-
含氯挥发性有机化合物(CVOCs)来源广泛,成分复杂,因具有高毒性和稳定性等特点受到广泛关注[1-2]。其中三氯乙烯(Trichloroethylene,TCE)作为原料、溶剂和脱脂剂广泛应用于制药、电子电镀等工业生产中[3],在制药和脱脂等过程中,可能会被释放到室内或室外空气中,对环境和人体健康造成危害。三氯乙烯已被世界卫生组织国际癌症研究机构列入一类致癌清单。CVOCs污染控制技术主要有吸附、吸收、催化氧化、生物氧化、等离子体等[4-5],相比而言,低温等离子体技术(Non-thermal Plasma,NTP)具有设备简单、启停便捷、反应迅速等优势而被广泛研究[6-7]。然而单一NTP技术在降解CVOCs时存在选择性低,容易形成O3和NOx等副产物等缺点。近年来研究表明,通过将低温等离子体技术和催化氧化技术耦合,可以提高CVOCs的去除率并减少副产物的产生[8-9]。
低温等离子体-催化氧化技术中,催化剂的选择对CVOCs降解效果有着很大的影响。过渡金属(Fe、Co、Mn、Ce、Cu)作为催化剂具有成本低、抗中毒、还原性好等优点而被广泛研究[10-11]。此外,许多研究表明通过将两种或多种过渡金属结合可以提高催化活性[6,9,12]。其中Mn基催化剂能够有效的将O3分解为活性氧,一方面促进了臭氧分解,另一方面分解得到的具有强氧化性的活性氧也可进一步促进CVOCs的氧化分解[13-14]。然而Mn基催化剂降解CVOCs时存在HCl和Cl2的总选择性低的问题[15],通过向Mn基催化剂中掺杂其他金属可以提高CVOCs的降解效果,降低副产物产量[16]。Co被认为是氧化CVOCs的一种有效催化剂,Boukha等[17]的研究结果显示Co催化剂能有效抑制1,2-二氯乙烷降解过程中产生的有机中间产物,提高HCl和Cl2的选择性。近年来研究表明Co-Mn双金属催化剂相比于单独的Co、Mn催化剂具有更高的催化活性[12,18-19]。考虑到Co和Mn两种金属之间存在协同效应,因此Co-Mn双金属催化剂耦合低温等离子体降解三氯乙烯具有研究意义。
本研究以γ-Al2O3为载体,采用等体积浸渍法制备不同比例的Co-Mn/γ-Al2O3双金属催化剂,并耦合NTP催化降解含三氯乙烯废气,从三氯乙烯去除率、CO2产率、CO产率、COx产率、氯平衡和副产物(O3和N2O)的产量为评价指标,探究不同Co:Mn比例对三氯乙烯降解性能的影响。通过比表面积及孔径分析(BET)、X射线衍射(XRD)、X射线光电子能谱(XPS)、H2程序升温还原(H2-TPR)方法对催化剂的物理化学性质进行表征,以阐明不同催化剂对三氯乙烯的降解差异,为NTP降解CVOCs系统中催化剂的优化及应用提供参考。
-
钴锰双金属催化剂采用等体积浸渍法制备,制备方法:选取Co(NO3)2·6H2O、Mn(NO3)2(50%wt水)(均为分析纯)为金属Co、Mn的前驱体,配置成不同Co:Mn比例(Co:Mn=1:0、1:3、1:1、3:1、0:1)的硝酸盐溶液,将其缓慢滴加在一定质量的γ-Al2O3上,静置12 h后,于120 ℃烘5 h,随后在马弗炉中以500 ℃焙烧5 h,制得金属氧化物负载量为10%wt的催化剂(Co/γ-Al2O3、Co3Mn1/γ-Al2O3、Co1Mn1/γ-Al2O3、Co1Mn3/γ-Al2O3、Mn/γ-Al2O3)。
使用V-Sorb 2800P比表面积测试仪对催化剂的BET比表面积进行表征,并采用Barrett Joyner Halenda(BJH)计算孔体积和孔径分布,催化剂在250 ℃真空条件下预处理2 h,以N2和He为吸附质,在液氮条件下进行测试。XRD使用日本理学UItima Ⅳ型X射线粉末衍射仪测定,选用聚焦光进行测定,CuKα射线,扫描速率10 (°)·min−1,扫描步长0.02,在2θ为5°—90°范围下扫描搜集XRD图谱。H2-TPR使用Chemisorb2720化学吸附仪对催化剂进行测定,反应过程中约100 mg的催化剂置于40 mL·min−1的H2中,以10 ℃·min−1的升温速率从50 ℃升至900 ℃。XPS使用美国热电K-Alpha X 射线光电子能谱仪对催化剂表面的元素组成、含量进行测定,光源为Al Kα(1486.6 eV)射线,以结合能284.8 eV的C1s峰进行校准,再利用XPS peak4.1软件对谱图进行分峰拟合。
-
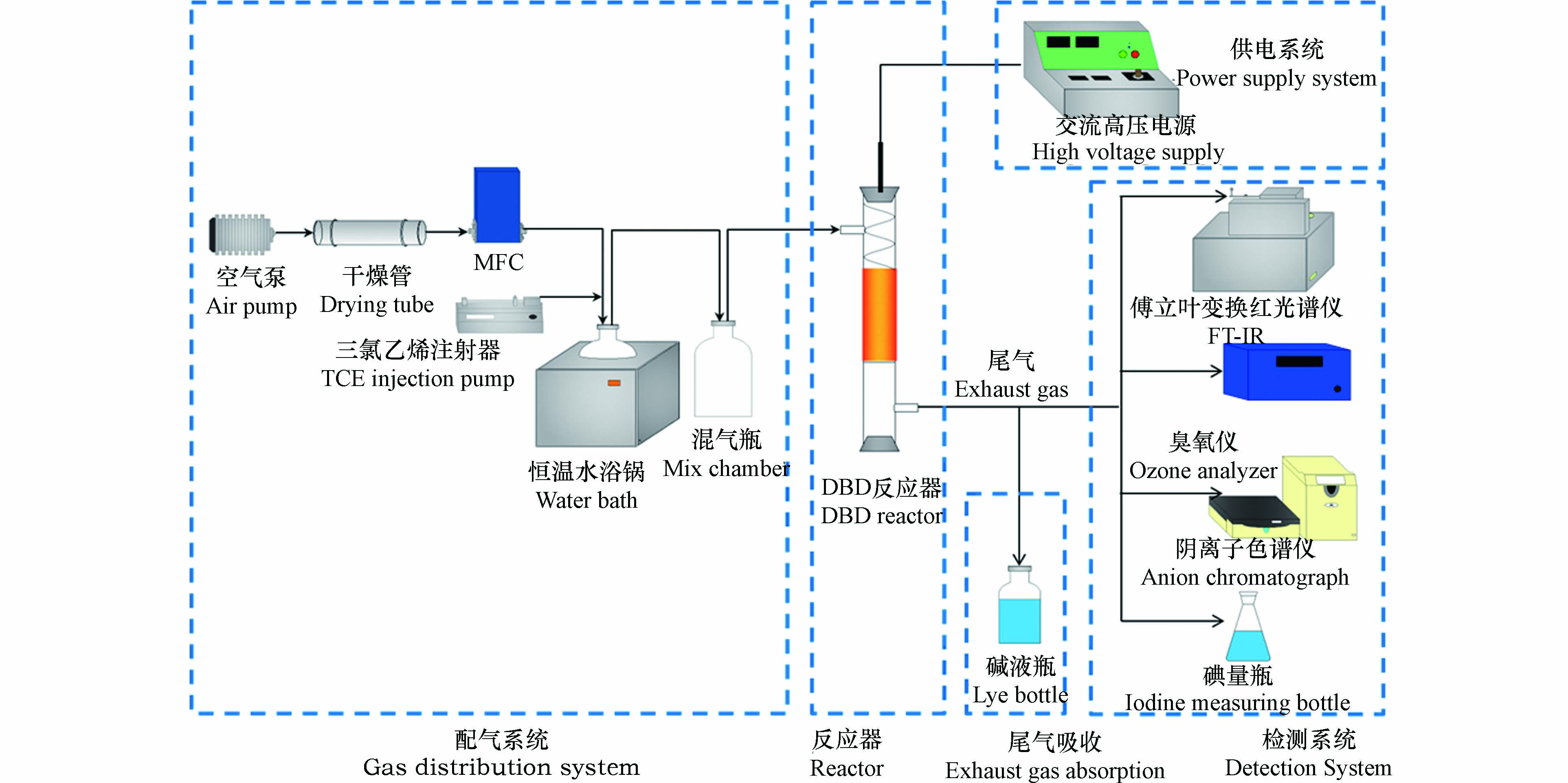
实验在常温常压下进行,实验系统主要由供气装置、高压交流供电系统、等离子体催化反应器、检测系统和尾气吸收系统组成,具体工艺流程见图1。
使用注射泵(LSP02-D)将液态三氯乙烯注入置于恒温水浴锅(85 ℃)中的玻璃瓶内,同时通过质量流量计(CS200D,北京七星华创电子股份有限公司)控制干燥除杂后的空气以1 L·min−1速率通入该玻璃瓶内使其带出含三氯乙烯气体。通过改变注射器的推进速度将反应器入口处的三氯乙烯浓度保持在1760 mg·m−3。反应管内填充催化剂2 g,用石英棉固定在放电间隙,实验电压为6、7、8、9、10 kV。反应器为自制的线管式介质阻挡放电反应器,反应管材质为石英玻璃(内径为22 mm、壁厚1 mm、长300 mm),高压电极为不锈钢线圈(外径20 mm,线径2 mm,长170 mm),接地电极为缠绕在反应器外壁的铜皮(厚0.05 mm,长100 mm)。实验所用电源为50 Hz高压交流电源(GJTK-0.01/30K,上海南罡电除尘器有限公司)。三氯乙烯及其降解过程中产生的CO2、CO、N2O的浓度由傅里叶变换红外光谱仪(Nicolet Antaris IGS,Thermo Scientific Company)来测定,HCl的浓度采用阴离子色谱仪(ICS1100)测定,通过碘量法测定Cl2浓度,O3浓度通过臭氧检测仪(2B Technologies Model 106-M)测得。
-
为了比较不同比例Co-Mn双金属催化剂耦合低温等离子体对三氯乙烯的催化氧化性能,通过三氯乙烯的去除率、COx产率、HCl和Cl2产率、碳平衡B进行评价,公式如下:
式中,P为输入功率W;f为频率,取值50 Hz;A为示波器所测李萨如图面积;C为电容,取值0.47 μF;[TCE]in、[TCE]out为三氯乙烯入口和出口浓度mol·m−3;[CO2]、[CO]为出口处的CO2和CO浓度mol·m−3;[HCl]、[Cl2]为出口处的Cl2和HCl浓度mol·m−3;Ey为反应器能量效率g·(kWh)−1;Q为三氯乙烯气体流量,取值1 L·min−1。
-
表1为催化剂的BET比表面积、孔容、孔径等物理性质。结果显示,负载金属的γ-Al2O3的BET比表面积、孔容、孔径均小于γ-Al2O3,这可能是由于负载的活性组分堵塞了一部分γ-Al2O3的孔道或受到催化剂烧结的影响[20-21]。负载金属的催化剂中Co3Mn1/γ-Al2O3的比表面积最大,达到146.15 m2·g−1。催化剂为低温等离子体催化反应提供了反应界面,催化剂的比表面积越大,三氯乙烯和等离子体放电产生的活性粒子越容易在催化剂表面吸附和富集,三氯乙烯与活性粒子有效碰撞几率以及发生表面反应的可能性提高[22],从而有利于三氯乙烯的降解。
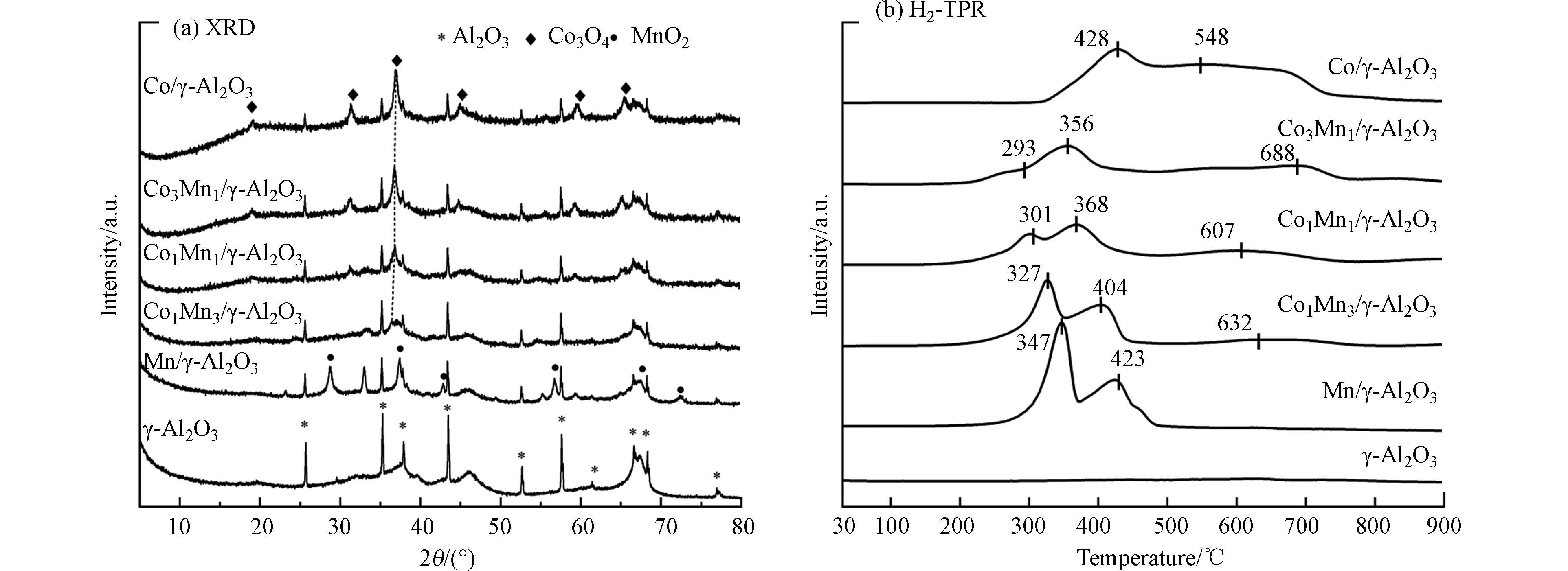
催化剂的XRD分析结果如图2(a)所示, 可以观察到所有的催化剂在2θ=25.6°、35.2°、37.8°、43.4°、52.5°、57.5°、66.5°、68.2°存在明显的Al2O3衍射峰,样品呈现典型的γ-Al2O3晶体的立方结构(PDF#78-2426),这说明钴锰金属的负载未改变氧化铝的结构和晶型。在Mn/γ-Al2O3的XRD图谱中可以观察到在2θ=28.6°、37.3°、42.7°、56.6°存在明显的MnO2衍射峰,样品呈现典型的MnO2软锰矿型晶体结构(PDF#81-2261)。在Co/γ-Al2O3的XRD图谱可以观察到在2θ=19.0°、31.3°、36.9°、44.8°、59.4°、65.2°存在明显的Co3O4衍射峰,样品呈现典型的Co3O4尖晶石型晶体结构(PDF#74-2120)。在Co-Mn双金属催化剂中均未观察到MnO2的衍射峰,从图2(a)可以看出,Co3O4的衍射峰随着Mn含量的增加衍射强度逐渐减弱。此外,相比于Co,Co-Mn双金属催化剂中Co3O4 (311)的晶面衍射峰从36.9°处偏移至36.3°处,这可能是由于Co、Mn之间发生相互作用,MnOx掺杂到Co3O4中,形成Co-Mn固溶体[18,23]。在2θ=36.9°(311)处Co3O4的晶粒尺寸大小排序为Co (18.5 nm)>Co1Mn1 (15.7 nm)>Co1Mn3 (14.6 nm)>Co3Mn1 (14.2 nm),晶粒尺寸越小,产生的晶格缺陷越多[23],表面氧空位数目随之增加,可以为三氯乙烯催化反应提供大量表面吸附氧物种[24]。从而提高Co-Mn催化剂耦合NTP降解三氯乙烯效果。
H2-TPR用于分析催化剂的氧化还原性,分析结果如图2(b)所示。在Co/γ-Al2O3催化剂中可以观察到两个还原峰,第一个峰位于428 ℃左右对应Co3+还原为Co2+,第二个峰位于548 ℃左右对应Co2+转化为金属Co[12,23]。Mn/γ-Al2O3催化剂在327 ℃和423 ℃处的两个还原峰对应于Mn3O4/MnO2的两步还原(MnO2→Mn2O3→Mn3O4;Mn3O4→MnO)[18,25]。与单组份催化剂相比,Co-Mn双金属催化剂的还原峰向更低的温度区间偏移。对于Co-Mn双金属催化剂可见3个还原峰,最低温度下的还原峰来自Co-Mn氧化物产生的易还原高度分散的Mn4+被还原为Mn8/3+,第二个还原峰是Mn3O4和Co3O4分别被还原为MnO和CoO [13,24],第三个还原峰是Co2+被还原为金属Co[26]。一般而言,还原温度越低,催化剂的氧化还原性能越好。从图2(b)可以看出,与单独的Co、Mn催化剂相比,Co-Mn双金属催化剂的还原峰向低温峰方向偏移,表明Co-Mn双金属催化剂提高了低温活性,其中Co3Mn1/γ-Al2O3的还原峰出现在更低的温度,说明Co3Mn1/γ-Al2O3催化剂具有更高的还原性。
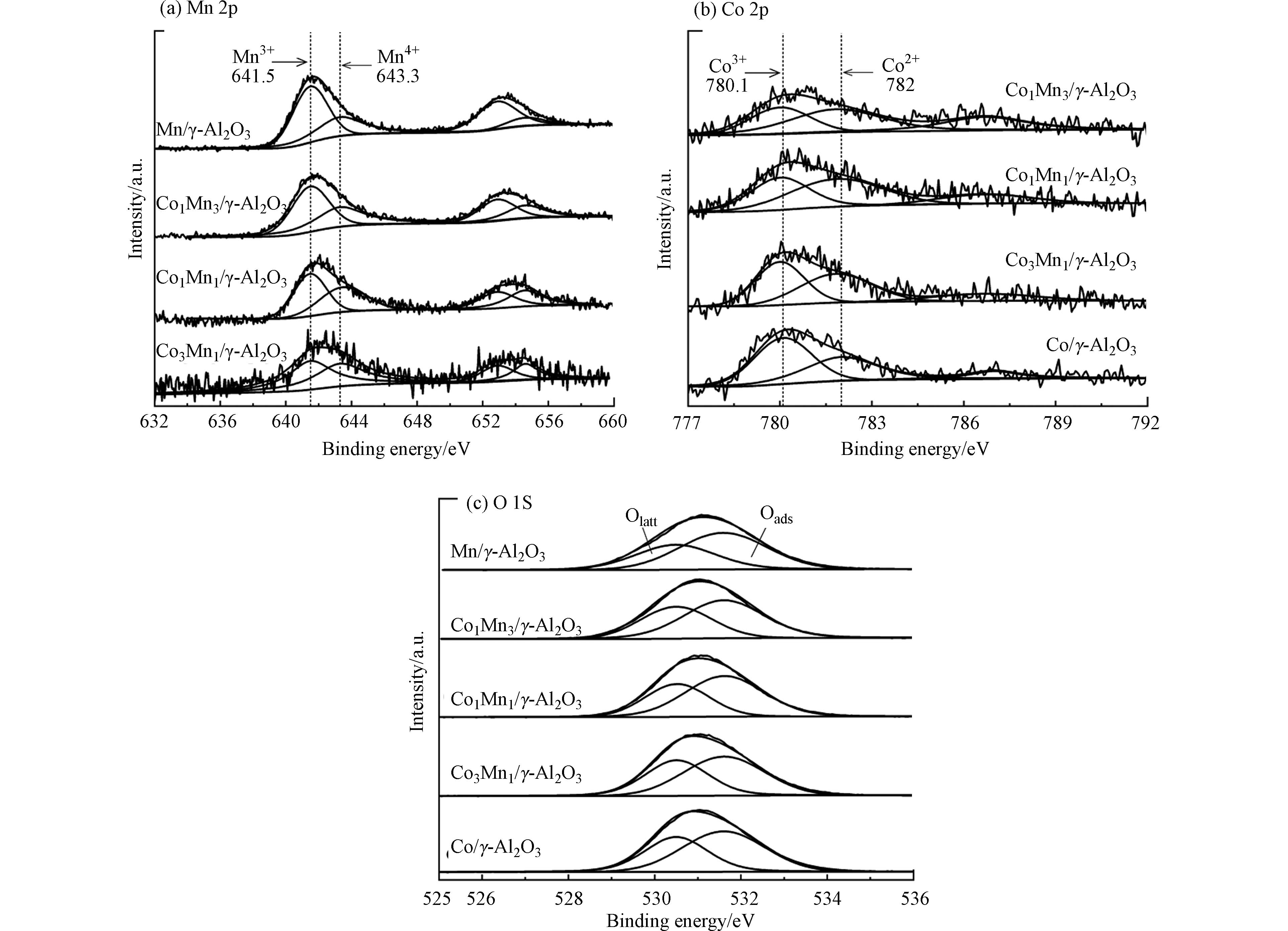
图3为不同催化剂Mn2p、Co2p、O1s的XPS谱图,表2为催化剂表面的Mn4+、Co3+、Oads含量。
图3a为不同催化剂的Mn2p XPS光谱,在635—648 eV和650—660 eV区域的结合能分别属于Mn2p3/2和Mn2p1/2。Mn2p3/2的XPS谱图由两个结合能643.3 eV和641.5 eV为中心的特征峰组成,分别代表Mn4+和Mn3+[27]。催化剂表面的MnOx还原顺序为:MnO2/Mn2O3→Mn2O3→MnO。Mn4+的取代,为三氯乙烯降解提供了更多的氧空位,表现出更高的催化活性[28]。从表2可见,Mn4+/Mn3+随着Co含量的增加而增加。图3b中Co2p3/2的XPS谱图由两个结合能780.1 eV和782 eV为中心的特征峰组成,分别代表Co3+和Co2+[27]。表2中Co3+/Co2+按照Co1Mn1/γ-Al2O3≈Co1Mn3/γ-Al2O3<Co3Mn1/γ-Al2O3<Co/γ-Al2O3的顺序增加,催化剂中更多Co3+有利于提高催化剂的氧化性能。图3c催化剂O1s的XPS谱图由两个531.6 eV和530.5 eV为中心的特征峰组成,前者主要是表面吸附氧(Oads),后者为表面晶格氧(Olatt)[29]。催化剂的Oads/(Oads+Olatt)由小到大排序为Co/γ-Al2O3< Co1Mn3/γ-Al2O3< Mn/γ-Al2O3< Co1Mn1/γ-Al2O3< Co3Mn1/γ-Al2O3,Co-Mn双金属催化剂中Co3Mn1/γ-Al2O3具有最高的表面吸附氧占比,而表面吸附氧含量越高,越有利于对三氯乙烯的催化氧化。
-
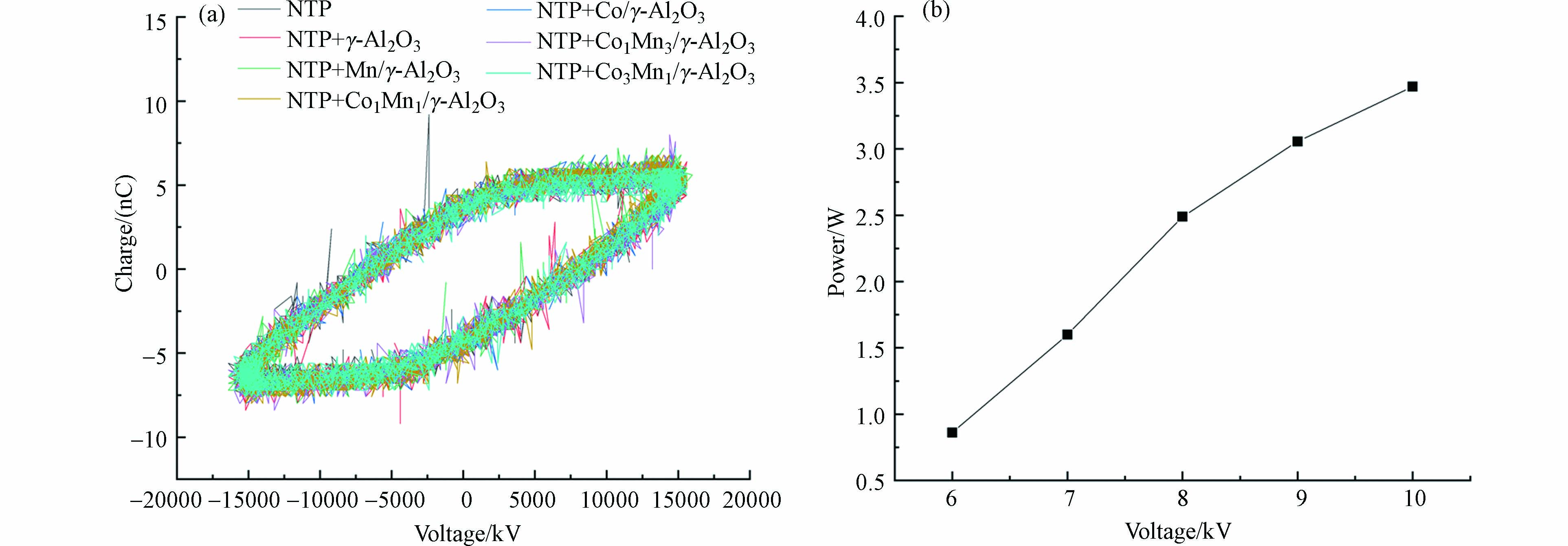
图4(a)为电压10kV时NTP耦合不同催化剂的李萨如图,NTP耦合不同催化剂的李萨如图与单一NTP相似,说明催化剂的填充对反应器中电荷转移量没有明显的影响,这可能是由于催化剂的填充量较少,因此催化剂的填充没有改变反应器的放电特性。图4(b)为NTP耦合Co3Mn1/γ-Al2O3催化剂时反应器的放电功率随电压变化的曲线图,电压从6 kV升至10 kV时,反应器的放电功率为0.86—3.47 W.
-
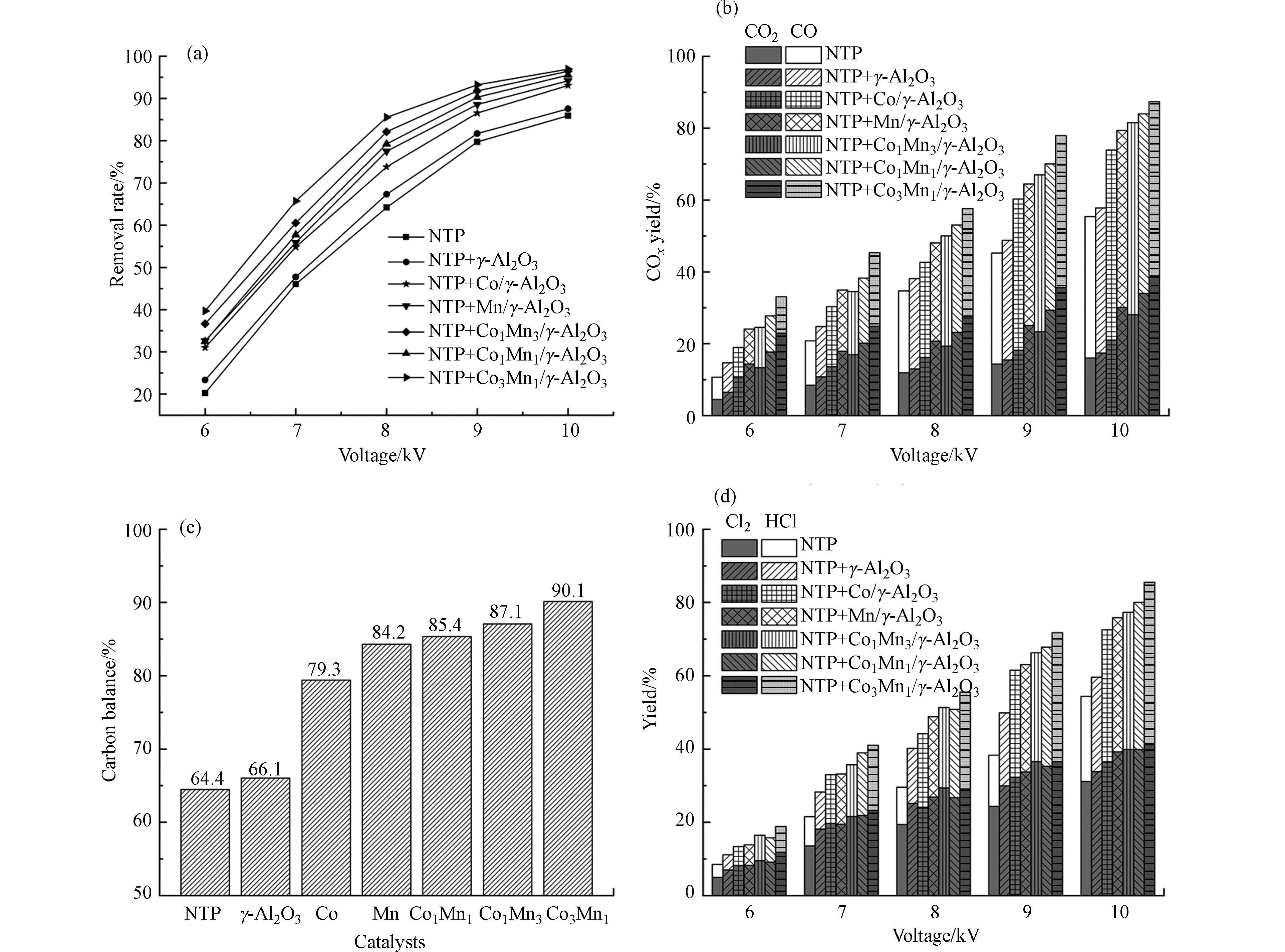
图5为单一NTP体系和NTP耦合催化体系的三氯乙烯去除率、COx产率、HCl和Cl2产率随电压的变化。在不同NTP体系中,三氯乙烯去除率、COx产率、HCl和Cl2产率都随着电压的升高而升高。电压升高,产生更多的丝状放电,导致各种活性物种的数量显著增加,从而提高了活性物种与三氯乙烯分子的碰撞几率[30-31],使得三氯乙烯的去除率、COx产率、HCl和Cl2产率均有所提高。另外,NTP耦合催化系统中三氯乙烯去除率、COx产率、碳平衡、HCl和Cl2产率明显高于单一NTP降解体系。电压为6—10 kV时,单一NTP降解体系中三氯乙烯去除率为20.2%—85.1%。NTP耦合催化剂降解体系中,当催化剂分别为γ-Al2O3、Co/γ-Al2O3、Mn/γ-Al2O3、Co1Mn3/γ-Al2O3、Co1Mn1/γ-Al2O3、Co3Mn1/γ-Al2O3时,对应的三氯乙烯去除率为23.3%—87.5%、31.1%—93.1%、32.5%—85.5%、36.7%—96.4%、32.6%—94.2%、39.7%—96.94%。催化剂的加入明显提高了三氯乙烯的去除效果,这是因为催化剂的加入使三氯乙烯和活性粒子吸附在催化剂表面,提高三氯乙烯与活性粒子有效碰撞几率,从而有利于三氯乙烯的降解[22]。并且催化剂的加入使得放电模式由单一NTP的丝状放电变为丝状放电与催化剂表面放电相结合[32]。催化剂表面放电产生的放电通道具有紧贴催化剂表面并向外延伸的特点,沿催化剂表面出现强化电离效应,有助于等离子体放电通道中产生的活性粒子在催化剂表面的有效利用,增强等离子体催化反应性能[33],因此NTP耦合催化系统中三氯乙烯的去除率高于单一NTP。此外催化剂的加入显著提高了COx产率与CO2产率,促进了三氯乙烯的深度氧化。电压为10 kV时,单一NTP体系的COx产率与CO2产率分别为55.4%与16.4%,NTP耦合催化体系中Co3Mn1/γ-Al2O3的COx产率与CO2产率分别为87.4%与38.6%。
电压为6—10 kV时,负载金属的催化剂中Co-Mn双金属催化剂耦合NTP降解三氯乙烯的效果高于单一Co、Mn催化剂。钴和锰的相互作用显著提高了三氯乙烯降解效果,双金属等离子体催化系统对三氯乙烯的降解性能顺序为Co3Mn1>Co1Mn3>Co1Mn1,这表明双金属催化剂中Co和Mn的负载比例对等离子体催化降解三氯乙烯性能有重要影响。根据H2-TPR的分析,相比于单一负载,Co-Mn双金属催化剂的还原性得到了提高,其中Co3Mn1催化剂的还原温度最低。根据XRD分析,MnOx掺杂到Co3O4中,形成Co-Mn固溶体,Co3O4的晶粒尺寸降低,可产生更多的氧空位[12]。此外,通过XPS分析,在Co3Mn1催化剂中观察到最高的Co3+/Co2+、Mn4+/Mn3+和Oads/(Oads+Olatt)比率,表明Co3Mn1催化剂具有更多的氧空位和更高的氧物种迁移率。因此Co3Mn1/γ-Al2O3催化剂用于降解三氯乙烯具有较高的催化活性。
-
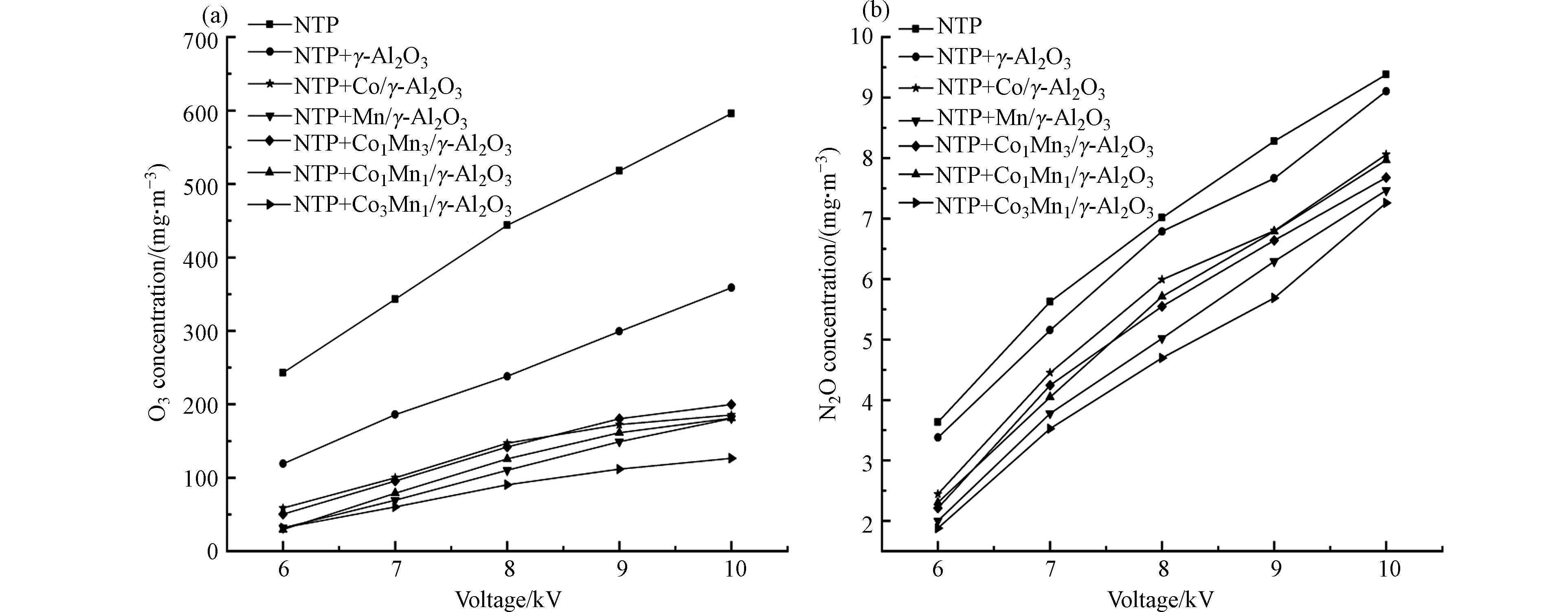
NTP降解三氯乙烯时,不可避免地会产生O3和N2O等副产物。在NTP降解三氯乙烯过程中,O3和N2O的产生过程如式(7)-(9),空气中的O2与高能电子碰撞生成O·,生成的O·与O2结合生成O3[34],N2与高能电子碰撞使其处于不稳定的激发态氮,进而被氧化为N2O[35]。需要说明的是,本研究中除N2O外,FTIR没有检测到其他NOx(NO和NO2),这主要是因为N2O的分解反应是吸热反应,而NO和NO2的分解反应是放热反应,因此在低温条件下NO和NO2可以自发分解,Shou等[36]也得到了相同的结果。
图6为副产物O3和N2O产量随电压变化图。由图6可知,O3和N2O产量随着输入电压的升高而升高。当电压为6—10 kV时,单一NTP系统产生O3和N2O的浓度分别为243.1—596.1 mg·m−3和3.7—9.4 mg·m−3,催化剂耦合NTP后O3和N2O的浓度明显降低,分别在119.1—358.6 mg·m−3和3.3—9.1 mg·m−3以下,其中Co3Mn1/γ-Al2O3催化剂对O3和N2O的抑制效果最好。填充催化剂后有助于O3的分解使出口O3浓度显著下降,这是由于O3充当了放电产生的电子的受体而被消耗,并产生活性氧[37](如式(10))。产生的O·进一步与N2O反应生成NO或N2和O2[38],从而降低O3和N2O的产量。
-
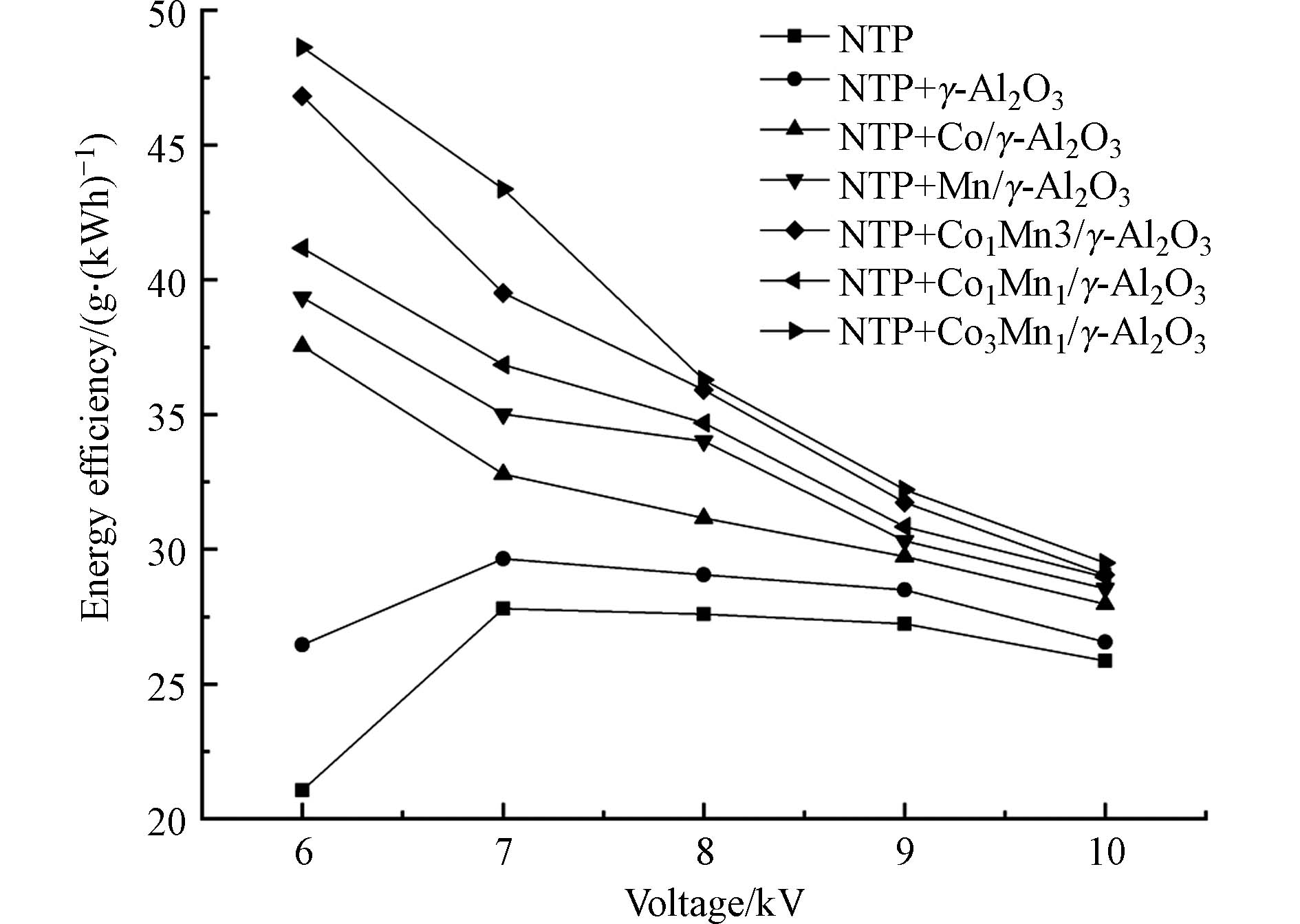
图7为不同NTP体系能量效率随电压变化的曲线图。单一NTP与NTP耦合γ-Al2O3的能量效率先升高后降低,因为在低电压时不能产生足够的活性物质来降解三氯乙烯从而导致能量效率低,随着电压的升高,活性物质的数量增加从而能量效率升高,随着电压的持续升高,更多的能量被用于加热气体而耗散掉,能量效率降低[39]。当催化剂分别为Co/γ-Al2O3、Mn/γ-Al2O3、Co1Mn3/γ-Al2O3、Co1Mn1/γ-Al2O3、Co3Mn1/γ-Al2O3时能量效率随电压升高而降低,这是由于在低电压时大部分三氯乙烯在NTP耦合催化体系中也会被分解。当电压为6—10 kV时Co3Mn1/γ-Al2O3催化剂的能量效率最高,为29.5—48.6 g·(kWh)−1。
-
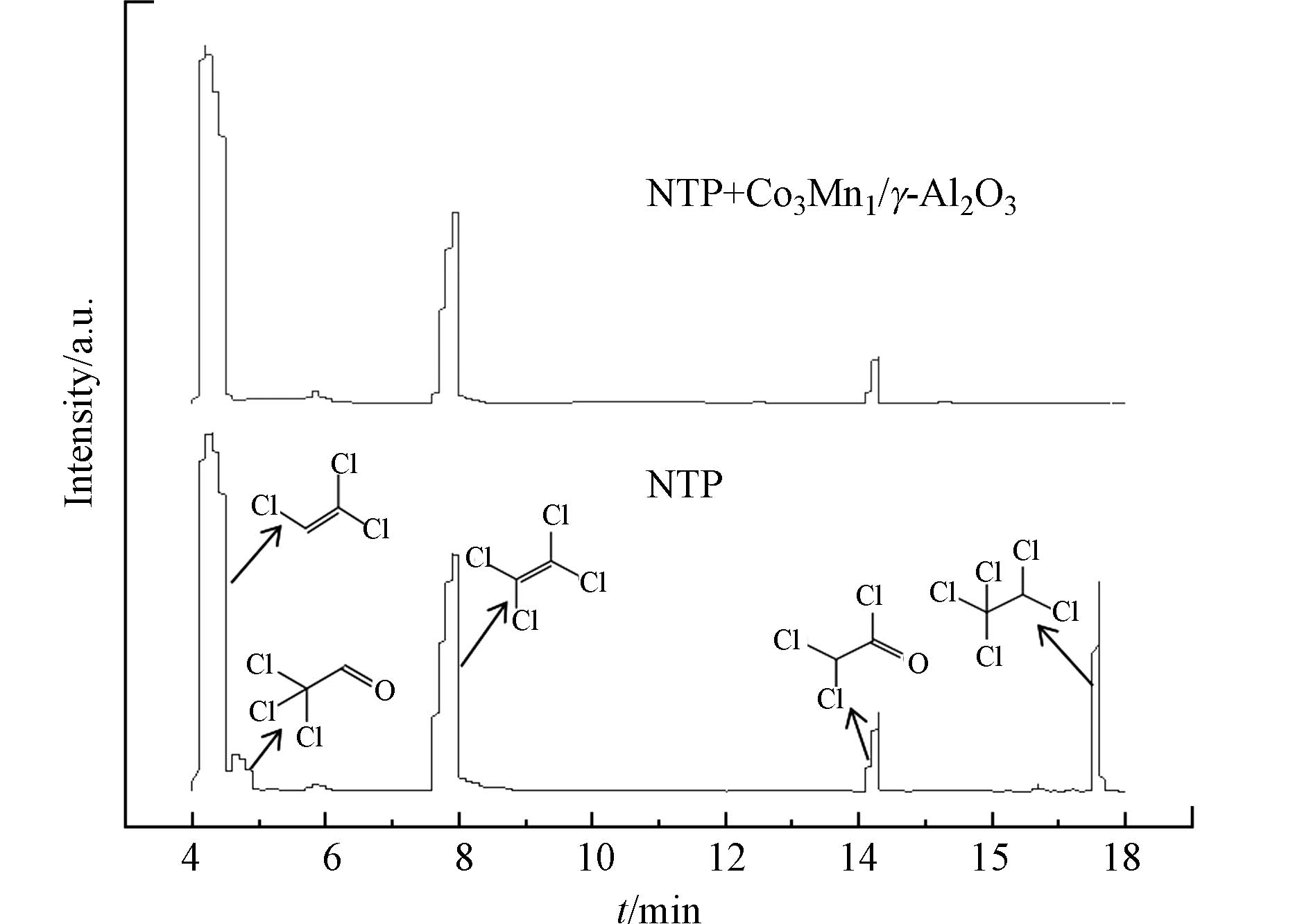
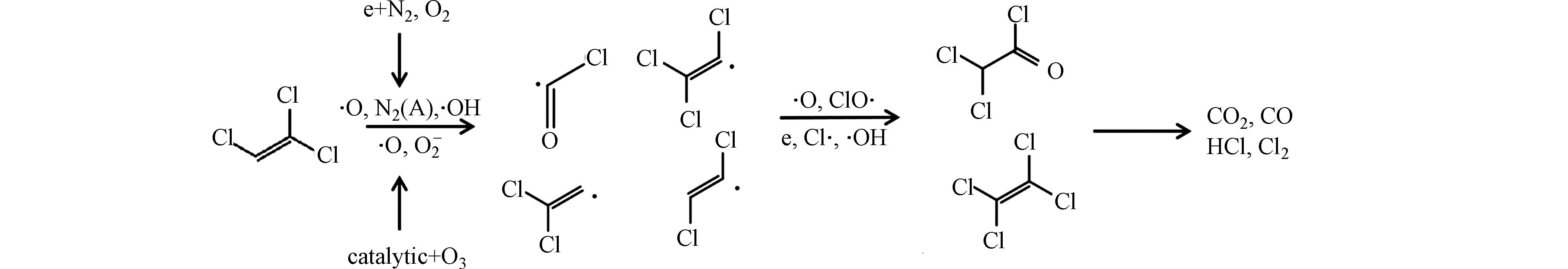
NTP耦合催化降解三氯乙烯过程中,不可避免会产生有机副产物,因此利用气相色谱-质谱联用仪(GC-MS)对输入电压为8 kV时反应产生的有机副产物进行分析。图8为单一NTP和NTP耦合Co3Mn1/γ-Al2O3降解三氯乙烯时尾气中的有机副产物GC-MS谱图。单一NTP去除三氯乙烯,反应产物中生成了二氯乙酰氯、三氯乙醛、四氯乙烯、五氯乙烷等物质,而在NTP耦合Co3Mn1/γ-Al2O3的系统中仅检测到了二氯乙酰氯、四氯乙烯等物质,这说明催化剂的加入可以有效减少有机副产物的产生,提高三氯乙烯降解效率。NTP耦合Co3Mn1/γ-Al2O3降解三氯乙烯的路径如图9所示,反应过程主要分为两部分:(1)放电过程中产生的O·、O2−、羟基自由基、电子和激发态氮等多种活性粒子,直接与三氯乙烯发生碰撞。(2)O3在催化剂表面分解产生的活性氧与三氯乙烯反应。随后,前两步生成的COCl·、CCl2Cl·、CHClCCl·、CCl2CH·等中间体进一步被氧化为二氯乙酰氯、四氯乙烯等有机物,最终被矿化成CO2、CO、HCl、Cl2[40-41]。
-
本文比较了不同Co-Mn催化剂耦合低温等离子体降解三氯乙烯性能的差异的影响,并通过多种表征手段分析催化剂的化学特性和物理结构,提出了其反应机理,主要结论如下。
(1) 相比单一NTP降解体系,NTP耦合催化降解体系显著提高了三氯乙烯的降解性能。而Co-Mn双金属催化剂的催化性能优于单一Co和Mn催化剂,其中Co3Mn1/γ-Al2O3催化剂在耦合NTP处理三氯乙烯时表现出优异的降解性能,在10 kV时去除率、COx产率、碳平衡、HCl和Cl2产率,分别可达到96.94%、87.36%、90.1%、84.41%。
(2) 随着输入电压升高,O3和N2O的产量增加,催化剂的加入明显减少了O3和N2O的产量,Co3Mn1/γ-Al2O3催化剂对O3和N2O的抑制效果最好。
(3) 通过 BET、XRD、XPS和H2-TPR表征可知与钴、锰单金属催化剂相比,Co-Mn双金属催化剂具有较高的比表面积、较低的氧化还原温度,其中Co3Mn1/γ-Al2O3的比表面积、吸附氧、Co3+、Mn4+均高于其他钴锰催化剂,表明Co3Mn1/γ-Al2O3催化活性较好。
低温等离子体耦合Co-Mn双金属催化剂降解三氯乙烯
Removal of trichloroethylene by non-thermal plasma combined with Co-Mn bimetallic catalyst
-
摘要: 以γ-Al2O3为载体,采用等体积浸渍法制备钴锰催化剂(Co/γ-Al2O3、Mn/γ-Al2O3、Co3Mn1/γ-Al2O3、Co1Mn1/γ-Al2O3、Co1Mn3/γ-Al2O3),研究不同催化剂耦合低温等离子体(NTP)对三氯乙烯的降解性能。与单一NTP相比,NTP耦合催化剂可显著提高三氯乙烯的去除率、COx产率、碳平衡、HCl和Cl2产率,减少副产物的产生。Co-Mn双金属催化剂催化性能高于单一Co和Mn负载的催化剂,其中Co3Mn1/γ-Al2O3具有最高的催化活性,电压为6—10 kV,Co3Mn1/γ-Al2O3耦合NTP对三氯乙烯去除率(39.7%—96.94%)与COx(33.04%—87.36%)产率最高。通过BET、XRD、H2-TPR、XPS表征催化剂的物化性质,以阐明不同催化剂耦合NTP降解三氯乙烯效果存在差异的原因。结果显示,Co3Mn1/γ-Al2O3催化剂具有较高的比表面积,较小的Co3O4晶粒尺寸,较低的还原温度,且催化剂表面上的Mn4+、Co3+和Oads含量更高,有利于三氯乙烯的降解。最后通过GC-MS分析三氯乙烯降解过程中产生的有机中间体,推测了低温等离子体降解三氯乙烯的反应路径。Abstract: The cobalt-manganese catalysts (Co/γ-Al2O3、Mn/γ-Al2O3、Co3Mn1/γ-Al2O3、Co1Mn1/γ-Al2O3、Co1Mn3/γ-Al2O3) were prepared by equal volume impregnation method using γ-Al2O3 as the carrier to study the degradation performance of trichloroethylene by different catalysts coupled with non-thermal plasma (NTP). Compared with single NTP, the NTP-coupled catalyst significantly improved the removal of trichloroethylene、COx yield、Carbon balance、HCl and Cl2 yield、and reduced by-product generation. The catalytic performance of Co-Mn bimetallic catalysts is higher than that of single Co and Mn loaded catalysts, and Co3Mn1/γ-Al2O3 has the highest catalytic activity.At the voltage of 6—10 kV, Co3Mn1/γ-Al2O3 coupled with NTP has the highest removal rate of trichloroethylene (39.7%—96.94%) and the highest COx yield (33.04%—87.36%). BET、XRD、H2-TPR、XPS were used to characterize the physical and chemical properties of the catalysts to clarify the reasons for the differences in the performance of different catalysts coupled with NTP in the degradation of trichloroethylene. The results showed that the Co3Mn1/γ-Al2O3 catalyst had higher specific surface area, smaller Co3O4 grain size, lower reduction temperature, and higher content of Mn4+、Co3+ and Oads on the catalyst surface, which were beneficial to the degradation of trichloroethylene. Finally, the organic intermediates produced during the degradation of trichloroethylene were analyzed by GC-MS to speculate the reaction path of trichloroethylene degradation by non-thermal plasma.
-
Key words:
- non-thermal plasma /
- Co-Mn bimetallic catalyst /
- trichloroethylene /
- removal efficience /
- by-product
-

-
表 1 催化剂的比表面积及孔结构性质
Table 1. BET surface area and pore structure of catalyst
催化剂
CatalystBET比表面积/(m2·g−1)
Specific surface area孔容/(cm3·g−1)
Pore volume孔径/nm
Average diameterγ-Al2O3 151.78 0.6657 17.5426 Co/γ-Al2O3 129.13 0.4214 13.0531 Mn/γ-Al2O3 132.88 0.4068 12.2458 Co1Mn3/γ-Al2O3 137.84 0.3833 12.122 Co1Mn1/γ-Al2O3 137.44 0.3763 10.9505 Co3Mn1/γ-Al2O3 146.15 0.3809 10.4237 表 2 催化剂的XPS结果
Table 2. XPS results of the catalysts
Mn4+/Mn3+ Co3+/Co2+ Oads/(Oads+Olatt) Co/γ-Al2O3 — 1.261 0.577 Co3Mn1/γ-Al2O3 0.913 1.018 0.611 Co1Mn1/γ-Al2O3 0.839 0.807 0.605 Co1Mn3/γ-Al2O3 0.797 0.848 0.588 Mn/γ-Al2O3 0.635 — 0.601 -
[1] YANG P, FAN S K, CHEN Z Y, et al. Synthesis of Nb2O5 based solid superacid materials for catalytic combustion of chlorinated VOCs [J]. Applied Catalysis B:Environmental, 2018, 239: 114-124. doi: 10.1016/j.apcatb.2018.07.061 [2] LI H F, LU G Z, DAI Q G, et al. Efficient low-temperature catalytic combustion of trichloroethylene over flower-like mesoporous Mn-doped CeO2 microspheres [J]. Applied Catalysis B:Environmental, 2011, 102(3/4): 475-483. [3] 钱翌, 岳飞飞, 褚衍洋. 三氯乙烯环境污染修复技术研究进展 [J]. 环境化学, 2012, 31(9): 1335-1343. QIAN Y, YUE F F, CHU Y Y. Advances in environmental remediation technologies for trichloroethylene pollution [J]. Environmental Chemistry, 2012, 31(9): 1335-1343(in Chinese).
[4] TIAN M J, GUO X, DONG R, et al. Insight into the boosted catalytic performance and chlorine resistance of nanosphere-like meso-macroporous CrOx/MnCo3Ox for 1, 2-dichloroethane destruction [J]. Applied Catalysis B:Environmental, 2019, 259: 118018. doi: 10.1016/j.apcatb.2019.118018 [5] HOLZER F, ROLAND U, KOPINKE F D. Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds: Part 1. Accessibility of the intra-particle volume [J]. Applied Catalysis B:Environmental, 2002, 38(3): 163-181. doi: 10.1016/S0926-3373(02)00040-1 [6] VEERAPANDIAN S K P, YE Z P, GIRAUDON J M, et al. Plasma assisted Cu-Mn mixed oxide catalysts for trichloroethylene abatement in moist air [J]. Journal of Hazardous Materials, 2019, 379: 120781. doi: 10.1016/j.jhazmat.2019.120781 [7] DANG X Q, LI S J, YU X, et al. Kinetic characterization of adsorbed toluene removal involving hybrid material catalysts—[M/13X-γ-Al2O3 (M: Ag, Ce, Mn, and Co)] in a sequential non-thermal plasma system [J]. Chemical Engineering Research and Design, 2020, 155: 80-87. doi: 10.1016/j.cherd.2019.12.025 [8] NGUYEN DINH M T, GIRAUDON J M, VANDENBROUCKE A M, et al. Manganese oxide octahedral molecular sieve K-OMS-2 as catalyst in post plasma-catalysis for trichloroethylene degradation in humid air [J]. Journal of Hazardous Materials, 2016, 314: 88-94. doi: 10.1016/j.jhazmat.2016.04.027 [9] 姜理英, 张瑜芬, 胡俊, 等. NTP协同双金属锰基催化剂降解氯苯的性能研究 [J]. 环境科学学报, 2021, 41(3): 922-931. JIANG L Y, ZHANG Y F, HU J, et al. Removal of chlorobenzene by non-thermal plasma combined with bimetallic manganese-based catalyst [J]. Acta Scientiae Circumstantiae, 2021, 41(3): 922-931(in Chinese).
[10] SULTANA S, VANDENBROUCKE A M, MORA M, et al. Post plasma-catalysis for trichloroethylene decomposition over CeO2 catalyst: Synergistic effect and stability test [J]. Applied Catalysis B:Environmental, 2019, 253: 49-59. doi: 10.1016/j.apcatb.2019.03.077 [11] LIN F W, ZHANG Z M, LI N, et al. How to achieve complete elimination of Cl-VOCs: A critical review on byproducts formation and inhibition strategies during catalytic oxidation [J]. Chemical Engineering Journal, 2021, 404: 126534. doi: 10.1016/j.cej.2020.126534 [12] TODOROVA S, BLIN J L, NAYDENOV A, et al. Co3O4-MnOx oxides supported on SBA-15 for CO and VOCs oxidation [J]. Catalysis Today, 2020, 357: 602-612. doi: 10.1016/j.cattod.2019.05.018 [13] 白文文, 秦彩虹, 郑洋, 等. 介质阻挡放电联合锰基催化剂对乙酸乙酯的降解效果 [J]. 环境工程学报, 2020, 14(5): 1294-1303. doi: 10.12030/j.cjee.201907089 BAI W W, QIN C H, ZHENG Y, et al. Degradation of ethyl acetate by dielectric barrier discharge combined with manganese-based catalyst [J]. Chinese Journal of Environmental Engineering, 2020, 14(5): 1294-1303(in Chinese). doi: 10.12030/j.cjee.201907089
[14] ODA T, KURAMOCHI H, ONO R. Non-thermal plasma processing for dilute VOCs decomposition combined with the catalyst[C]. 11th International Conference on Electrostatic Precipitation. Hangzhou: Springer Berlin Heidelberg, 2009: 638-643. [15] ZHANG Z M, XIANG L, LIN F W, et al. Catalytic deep degradation of Cl-VOCs with the assistance of ozone at low temperature over MnO2 catalysts [J]. Chemical Engineering Journal, 2021, 426: 130814. doi: 10.1016/j.cej.2021.130814 [16] YAO X H, ZHANG J, LIANG X Y, et al. Niobium doping enhanced catalytic performance of Mn/MCM-41 for toluene degradation in the NTP-catalysis system [J]. Chemosphere, 2019, 230: 479-487. doi: 10.1016/j.chemosphere.2019.05.075 [17] BOUKHA Z, GONZÁLEZ-PRIOR J, de RIVAS B, et al. Synthesis, characterisation and behaviour of Co/hydroxyapatite catalysts in the oxidation of 1, 2-dichloroethane [J]. Applied Catalysis B:Environmental, 2016, 190: 125-136. doi: 10.1016/j.apcatb.2016.03.005 [18] SHI C, WANG Y, ZHU A M, et al. MnxCo3−xO4 solid solution as high-efficient catalysts for low-temperature oxidation of formaldehyde [J]. Catalysis Communications, 2012, 28: 18-22. doi: 10.1016/j.catcom.2012.08.003 [19] LI X, ZHENG J K, LIU S, et al. A novel wormhole-like mesoporous hybrid MnCoOx catalyst for improved ethanol catalytic oxidation [J]. Journal of Colloid and Interface Science, 2019, 555: 667-675. doi: 10.1016/j.jcis.2019.07.062 [20] 郭惠, 党小庆, 秦彩虹, 等. 低温等离子体催化降解甲苯的影响因素分析 [J]. 环境污染与防治, 2019, 41(12): 1422-1426. GUO H, DANG X Q, QIN C H, et al. Influencing factors analysis of toluene degradation by low temperature plasma catalysis [J]. Environmental Pollution & Control, 2019, 41(12): 1422-1426(in Chinese).
[21] 崔维怡, 王希越, 谭乃迪. 焙烧温度对Pt-FeOx/γ-Al2O3催化剂催化甲醛氧化性能的影响 [J]. 燃料化学学报, 2019, 47(8): 964-972. doi: 10.3969/j.issn.0253-2409.2019.08.009 CUI W Y, WANG X Y, TAN N D. Effect of calcination temperature on catalytic performance of Pt-FeOx/γ-Al2O3 catalysts for HCHO oxidation [J]. Journal of Fuel Chemistry and Technology, 2019, 47(8): 964-972(in Chinese). doi: 10.3969/j.issn.0253-2409.2019.08.009
[22] 陈春雨, 刘彤, 王卉, 等. 低温等离子体与MnOx/γ-Al2O3协同催化降解正己醛 [J]. 催化学报, 2012, 33(6): 941-951. CHEN C Y, LIU T, WANG H, et al. Removal of hexanal by non-thermal plasma and MnOx/γ-Al2O3 combination [J]. Chinese Journal of Catalysis, 2012, 33(6): 941-951(in Chinese).
[23] TANG W X, LI W H, LI D Y, et al. Synergistic effects in porous Mn–co mixed oxide nanorods enhance catalytic deep oxidation of benzene [J]. Catalysis Letters, 2014, 144(11): 1900-1910. doi: 10.1007/s10562-014-1340-3 [24] BO Z, ZHU J H, YANG S L, et al. Enhanced plasma-catalytic decomposition of toluene over Co–Ce binary metal oxide catalysts with high energy efficiency [J]. RSC Advances, 2019, 9(13): 7447-7456. doi: 10.1039/C9RA00794F [25] CAI T, HUANG H, DENG W, et al. Catalytic combustion of 1, 2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts [J]. Applied Catalysis B:Environmental, 2015, 166/167: 393-405. doi: 10.1016/j.apcatb.2014.10.047 [26] ZHOU G L, HE X L, LIU S, et al. Phenyl VOCs catalytic combustion on supported CoMn/AC oxide catalyst [J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 932-941. doi: 10.1016/j.jiec.2014.04.035 [27] LUO Y J, ZHENG Y B, ZUO J C, et al. Insights into the high performance of Mn-Co oxides derived from metal-organic frameworks for total toluene oxidation [J]. Journal of Hazardous Materials, 2018, 349: 119-127. doi: 10.1016/j.jhazmat.2018.01.053 [28] LIN X T, LI S J, HE H, et al. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation [J]. Applied Catalysis B:Environmental, 2018, 223: 91-102. doi: 10.1016/j.apcatb.2017.06.071 [29] BIESINGER M C, BROWN C, MYCROFT J R, et al. X-ray photoelectron spectroscopy studies of chromium compounds [J]. Surface and Interface Analysis, 2004, 36(12): 1550-1563. doi: 10.1002/sia.1983 [30] 姚志伟, 黄武, 陈颖, 等. 低温等离子体结合锰基催化剂去除乙酸乙酯的研究 [J]. 环境科学学报, 2021, 41(6): 2311-2319. YAO Z W, HUANG W, CHEN Y, et al. Removal of ethyl acetate by non-thermal plasma combined with manganese-based catalysts [J]. Acta Scientiae Circumstantiae, 2021, 41(6): 2311-2319(in Chinese).
[31] 于欣, 党小庆, 李世杰, 等. 单介质和双介质阻挡放电低温等离子体降解甲苯的比较 [J]. 环境工程学报, 2020, 14(4): 1033-1041. doi: 10.12030/j.cjee.201906002 YU X, DANG X Q, LI S J, et al. Comparison of single and double dielectric barrier discharge non-thermal plasma for toluene removal [J]. Chinese Journal of Environmental Engineering, 2020, 14(4): 1033-1041(in Chinese). doi: 10.12030/j.cjee.201906002
[32] 朱金辉. 低温等离子体耦合Co-Ce二元金属氧化物催化剂降解甲苯基础研究[D]. 杭州: 浙江大学, 2020. ZHU J H. Non-thermal plasma combined with co-Ce binary metal oxide catalysts for toluene decomposition[D]. Hangzhou: Zhejiang University, 2020(in Chinese).
[33] KIM H H, KIM J H, OGATA A. Microscopic observation of discharge plasma on the surface of zeolites supported metal nanoparticles [J]. Journal of Physics D:Applied Physics, 2009, 42(13): 135210. doi: 10.1088/0022-3727/42/13/135210 [34] LIANG W J, MA L, LIU H, et al. Toluene degradation by non-thermal plasma combined with a ferroelectric catalyst [J]. Chemosphere, 2013, 92(10): 1390-1395. doi: 10.1016/j.chemosphere.2013.05.042 [35] ZHENG C H, ZHU X B, GAO X, et al. Experimental study of acetone removal by packed-bed dielectric barrier discharge reactor [J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 2761-2768. doi: 10.1016/j.jiec.2013.11.004 [36] SHOU T Y, LI Y N, BERNARDS M T, et al. Degradation of gas-phase o-xylene via combined non-thermal plasma and Fe doped LaMnO3 catalysts: Byproduct control [J]. Journal of Hazardous Materials, 2020, 387: 121750. doi: 10.1016/j.jhazmat.2019.121750 [37] XU X X, WU J L, XU W C, et al. High-efficiency non-thermal plasma-catalysis of cobalt incorporated mesoporous MCM-41 for toluene removal [J]. Catalysis Today, 2017, 281: 527-533. doi: 10.1016/j.cattod.2016.03.036 [38] YU X, DANG X Q, LI S J, et al. A comparison of in- and post-plasma catalysis for toluene abatement through continuous and sequential processes in dielectric barrier discharge reactors [J]. Journal of Cleaner Production, 2020, 276: 124251. doi: 10.1016/j.jclepro.2020.124251 [39] LI S J, DANG X Q, YU X, et al. High energy efficient degradation of toluene using a novel double dielectric barrier discharge reactor [J]. Journal of Hazardous Materials, 2020, 400: 123259. doi: 10.1016/j.jhazmat.2020.123259 [40] KIRKPATRICK M, FINNEY W, LOCKE B. Chlorinated organic compound removal by gas phase pulsed streamer Corona electrical discharge with reticulated vitreous carbon electrodes [J]. Plasmas and Polymers, 2003, 8(3): 165-177. doi: 10.1023/A:1024845721132 [41] VANDENBROUCKE A M, DINH M T N, GIRAUDON J M, et al. Qualitative by-product identification of plasma-assisted TCE abatement by mass spectrometry and Fourier-transform infrared spectroscopy [J]. Plasma Chemistry and Plasma Processing, 2011, 31(5): 707-718. doi: 10.1007/s11090-011-9310-7 -



 下载:
下载: