-
唑类是环状有机分子,根据化学结构可分为咪唑类(两个氮原子)和三唑类(三个氮原子)[1-2]。由于其广谱的抗真菌活性和高稳定性,被广泛用于农业杀菌剂、人类和兽医药品等各种产品中[3]。随着全球人口的增加导致对农业的需求增加,为满足日益增长的消费需求,进一步提高生产力,主要是通过使用农药来减少害虫病菌[4]。尽管欧洲疾病预防控制中心(ECDC)早在2013年在报告就提到,应该减少农业杀真菌剂的使用[5-6],但唑类杀菌剂在世界范围内仍然被广泛使用[6-7]。虽然唑类杀真菌剂可用于控制植物病原体,但这些化合物的广泛使用和过度暴露会导致害虫病菌的敏感性降低[8],这种趋势会不断加大环境中农药负荷,从而增加人类通过水、空气、土壤和食物暴露的风险[9]。除农业外,医疗、工业和家庭活动都会将唑类排放到环境中,造成了其污染[10],如唑类药物的局部给药(克霉唑)、口服(氟康唑)和静脉内注射(咪康唑)等,大部分残留成分会通过清洗或尿液排泄进入环境中,这是唑类化合物进入城市废水的主要途径[3, 11-13]。而在日常生活中,酮康唑在护发配方中用作去屑剂,甘宝素不仅用作去屑活性成分,还用作抗霉菌防腐剂[1-2]。除了用于生活用品和个人护理产品,唑类化合物还用作飞机上的腐蚀抑制剂。这些化合物的广泛使用导致空气、植物和土壤的污染[14],欧洲已在废水和地表水中检测到了唑类抗菌剂[3]。CREUSOT 等[15]调查了瑞士高原的地表水、城市污水、沉积物、土壤和地下水、生物被膜和鱼样品,结果均存在抗真菌唑类及其某些(生物)转化产物。Chiaia等[16]分析了城市和农业污染湖的沉积物核心,其中最常见的是唑类。Chen等[17]实验发现唑类在土壤环境中具有高度持久性。唑类杀菌剂在环境中的存在对人类的潜在危害和其生态毒理学威胁成为新出现的问题之一[18-19]。作为最大的一类人工合成抗真菌药物,唑类抗真菌药物已在全球范围内的水生环境中均有所检出[20]。然而目前对这些唑类抗真菌药物毒性研究不太全面,且多数停留在急性微板毒性测试,对于作用机理和响应机制研究较为缺乏。因此,研究唑类杀菌剂对水生生态系统中的初级生产者(淡水藻类)的生理生化响应机制具有重要现实意义。
本研究以较为广泛使用的2种唑类杀菌剂(氟康唑和酮康唑)为研究对象,以蛋白核小球藻为受试生物,以确定三唑类(氟康唑)和咪唑类(酮康唑)杀菌剂对淡水绿藻的潜在毒性作用。实验研究了2种唑类杀菌剂对藻细胞光合色素叶绿素a(Chla)、叶绿素b(Chlb)和类胡萝卜素(Car)的影响,测定了氧化应激相关的变化(超氧化物歧化酶(SOD)和过氧化氢酶(CAT)、活性氧(ROS)和丙二醛(MDA)含量)以及总蛋白(TP)和细胞凋亡。本研究旨在揭示唑类杀菌剂对淡水绿藻的作用机制,为评价和控制水环境中氟康唑和酮康唑以及唑类的潜在风险提供有用的信息。
-
研究选取了三唑类杀菌剂氟康唑(CAS No.86386-73-4,纯度为98.00%)和咪唑类杀菌剂酮康唑(CAS No.65277-42-1,纯度为97.70%),以上药品均购自Toronto Research Chemicals。储备液用Mill-Q水和体积浓度不超过5‰的HPLC级二甲亚砜(DMSO)配制。生理指标测定等试剂盒购自南京建成生物工程研究所。
-
蛋白核小球藻(Chlorella pyrenoidosa,[FACHB]-5)购自中国科学院武汉水生生物研究所淡水藻类菌种库(FACHB),参照OECD 201藻类生长抑制实验方法[21],采用灭菌BG-11营养培养基进行连续培养[22]。藻类的培养温度为22 ℃,光强为2500 lx,光/暗周期为12 h/12 h。选择初始细胞密度约为8×105 cells·mL−1的指数生长期藻类细胞开展实验。
-
根据预实验结果和毒性试验单一目标污染物的EC50,将氟康唑和酮康唑标准品配制成高浓度的储备液,再用BG-11培养基以0.2和0.5的稀释倍数对其逐级稀释,得到梯度浓度试验溶液。
将培养至对数生长期的蛋白核小球藻接种到250 mL锥形瓶中,试验初始藻细胞密度约4×105个cell·mL−1,然后向各锥形瓶中依次加入对应浓度配置好的氟康唑和酮康唑溶液。将加好氟康唑和酮康唑溶液后的实验藻种置于原培养条件下进行暴露处理。每天定时摇晃藻液3—4次并随机更换位置,连续培养至96 h。每24 h在波长为681 nm的光密度(OD681)下用紫外可见分光光度计测定其藻液吸光度[23],并计算不同浓度暴露后的生长抑制率(式1)。实验采用两参数非线性函数Logit(式2)对浓度-抑制率数据进行非线性最小二乘拟合,以确定系数(R2)和均方根误差(RMSE)评价拟合效果[24]。
式中,ODti为第i(i=0,96 h)时刻污染物处理组藻液的OD值,OD0i为i时刻空白对照组藻液的OD值,α和β是Logit函数的位置与斜率参数,E为效应即污染物对绿藻的生长抑制率,C是单个污染物的浓度。
-
在藻类毒性暴露实验的第96 h取30 mL藻液,于4 ℃ 10000 r·min−1下冷冻离心10 min,收集藻细胞。加入1.5 mL 0.05 mol·L−1的磷酸盐缓冲盐溶液(pH为7.41,PBS缓冲液)进行吹打,移入1.5 mL离心管,并迅速用全自动样品快速研磨机于75 Hz、4 ℃下反复研磨10次,每次60 s。将破碎后的藻液于4 ℃、10000 r·min−1下离心10 min,其上清液即为粗酶液,可于低温下保存。
-
叶绿素含量的测定采用乙醇提取法[25]。取经氟康唑和酮康唑暴露96 h后的藻液30 mL,在4 ℃ 10000 r·min−1下冷冻离心10 min,分离藻体细胞,加入15 mL的乙醇(95%)溶液于4 ℃下超声破碎20 min,振荡摇匀,4 ℃黑暗条件下提取24 h后,同上述条件离心10 min,提取上清液。用紫外分光光度计在470、649、665 nm波长下进行测定,计算公式如下:
-
利用1.3.2节中提取的粗酶液作为待测样品测定,计算超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、总蛋白(TP)和丙二醛(MDA)的含量。SOD的活性采用羟胺法测定[26],CAT利用钼酸铵比色法测定[27],TP采用考马斯亮蓝法[28],MDA的含量采用硫代巴比妥酸TBA比色法测定[29]。以上实验均采用南京建成生物工程研究所提供的测试方法完成测定,并根据式(6)计算抑制率。
式中,Cti为第i(i=0,96 h)时刻污染物处理组藻液各项生理指标的浓度,C0i为i时刻空白对照组的藻液各项生理指标的浓度。
-
使用ROS检测试剂盒(南京建成生物工程研究所)提供的方法和Knauert和Knauer[30]采用的化学荧光法改进检测蛋白核小球藻活性氧含量。取经氟康唑和酮康唑暴露96 h后的均匀藻液5 mL转移到离心管,依次对其冷冻离心,洗涤。藻细胞染色后置于电热恒温培养箱中孵育(避光,37 ℃,20 min),每5 min倒置3次,使药物和藻细胞充分混匀。孵育后,用PBS缓冲液冲洗过滤获取待测藻液,用流式细胞仪检测。
-
使用Annexin V-FITC/PI细胞凋亡试剂盒(南京建成生物工程研究所)提供的方法完成蛋白核小球藻细胞凋亡的测定。取经氟康唑和酮康唑暴露96 h后的藻液5 mL转移到离心管后离心处理(10 min,4 ℃,10000 r·min−1),洗涤浓缩,Annexin V-FITC染色。然后置于电热恒温培养箱中孵育20 min(避光,37 ℃),用碘化丙啶(PI)染色获得染色藻液,过滤后用流式细胞仪检测。
-
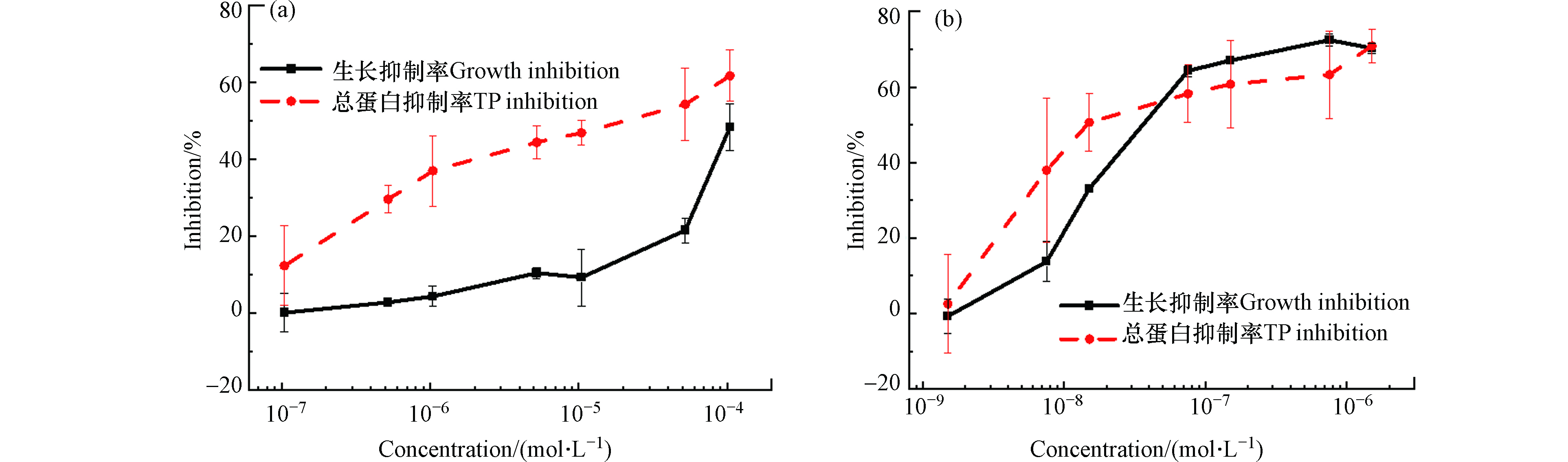
氟康唑和酮康唑对蛋白核小球藻暴露96 h浓度-效应曲线(CRC)如图1所示。通过Logit函数拟合氟康唑和酮康唑对蛋白核小球藻在96 h的浓度-效应数据,其中R2均大于0.90,RMSE均小于0.09,说明Logit函数的拟合效果良好。以pEC50数值(pEC50为EC50的负对数)作为毒性大小指标,氟康唑和酮康唑对蛋白核小球藻暴露96 h毒性大小顺序为酮康唑(7.09)>氟康唑(3.86)。从图1可以看出,氟康唑和酮康唑对蛋白核小球藻的毒性效应从低浓度到高浓度均表现出抑制作用,而且随着浓度升高抑制率增大。
-
氟康唑和酮康唑对蛋白核小球藻暴露96 h后,藻细胞中Chla、Chlb和Car的含量的变化如图2。从图2中可以看出,藻细胞中的叶绿素对不同浓度的氟康唑和酮康唑都作出了相应的响应变化。氟康唑和酮康唑对藻细胞叶绿素的合成抑制率与毒性抑制率整体呈现出良好的正相关(rChla=0.97,rChlb=0.92,rCar=0.97),但是它们响应的程度有所不同。
由图2(a)可知,氟康唑对叶绿素的合成具有抑制作用,随着浓度的提高抑制作用越明显。氟康唑对蛋白核小球藻的Chla的合成具有抑制作用,在浓度为0.00—1.04×10−5 mol·L−1范围内蛋白核小球藻Chla抑制率与氟康唑浓度呈负相关,但整体上还是为抑制作用。图1在浓度区间为0.00—1.04×10−5 mol·L−1范围内氟康唑对蛋白核小球藻抑制不明显,可能是藻类细胞可以使用低浓度的杀真菌剂作为碳源[31],增强光合效率。当氟康唑浓度大于1.04×10−5 mol·L−1时,对Chla抑制随浓度提高而增大。当氟康唑浓度浓度达到5.22×10−5 mol·L−1时,Chla、Chlb和Car含量急剧下降,显著低于空白及低浓度下培养下的蛋白核小球藻,表明在大于此浓度作用下,叶绿素的生理功能丧失,藻类大量死亡。
不同浓度酮康唑对蛋白核小球藻处理96 h后,随着酮康唑浓度的升高,对蛋白核小球藻细胞中Chla、Chlb和Car的抑制增强(图2b)。浓度越高,对合成Chla、Chlb和Car的抑制作用越强,表明酮康唑对蛋白核小球藻Chla、Chlb和Car的合成具有浓度依赖毒性。当酮康唑大于1.51×10−9 mol·L−1时,对藻细胞中Chla、Chlb和Car抑制明显高于空白。从图2还可以得知,在相同浓度下酮康唑对绿藻的毒性比氟康唑大。当酮康唑暴露浓度大于7.53×10−7 mol·L−1时,酮康唑对叶绿素的抑制下降,同样也体现对绿藻生长抑制下降,与图1变化趋势一致,表明叶绿素含量的变化与蛋白核小球藻生长密切相关。
实验结果表明对Chla、Chlb和Car合成的抑制随浓度的升高而增大,经氟康唑和酮康唑处理后的叶绿素和类胡萝卜素的减少呈浓度依赖性,这一结果与Saladin的结论是一致的[32]。此外,唑类还能影响藻细胞光合作用相关基因表达,损害细胞的完整性并破坏叶绿体结构从而抑制藻的活性[33-34],Artigas等[35]实验发现唑类对生物膜功能(抑制底物诱导的呼吸和光合活性)的影响尤其明显。
-
由图3可知,不同浓度的氟康唑和酮康唑胁迫蛋白核小球藻96 h后,随着氟康唑和酮康唑浓度的升高,对蛋白核小球藻的生长及蛋白质合成的抑制呈升高趋势。
在图3(a),当氟康唑浓度在5.22×10−6—1.04×10−5 mol·L−1时,对蛋白质小球藻生长抑制率出现轻微的下降,但总体抑制率对藻的生长和总蛋白均呈上升趋势,随着氟康唑浓度的升高,蛋白核小球藻生长抑制率和总蛋白含量的抑制率也随之升高,且藻细胞生长抑制率与总蛋白含量呈现良好的正相关(r=0.81)。图3(b)中,酮康唑浓度对蛋白核小球藻生长抑制率和总蛋白抑制率呈正相关性(r=0.93),当酮康唑浓度为7.53×10−7 mol·L−1对蛋白核小球藻生长抑制率高达72.46%,而浓度为1.51×10−6 mol·L−1时对藻细胞抑制率为70.31%。表明唑类对总蛋白抑制效果具有浓度依赖性,浓度越高,对蛋白质抑制越明显。有相关报道,唑类抗真菌药物能够以绿藻中一些酶为靶点来抑制相应蛋白质的合成[36]。
-
细胞的保护酶主要有SOD、CAT等[37],本研究检测了在氟康唑和酮康唑胁迫作用下,蛋白核小球藻的SOD和CAT的变化(图4)。如图4(a)所示,随着氟康唑对蛋白核小球藻暴露的浓度增加,对SOD的抑制效果增强,SOD含量的抑制率与蛋白核小球藻的生长抑制率呈正相关(r=0.90)。当氟康唑浓度为1.04×10−4 mol·L−1时对SOD抑制率达47.93%。而对于CAT,随着氟康唑浓度的提高,CAT的活性不断增强,与蛋白核小球藻生长抑制率呈负相关(r=−0.92),相同浓度下对CAT抑制率为−312.79%,此时胁迫程度最大,对蛋白核小球藻抑制率达48.53%。
酮康唑处理对这两种抗氧化酶活性的影响规律与氟康唑类似(图4b),对SOD活性的抑制随酮康唑浓度的增加而升高,呈良好的正相关性(r=0.97)。当酮康唑浓度为7.53×10−7mol·L−1对SOD抑制率达到最大,为96.50%。酮康唑暴露浓度高于7.53×10−7mol·L−1时,对SOD抑制率85.68%,而对蛋白核小球藻生长抑制率也由72.45%降至70.31%,说明大于此浓度藻类大量死亡或者酶系统失活。CAT抑制率与蛋白核小球藻生长抑制率呈负相关性(r=−0.92),高浓度氟康唑、酮康唑处理蛋白核小球藻时对藻细胞内CAT活性表现为促进,CAT活性的增加以及SOD活性的降低表明氟康唑、酮康唑可能通过诱导氧化应激而伤害蛋白核小球藻[38]。产生这种原因可能是绿藻受到胁迫后产生大量自由基,在氟康唑、酮康唑刺激下藻细胞内一些抗氧化酶(CAT)被激活,CAT能清除分子结构里带有亲电基团的化合物[39],从而清除藻细胞内过量积累的ROS,提高绿藻抗逆性[40]。氟康唑、酮康唑浓度提高后CAT活性的迅速升高,表明了氟康唑、酮康唑在蛋白核小球藻体内具有较高的清除率。随着处理蛋白核小球藻的氟康唑和酮康唑浓度越高,对细胞内SOD的活力抑制越强,但对CAT酶活性具有促进作用,说明在唑类污染物胁迫下藻细胞内产生了ROS和氧化性损伤,对细胞中MDA含量检测也进一步验证了高浓度的唑类污染物会加剧藻细胞脂质过氧化的程度。
-
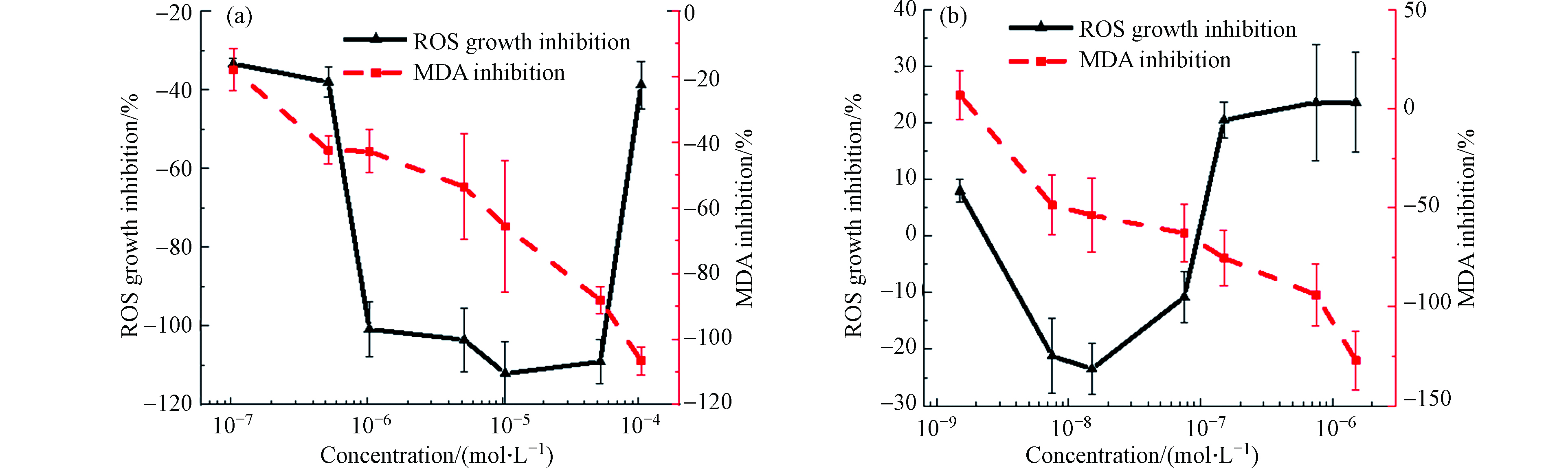
如图5(a)所示,不同浓度梯度的氟康唑胁迫蛋白核小球藻96 h后,藻细胞内ROS的含量随氟康唑的升高而增加,对藻细胞内ROS的产生促进作用增强。氟康唑浓度分别为5.22×10−7、1.04×10−6 mol·L−1时,与对照组相比,对藻细胞内ROS含量抑制率分别为−38.08%,−100.84%。藻细胞内ROS水平均明显升高,表明低浓度氟康唑能够引起蛋白核小球藻细胞内活性氧水平升高,使藻细胞产生氧化胁迫。浓度升高,抑制率增大,ROS水平与藻细胞生长抑制率整体上呈为正相关性(r=0.84),藻细胞在逆环境下活性氧大量产生,对细胞产生毒害作用。当浓度为1.04×10−5 mol·L−1对ROS生成的促进最大,抑制率达−112.10%。但随着浓度进一步提高,对细胞内ROS促进减弱,ROS的浓度开始降低,此时ROS抑制率与藻细胞毒性相关性为正,可能原因是ROS大量累积引起细胞稳态失衡,部分细胞裂解或死亡。也可能是在逆环境下触发了细胞内的抗氧化机制,清除细胞内过量的ROS,降低了细胞内ROS的浓度。氟康唑对MDA含量的影响随呈正相关,MDA随氟康唑浓度的升高而升高,蛋白核小球藻毒性抑制率与MDA抑制率呈负相关性(r=−0.91)。
不同浓度梯度的酮康唑胁迫蛋白核小球藻96 h时的ROS和MDA含量影响如图5(b)所示。酮康唑浓度为1.51×10−9—7.53×10−8 mol·L−1范围内对藻细胞ROS呈促进作用,酮康唑浓度为1.51×10−8 mol·L−1对ROS促进效果最强。当浓度超过此浓度时,藻细胞内对ROS生成抑制增强,抑制效果随浓度的增高而增强。在整个浓度区间上ROS抑制率与藻细胞毒性抑制率之间呈正相关关系(r=0.60),主要原因是增长的ROS引起了藻细胞内抗氧化酶活性响应,清除了部分ROS。随着酮康唑浓度的升高,对ROS的生成不是单调的促进或抑制,有相关报道表明,在逆境条件下,植物中的ROS的生成和自我清除机制失衡会改变CAT和SOD在内的几种抗氧化酶的活性[41]。MDA的含量随酮康唑浓度的提高而增加,呈现良好的的负相关(r=−0.91),在1.51×10−6 mol·L−1时促进最大,表明细胞膜发生了氧化应激,产生了大量的MDA。
-
细胞凋亡是细胞为维持内环境稳定,自主进行的有序死亡[42]。实验中通过细胞凋亡抑制率来评估氟康唑和酮康唑对蛋白核小球藻的毒性作用,如图6所示。
不同浓度的氟康唑(a)和酮康唑(b)对藻细胞活性产生了不同程度的影响,浓度越高,对藻细胞生长抑制越大。其中细胞凋亡抑制率与绿藻的生长抑制率变化程度相关(r氟康唑=−0.97,r酮康唑=−0.94),即对细胞生长抑制率越高,细胞凋亡越明显。细胞凋亡是由多种因素共同作用的结果,除内坏境失衡外,有研究表明,光合色素和蛋白含量下降也会对藻的光合系统和细胞的相关代谢结构产生破坏作用[43],最终导致细胞凋亡。结合图1中浓度-效应关系以及图6中浓度-细胞凋亡抑制率对氟康唑和酮康唑毒性进行定量分析,可知蛋白核小球藻对高浓度酮康唑(1.51×10−7 mol·L−1)比低浓度氟康唑(5.22×10−7 mol·L−1)更为敏感,对蛋白核小球藻生长抑制率分别为67.06%,2.83%,藻细胞的细胞凋亡抑制率分别为−2939.20%,−25.52%。表明酮康唑毒性比氟康唑大。
氟康唑(图7a)和酮康唑(图7b)对蛋白核小球藻暴露96 h后用流式细胞仪检测藻细胞凋亡结果如图7所示。由图7可以看出,经氟康唑和酮康唑处理后的藻细胞出现明显的细胞凋亡现象。
-
目前,唑类在水生生态环境中的存在对水生生物及非目标生物(人类)的持续影响受到普遍关注。曾有研究报道单一及多元混合唑类对绿藻的急性毒性[44],但对绿藻生理生化响应机制的研究信息有限,可能通过多种途径抑制藻类的生长。实验通过使蛋白核小球藻暴露于不同浓度的氟康唑和酮康唑96 h后,研究对藻细胞的生理(如光合作用(Chla、Chlb和Car),细胞膜稳定性(ROS和MDA)、生化(SOD、CAT和TP)机制和细胞凋亡的影响。由于唑类杀菌剂对蛋白核小球藻的致毒机理涉及多个指标,且各生理指标响应成因复杂。因此,借助于模型的手段,建立基于藻细胞的毒性-生理生化指标响应的模型。本研究通过主成分分析(PCA)对蛋白核小球藻各指标进行分析,以毒性抑制率为因变量(y),以各机理指标为自变量(x),建立PCR线性回归模型(表1),通过最佳主成分(PC)解释毒性机理。
从表1可以看出,PCR模型的R2均大于0.89,RMSE均小于0.05,RMSP均小于0.05,说明PCR拟回归模型的预测能力较优。从表1氟康唑的线性回归模型可知,对藻细胞内TP、SOD和叶绿素(Chla、Chlb和Car)的抑制越高,对蛋白核小球藻的生长抑制越大,而ROS、MDA含量越低,CAT和CAT活性越高,越有利藻细胞生长,细胞凋亡抑制率越低。同理可知,酮康唑对蛋白核小球藻毒性抑制率与TP、ROS、SOD和叶绿素(Chla、Chlb和Car)的抑制率呈正比,与MDA、CAT和Apoptosis的抑制率呈反比。这个线性回归模型预测与实验结果的相关性分析一致,说明该最优模型可信度较高。
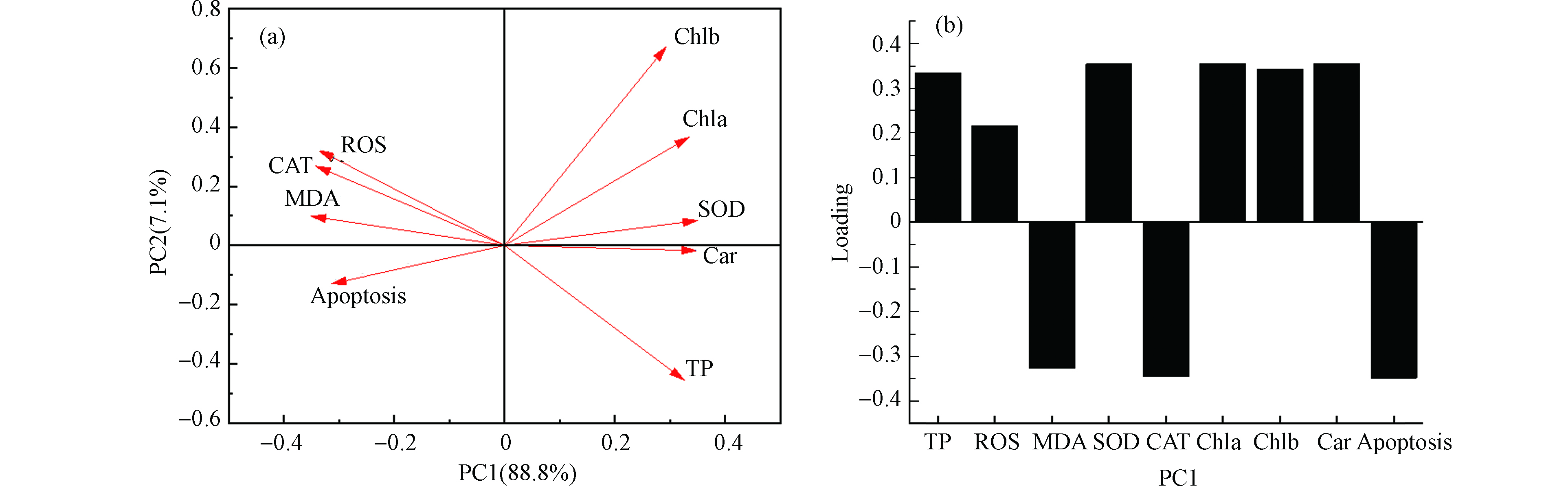
图8(a)是氟康唑胁迫蛋白核小球藻96 h的PCA分析结果,PC1和PC2分别描述了总方差的88.8%和7.1%。前两个主成分累计占总方差的95.9%,能够反映数据的大部分信息。其中叶绿素(Chla、Chlb、Car)、SOD和TP在PC1上具有较高的载荷,表明叶绿素、SOD和TP指标对毒性抑制率的贡献最大。PC2在Chla、Chlb、ROS和CAT上最具有代表性。在相关性分析的基础上,对各机理指标进行聚类分析,在PC1的维度上,Chla可以和Chlb、Car、SOD归为一类(Chla与Chlb、Car之间的相关性分别为0.92、0.95、0.96),Car和SOD为一类(Car与SOD相关性为0.97)。对于PC2,Chla、Chlb、ROS和CAT之间相关性强,可以划为一类,但PC2占的总体方差的信息量较少(7.1%),可以忽略不计。氟康唑对蛋白核小球藻毒性抑制率的影响主要在PC1这个维度上,说明各生理指标间对毒性大小贡献差异较大。对于酮康唑,由表1可知,PC=1时能概括所构成的信息量占总信息量的98.89%,具有良好的说明性。由图8(b)可以看出,叶绿素(Chla,Chlb,Car)、SOD和TP均有较高的载荷,说明叶绿素、SOD和TP对毒性抑制率的贡献较大。
-
综上所述,氟康唑和酮康唑对蛋白核小球藻暴露96 h的毒性顺序大小为酮康唑>氟康唑。氟康唑和酮康唑对蛋白核小球藻暴露96 h后,绿藻细胞活性随暴露浓度的升高而降低,浓度越高,毒性越大,细胞凋亡的程度越严重。不同浓度的氟康唑和酮康唑对蛋白核小球藻的生长均有抑制效果,具有浓度依赖性。高浓度的氟康唑(≥1.04×10−5 mol·L−1)和酮康唑(≥7.53×10−9 mol·L−1)对Chla、Chlb和Car均表现出抑制作用,使其光合效率降低。蛋白核小球藻SOD活性随氟康唑和酮康唑浓度的增高而降低,表明对藻细胞的氧化损伤加剧,而CAT酶在歧化反应生成物的刺激下,活性增强。氟康唑和酮康唑对藻细胞总蛋白合成具有抑制作用,同时使得藻细胞处于氧化应激的状态,藻细胞内MDA水平持续增高,提高氟康唑浓度会对加剧藻细胞脂质过氧化受损,ROS水平急剧增加。
蛋白核小球藻对氟康唑和酮康唑的生理生化响应
Mechanisms of physiological and biochemical responses of fluconazole and ketoconazole to Chlorella pyrenoidosa
-
摘要: 氟康唑和酮康唑是水生环境中普遍存在两种唑类杀菌剂,对水生生态系统具有潜在的生态风险。目前,它们对绿藻的作用机理研究较为缺乏。本研究目的是揭示氟康唑和酮康唑暴露蛋白核小球藻96 h后对藻细胞生长、叶绿素、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、总蛋白(TP)、丙二醛(MDA)、活性氧(ROS)含量以及细胞凋亡的生理生化响应机制。结果表明,两种物质暴露96 h后对藻细胞各组分损伤随浓度提高而增大,且酮康唑毒性比氟康唑大。氟康唑和酮康唑对叶绿素合成具有抑制作用,呈浓度依赖性。对SOD活性和TP含量具有显著抑制,其中酮康唑浓度为7.53×10-7 mol·L-1对SOD抑制率达96.50%。氟康唑和酮康唑对CAT活性均具有促进作用。氟康唑和酮康唑可以刺激藻细胞内ROS的含量增加,但高浓度酮康唑对ROS的产生具有抑制作用。MDA含量随胁迫藻细胞的氟康唑和酮康唑浓度的上升而升高。氟康唑和酮康唑浓度越大对藻细胞活性影响越大,出现细胞凋亡现象越明显。该研究结果将为氟康唑和酮康唑对水生生态系统影响的潜在风险评估提供科学依据。Abstract: Fluconazole and ketoconazole are two azole pollutants commonly found in the aquatic environment. They have the potential ecological risk to aquatic ecosystems. Currently, there is little research on their mechanism of action on green algae. The purpose of this study was to reveal mechanisms of physiological and biochemical responses of fluconazole and ketoconazole exposed to Chlorella pyrenoidosa at 96 h. The physiological and biochemical parameters include the algae cell growth, chlorophyll superoxide dismutase (SOD), catalase (CAT), total protein (TP), the content of malondialdehyde (MDA), reactive oxygen species (ROS), and cell apoptosis. The results showed that the damage to cell components increased with the increasing concentration of two compounds after exposure for 96 hours. Ketoconazole was more toxic than fluconazole. The inhibitory effects of fluconazole and ketoconazole on chlorophyll synthesis were concentration-dependent. The SOD activity and TP content were significantly inhibited, where 7.53×10-7 mol·L-1 ketoconazole inhibits SOD up to 96.50%. Both fluconazole and ketoconazole had catalytic effects on CAT activity. Fluconazole and ketoconazole could stimulate the increase of ROS content in algal cells, but the high concentration of ketoconazole could inhibit ROS production. MDA content increased with the increasing concentrations of fluconazole and ketoconazole. The higher the concentrations of fluconazole and ketoconazole, the greater the effects on the activity of algal cells and the more obvious the phenomenon of apoptosis. The results of this study may provide a scientific basis for potential risk assessment of fluconazole and ketoconazole impacts on aquatic ecosystems.
-
Key words:
- fluconazole /
- ketoconazole /
- Chlorella pyrenoidosa /
- toxic mechanism
-

-
表 1 主成分分析的模型统计量
Table 1. Model statistics for principal component analysis
化合物
Compound模型
Model模型参数
Model parameters氟康唑 y=−0.0053+0.0493TP−0.0189ROS
−0.0580MDA+0.1153SOD
-0.0131CAT+0.2447Chla
+0.2076Chlb+0.2931Car
−0.0183ApoptosisPC=2
R2=0.90,RMSE=0.05
F=17.62
q2=0.74,RMSP=0.08酮康唑 y=−0.0128+0.1432TP
+0.1015ROS−0.0777MDA
+0.1211SOD
−0.0428CAT+0.1819Chla
+0.2110Chlb+0.1506Car
−0.0024ApoptosisPC=1
R2=0.99,RMSE=0.03 F=447.39
q2=0.99,RMSP=0.03注:PC和R2分别是最佳主成分和确定系数;RMSE和RMSP分别是估计均方根误差和留一法(LOO)交叉验证均方根误差;F和q2分别是Fischer统计量和LOO交叉验证相关系数.
Note: PC and R2 are the best principal components and coefficient of determination, respectively; RMSE and RMSP are the estimated root mean square error and the root mean square error of the leave-one-out (LOO) cross-validation, respectively; F and q2 are Fischer statistic and LOO cross-validation correlation coefficient, respectively. -
[1] BHAGAT J, SINGH N, NISHIMURA N, et al. A comprehensive review on environmental toxicity of azole compounds to fish [J]. Chemosphere, 2021, 262: 128335. doi: 10.1016/j.chemosphere.2020.128335 [2] CHEN Z F, YING G G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review [J]. Environment International, 2015, 84: 142-153. doi: 10.1016/j.envint.2015.07.022 [3] PENG X Z, HUANG Q X, ZHANG K, et al. Distribution, behavior and fate of azole antifungals during mechanical, biological, and chemical treatments in sewage treatment plants in China [J]. Science of the Total Environment, 2012, 426: 311-317. doi: 10.1016/j.scitotenv.2012.03.067 [4] BRAUER V S, REZENDE C P, PESSONI A M, et al. Antifungal agents in agriculture: Friends and foes of public health [J]. Biomolecules, 2019, 9(10): 521. doi: 10.3390/biom9100521 [5] KLEINKAUF N, VERWEIJ P E, ARENDRUP M C, et al. Risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species. Stockholm: ECDC; 2013 [R]. European Centre for Disease Prevention and Control Technical Report, 2013 Stockholm: ECDC, 2013: 8-13. [6] ROCCHI S, REBOUX G, MILLON L. Résistance Aux antifongiques azolés d’origine environnementale: Quelles alternatives pour l’avenir ? [J]. Journal De Mycologie Médicale, 2015, 25(4): 249-256. [7] SHARMA A, KUMAR V, SHAHZAD B, et al. Worldwide pesticide usage and its impacts on ecosystem [J]. SN Applied Sciences, 2019, 1(11): 1446. doi: 10.1007/s42452-019-1485-1 [8] PRICE C L, PARKER J E, WARRILOW A G, et al. Azole fungicides - understanding resistance mechanisms in agricultural fungal pathogens [J]. Pest Management Science, 2015, 71(8): 1054-1058. doi: 10.1002/ps.4029 [9] LINHART C, PANZACCHI S, BELPOGGI F, et al. Year-round pesticide contamination of public sites near intensively managed agricultural areas in South Tyrol [J]. Environmental Sciences Europe, 2021, 33(1): 1-12. doi: 10.1186/s12302-020-00446-y [10] BARBIERI M V. Pesticides in the environment: analysis, occurrence, impact and recommendations for their attenuation [D]. Barcelona: University of Barcelona, 2021.13-18. [11] LETZEL M, METZNER G, LETZEL T. Exposure assessment of the pharmaceutical diclofenac based on long-term measurements of the aquatic input [J]. Environment International, 2009, 35(2): 363-368. doi: 10.1016/j.envint.2008.09.002 [12] GARCÍA-VALCÁRCEL A I, TADEO J L. Influence of moisture on the availability and persistence of clotrimazole and fluconazole in sludge-amended soil [J]. Environmental Toxicology and Chemistry, 2012, 31(3): 501-507. doi: 10.1002/etc.1711 [13] FROMTLING R A. Overview of medically important antifungal azole derivatives [J]. Clinical Microbiology Reviews, 1988, 1(2): 187-217. doi: 10.1128/CMR.1.2.187 [14] AZEVEDO M M, FARIA-RAMOS I, CRUZ L C, et al. Genesis of azole antifungal resistance from agriculture to clinical settings [J]. Journal of Agricultural and Food Chemistry, 2015, 63(34): 7463-7468. doi: 10.1021/acs.jafc.5b02728 [15] CREUSOT N, CASADO-MARTINEZ C, CHIAIA-HERNANDEZ A, et al. Retrospective screening of high-resolution mass spectrometry archived digital samples can improve environmental risk assessment of emerging contaminants: A case study on antifungal azoles [J]. Environment International, 2020, 139: 105708. doi: 10.1016/j.envint.2020.105708 [16] CHIAIA-HERNÁNDEZ A C, SCHERINGER M, MÜLLER A, et al. Target and suspect screening analysis reveals persistent emerging organic contaminants in soils and sediments [J]. Science of the Total Environment, 2020, 740: 140181. doi: 10.1016/j.scitotenv.2020.140181 [17] CHEN Z F, YING G G, MA Y B, et al. Typical azole biocides in biosolid-amended soils and plants following biosolid applications [J]. Journal of Agricultural and Food Chemistry, 2013, 61(26): 6198-6206. doi: 10.1021/jf4013949 [18] SUBBIAH S, RAMESH M, ASHOKAN A P, et al. Acute and sublethal toxicity of an azole fungicide tebuconazole on ionic regulation and Na+/K+-ATPase activity in a freshwater fish Cirrhinus mrigala [J] International Journal of Fisheries and Aquatic Studies 2020; 8(3): 361-371. [19] ASSRESS H A, NYONI H, MAMBA B B, et al. Occurrence and risk assessment of azole antifungal drugs in water and wastewater [J]. Ecotoxicology and Environmental Safety, 2020, 187: 109868. doi: 10.1016/j.ecoenv.2019.109868 [20] 黄秋鑫, 王志方, 王春维, 等. 珠江三角洲城市污水及其接纳水体中唑类抗真菌药物的手性特征[C]//2015年中国环境科学学会学术年会论文集. 深圳, 2015: 930-939. HUANG Q X, WANG Z F, WANG C W, et al. Chiral characteristics of azole antifungal drugs in urban sewage and receiving waters in the Pearl River Delta[C]//. Proceedings of the 2015 Annual Conference of the Chinese Society for Environmental Sciences. Shenzhen, 2015: 930-939 ( in Chinese).
[21] GENERAL C. Test No. 201: Freshwater alga and cyanobacteria, growth inhibition test [J]. OECD Guidelines for the Testing of Chemicals, 2006, 1(2): 1-1. [22] BARAHOEI M, HATAMIPOUR M S, AFSHARZADEH S. Direct brackish water desalination using Chlorella vulgaris microalgae [J]. Process Safety and Environmental Protection, 2021, 148: 237-248. doi: 10.1016/j.psep.2020.10.006 [23] ZHONG X Q, ZHU Y L, WANG Y J, et al. Effects of three antibiotics on growth and antioxidant response of Chlorella pyrenoidosa and Anabaena cylindrica [J]. Ecotoxicology and Environmental Safety, 2021, 211: 111954. doi: 10.1016/j.ecoenv.2021.111954 [24] 刘树深, 张瑾, 张亚辉, 等. APTox: 化学混合物毒性评估与预测 [J]. 化学学报, 2012, 70(14): 1511-1517. doi: 10.6023/A12050175 LIU S S, ZHANG J, ZHANG Y H, et al. APTox: assessment and prediction on toxicity of chemical mixtures [J]. Acta Chimica Sinica, 2012, 70(14): 1511-1517(in Chinese). doi: 10.6023/A12050175
[25] WANG X, HUANG J. Principles and techniques of plant physiological biochemical experiment [M]. Beijing: Higher Education Pres, 2006 . 260-267. [26] TAN S, HU X L, YIN P H, et al. Photosynthetic inhibition and oxidative stress to the toxic Phaeocystis globosa caused by a diketopiperazine isolated from products of algicidal bacterium metabolism [J]. Journal of Microbiology, 2016, 54(5): 364-375. doi: 10.1007/s12275-016-6012-0 [27] HUO D, SUN L N, RU X S, et al. Impact of hypoxia stress on the physiological responses of sea cucumber Apostichopus japonicus: Respiration, digestion, immunity and oxidative damage [J]. PeerJ, 2018, 6: e4651. doi: 10.7717/peerj.4651 [28] BRADFORD M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [J]. Analytical Biochemistry, 1976, 72(1/2): 248-254. [29] WANG F, WANG B, QU H, et al. The influence of nanoplastics on the toxic effects, bioaccumulation, biodegradation and enantioselectivity of ibuprofen in freshwater algae Chlorella pyrenoidosa [J]. Environmental Pollution, 2020, 263: 114593. doi: 10.1016/j.envpol.2020.114593 [30] KNAUERT S, KNAUER K. The role of reactive oxygen species in copper toxicity to two freshwater green algae(1) [J]. Journal of Phycology, 2008, 44(2): 311-319. doi: 10.1111/j.1529-8817.2008.00471.x [31] NONG Q Y, LIU Y A, QIN L T, et al. Toxic mechanism of three azole fungicides and their mixture to green alga Chlorella pyrenoidosa [J]. Chemosphere, 2021, 262: 127793. doi: 10.1016/j.chemosphere.2020.127793 [32] SALADIN G, MAGNÉ C, CLÉMENT C. Effects of fludioxonil and pyrimethanil, two fungicides used against Botrytis cinerea, on carbohydrate physiology in Vitis vinifera L [J]. Pest Management Science, 2003, 59(10): 1083-1092. doi: 10.1002/ps.733 [33] XI J J, SHAO J, WANG Y, et al. Acute toxicity of triflumizole to freshwater green algae Chlorella vulgaris [J]. Pesticide Biochemistry and Physiology, 2019, 158: 135-142. doi: 10.1016/j.pestbp.2019.05.002 [34] LIU R, DENG Y, ZHANG W J, et al. Enantioselective mechanism of toxic effects of triticonazole against Chlorella pyrenoidosa [J]. Ecotoxicology and Environmental Safety, 2019, 185: 109691. doi: 10.1016/j.ecoenv.2019.109691 [35] ARTIGAS J, PASCAULT N, BOUCHEZ A, et al. Comparative sensitivity to the fungicide tebuconazole of biofilm and plankton microbial communities in freshwater ecosystems [J]. Science of the Total Environment, 2014, 468/469: 326-336. doi: 10.1016/j.scitotenv.2013.08.074 [36] ZHOU W X, DEBNATH A, JENNINGS G, et al. Enzymatic chokepoints and synergistic drug targets in the sterol biosynthesis pathway of Naegleria fowleri [J]. PLoS Pathogens, 2018, 14(9): e1007245. doi: 10.1371/journal.ppat.1007245 [37] COLODETE C M, RUAS K F, BARBIRATO J, et al. Biochemistry characterization of proteins defense against oxidative stress in plants and their biosynthetic pathways of secondary metabolites [J] Natureza on line, 2015, 13: 195-204. [38] LIU L, ZHU B, WANG G X. Azoxystrobin-induced excessive reactive oxygen species (ROS) production and inhibition of photosynthesis in the unicellular green algae Chlorella vulgaris [J]. Environmental Science and Pollution Research, 2015, 22(10): 7766-7775. doi: 10.1007/s11356-015-4121-7 [39] NEMAT ALLA M M, HASSAN N M. Changes of antioxidants levels in two maize lines following atrazine treatments [J]. Plant Physiology and Biochemistry, 2006, 44(4): 202-210. doi: 10.1016/j.plaphy.2006.05.004 [40] LI F M, LIANG Z, ZHENG X, et al. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production [J]. Aquatic Toxicology, 2015, 158: 1-13. doi: 10.1016/j.aquatox.2014.10.014 [41] ZHANG W J, CHENG C, CHEN L, et al. Enantioselective toxic effects of cyproconazole enantiomers against Chlorella pyrenoidosa [J]. Chemosphere, 2016, 159: 50-57. doi: 10.1016/j.chemosphere.2016.05.073 [42] KURT O, ÖZDAL-KURT F, AKÇORA C, et al. Neurotoxic, cytotoxic, apoptotic and antiproliferative effects of some marine algae extracts on the NA2B cell line [J]. Biotechnic & Histochemistry, 2018, 93(1): 59-69. [43] WU Y, WANG Y J, LI Y W, et al. Effects of single-walled carbon nanotubes on growth and physiological characteristics of Microcystis aeruginosa [J]. Journal of Central South University, 2018, 25(7): 1628-1641. doi: 10.1007/s11771-018-3855-z [44] 农琼媛, 覃礼堂, 莫凌云, 等. 抗生素与三唑类杀菌剂混合物对羊角月牙藻的长期毒性相互作用研究 [J]. 生态毒理学报, 2019, 14(4): 140-149. NONG Q Y, QIN L T, MO L Y, et al. The toxic interactions of long-term effects involving antibiotics and triazole fungicides on Selenastrum capricornutum [J]. Asian Journal of Ecotoxicology, 2019, 14(4): 140-149(in Chinese).
-




 下载:
下载: