产物分支比分别为93.4%和6.60%,其中8AD的产物分支比为29.6%,其次为12AD(27.3%),超过10%还有1AD(12.2%)和16AD(14.9%),表明由·OH引发的反应中加成产物为主要物质。
Yang等[9]通过量子化学计算对SMX的非直接光解作用进行了研究,发现在水相中·OH与SMX的二级反应速率常数为1.28×108 L·mol−1·s−1,远低于本研究的结果。然而,早期的实验结果[7- 8, 23]显示,SMX与·OH的二级反应速率常数均在109 L·mol−1·s−1数量级水平,处于液相双分子扩散控制速率附近,并且不同形态SMX的二级反应速率常数无显著变化[7],均高于上述Yang等计算结果[9]。因此,为了考察液相条件下扩散限制对速率常数的影响,本研究采用Collins-Kimball理论对二级反应速率常数进行校正,通过Smoluchowski方程计算得出·OH与SMX的扩散限制速率常数为8.69×109 L·mol−1·s−1,最终得到的二级表观速率常数为8.68×109 L·mol−1·s−1。Boreen等[7]以苯乙酮为参考物质,通过实验得出SMX与·OH的反应速率常数为(5.8±0.2)×109 L·mol−1·s−1,实验结果与本研究基本一致,进一步证实了本研究方法的可靠性。
-
为了分析反应物和加成产物的生态毒性,本论文采用ECOSAR程序评估了目标化合物对藻类、水蚤及鱼类3种水生生物的急性毒性(LC50和EC50)和慢性毒性(ChV),研究结果如表2所示。
由表2可以看出,水蚤对各种物质均较为敏感,母体化合物SMX对水蚤和绿藻的急性毒性分别处于高风险和中等风险,对水蚤的慢性毒性处于高风险;加成产物对水生生物的毒性总体呈现下降趋势,但部分产物仍具有一定的风险,如1AD-1、8AD、12AD和16AD等反应产物对水蚤的急性和慢性毒性依然处于高风险级别。Trovó等[21]在研究光芬顿降解SMX过程中发现,SMX可发生矿化且反应过程中对大型溞的毒性下降,与本研究及Yang等[9]的结果一致。因此,在利用·OH处理水体中SMX残留过程中应加强对其母体及转化产物的生态毒性评估。
-
(1)本研究采用量子化学方法确定了·OH与SMX的加成反应与抽氢反应机理,通过ALIE分析推断出SMX上C8、C12和C16等位点的反应活性较高;大部分反应为放热反应且能垒较低,热力学和动力学角度均表明反应具有可行性。
(2)依据过渡态理论的计算得到了水相条件下·OH与SMX的反应的活化自由能和反应速率常数。在未考虑扩散控制因素情况下其反应速率常数远高于实验值,而实际过程因受扩散过程所控制导致其最终表观速率常数与实验值基本一致,结果表明在研究水相中自由基引发的氧化反应的动力学过程时应关注扩散作用的影响。
(3)采用ECOSAR软件预测和评估了加成产物对鱼类、蚤类及藻类的生态毒性,研究结果表明加成反应过程在一定程度上可降低母体化合物的生态毒性,但对水生生物仍存在一定的毒性风险。因此,在高级氧化过程中对有机污染物的转化产物评估至关重要。
水相中羟基自由基与磺胺甲恶唑反应机理的理论研究
Theoretical investigation on the mechanisms for reactions of hydroxyl radicals with sulfamethoxazole in aqueous phase
-
摘要: 本研究采用量子化学计算研究了羟基自由基引发的磺胺甲恶唑加成反应与抽氢反应的机理,系统考察了水相中的反应动力学,应用ECOSAR软件预测并评估了加成产物的生态毒性。研究结果表明,磺胺甲恶唑的C8、C12及C16等位点的平均局部离子化能较低,说明它们更易与羟基自由基发生反应。除C17和H21外,其他位点的反应过程均放热,并且C1位点的加成反应与C—S键的裂解存在协同效应;加成反应和抽氢反应的活化自由能在4.0—28.6 kcal·mol−1之间变化,最小值和最大值分别发生在C8和H9位点;虽然反应的速率常数达到4.97×1012 L·mol−1·s−1,但因受扩散控制使其在水相中的表观反应速率常数仅为8.68×109 L·mol−1·s−1。毒性预测结果显示,尽管大部分加成产物的生态毒性较母体化合物低,但C8、C12和C16位点上的加成产物仍具有较高的生态毒性风险。Abstract: The mechanisms of sulfamethoxazole addition and abstraction reactions initiated by hydroxyl radicals were investigated based on quantum chemistry calculation. Meanwhile, their reaction kinetics in aqueous phase were explored in details. Furthermore, the eco-toxicity of the addition products was predicted and assessed by using ECOSAR software. The results showed that the C8, C12 and C16 sites of sulfamethoxazole had low average local ionization energies, indicating their higher reactivity with hydroxyl radicals. With the exception of C17 and H21 sites, the reactions occurring at other sites were exothermal and the addition reaction at C1 site was synergic to C-S bond cleavage. The activated free energies of reactions varied in the range of 4.0 —28.6 kcal·mol−1 and the maximum and minimum values belonged to C8 and H9 sites, respectively. Although the total rate constant of second-order reaction for the addition and abstraction reactions was 4.97×1012 L·mol−1·s−1, the apparent rate constant was only 8.68 ×109 L·mol−1·s−1 in that the reactions were controlled by diffusion in aqueous phase. Further results of toxicity prediction indicated that most adducts had lower eco-toxicity than the parent compound. However, the adducts formed at the sites of C8, C12 and C16 still had high eco-toxicity risks to aqueous organisms.
-
Key words:
- hydroxyl radical /
- sulfamethoxazole /
- quantum chemistry /
- kinetics /
- eco-toxicity
-

-
表 1 毒性分级标准(mg·L−1)
Table 1. Standards of toxicity classification (mg·L−1)
表 2 不同物质的急性毒性和慢性毒性(mg·L−1)
Table 2. Acute toxicity and chronic toxicity of different chemicals
急性毒性Acute toxicity 慢性毒性Chronic toxicity 鱼(LC50)
Fish水蚤(LC50)
Daphnia绿藻(EC50)
Green algae鱼
Fish水蚤
Daphnia绿藻
Green algaeSMX 267a 6.43c 21.8 b 5 a 0.068 c 11.1 a 1AD-1 165 a 3.19 c 11.3 b 3.41 a 0.033 c 6.36 a 1AD-2 2.82×104 a 1.26×104 a 3.50×104 a 2.08×103 a 633 a 538 a 3AD 989 a 11.2b 43.8 b 26.1 a 0.11 b 31.5 a 7AD 989 a 11.2 b 43.8 b 26.1 a 0.11 b 31.5 a 8AD 584 a 9.08 c 33.5 b 13.3 a 0.092 c 20.9 a 10AD 5.94×103 a 22.7 b 109 a 256 a 0.199 b 129 a 12AD 584 a 9.08 c 33.5 b 13.3 a 0.092 c 20.9 a 14AD 2.01×103 a 181 a 261 a 276 a 11.3 a 70.7 a 16AD 584 a 9.08 c 33.5 b 13.3 a 0.092 c 20.9 a 17AD 3.17×103 a 17.7 b 79.3 b 115 a 0.162 b 78.6 a 20AD 1.44×103 a 133 a 182 a 182 a 8.52 a 50.2 a a:低风险;b:中风险;c:高风险;1AD-1/1AD-2:裂解产物.
a: low risk; b: medium risk; c: high risk; 1AD-1/1AD-2: breakdown product -
[1] SANTOS L H M L M, ARAÚJO A N, FACHINI A, et al. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment [J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 45-95. [2] CHEN F, WU X L, YANG L, et al. Efficient degradation and mineralization of antibiotics via heterogeneous activation of peroxymonosulfate by using graphene supported single-atom Cu catalyst [J]. Chemical Engineering Journal, 2020, 394: 124904. doi: 10.1016/j.cej.2020.124904 [3] LI W H, SHI Y L, GAO L H, et al. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China [J]. Chemosphere, 2012, 89(11): 1307-1315. doi: 10.1016/j.chemosphere.2012.05.079 [4] WANG J L, WANG S Z. Microbial degradation of sulfamethoxazole in the environment [J]. Applied Microbiology and Biotechnology, 2018, 102(8): 3573-3582. doi: 10.1007/s00253-018-8845-4 [5] AYDIN S, INCE B, CETECIOGLU Z, et al. Combined effect of erythromycin, tetracycline and sulfamethoxazole on performance of anaerobic sequencing batch reactors [J]. Bioresource Technology, 2015, 186: 207-214. doi: 10.1016/j.biortech.2015.03.043 [6] JIANG B C, LI A, CUI D, et al. Biodegradation and metabolic pathway of sulfamethoxazole by Pseudomonas psychrophila HA-4, a newly isolated cold-adapted sulfamethoxazole-degrading bacterium [J]. Applied Microbiology and Biotechnology, 2014, 98(10): 4671-4681. doi: 10.1007/s00253-013-5488-3 [7] BOREEN A L, ARNOLD W A, MCNEILL K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups [J]. Environmental Science & Technology, 2004, 38(14): 3933-3940. [8] YANG Y, LU X L, JIANG J, et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate [J]. Water Research, 2017, 118: 196-207. doi: 10.1016/j.watres.2017.03.054 [9] YANG J X, LV G C, ZHANG C X, et al. Indirect photodegradation of sulfamethoxazole and trimethoprim by hydroxyl radicals in aquatic environment: Mechanisms, transformation products and eco-toxicity evaluation [J]. International Journal of Molecular Sciences, 2020, 21(17): 6276. doi: 10.3390/ijms21176276 [10] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 16 Rev. C. 01[CP]. Wallingford, CT. 2016. [11] LU T, CHEN Q X. Shermo: A general code for calculating molecular thermochemistry properties [J]. Computational and Theoretical Chemistry, 2021, 1200: 113249. doi: 10.1016/j.comptc.2021.113249 [12] LEGAULT C Y. CYLVIEW20[CP]Université de Sherbrooke. 2020. [13] DIRECTIVE C. Council Directive 67/548/EEC of 27 June 1967 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances [J]. Official Journal of the European Communities, 1967, 196(16.8): 1. [14] 国家环境保护总局. 新化学物质危害评估导则: HJ/T 154—2004[S]. 北京: 中国环境科学出版社, 2004: 12. Ministry of Ecology and Environmental of the People’s Republic of China. The guidelines for the hazard evaluation of new chemical substances: HJ/T 154-2004[S]. Beijing: China Environment Publish Group, 2004: 12.
[15] POLITZER P, MURRAY J S, BULAT F A. Average local ionization energy: A review [J]. Journal of Molecular Modeling, 2010, 16(11): 1731-1742. doi: 10.1007/s00894-010-0709-5 [16] 付蓉, 卢天, 陈飞武. 亲电取代反应中活性位点预测方法的比较 [J]. 物理化学学报, 2014, 30(4): 628-639. doi: 10.3866/PKU.WHXB201401211 FU R, LU T, CHEN F W. Comparing methods for predicting the reactive site of electrophilic substitution [J]. Acta Physico-Chimica Sinica, 2014, 30(4): 628-639(in Chinese). doi: 10.3866/PKU.WHXB201401211
[17] LU T, CHEN F W. Multiwfn: A multifunctional wavefunction analyzer [J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. doi: 10.1002/jcc.22885 [18] HUMPHREY W, DALKE A, SCHULTEN K. VMD: Visual molecular dynamics [J]. Journal of Molecular Graphics, 1996, 14(1): 33-38. doi: 10.1016/0263-7855(96)00018-5 [19] HU L H, FLANDERS P M, MILLER P L, et al. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis [J]. Water Research, 2007, 41(12): 2612-2626. doi: 10.1016/j.watres.2007.02.026 [20] ZHANG R C, YANG Y K, HUANG C H, et al. UV/H2O2 and UV/PDS treatment of trimethoprim and sulfamethoxazole in synthetic human urine: Transformation products and toxicity [J]. Environmental Science & Technology, 2016, 50(5): 2573-2583. [21] TROVÓ A G, NOGUEIRA R F P, AGÜERA A, et al. Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation [J]. Water Research, 2009, 43(16): 3922-3931. doi: 10.1016/j.watres.2009.04.006 [22] DIRANY A, SIRÉS I, OTURAN N, et al. Electrochemical abatement of the antibiotic sulfamethoxazole from water [J]. Chemosphere, 2010, 81(5): 594-602. doi: 10.1016/j.chemosphere.2010.08.032 [23] ZHANG R C, SUN P Z, BOYER T H, et al. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS [J]. Environmental Science & Technology, 2015, 49(5): 3056-3066. -



 下载:
下载: