-
作为21世纪三大科学之一的纳米科学,从20世纪80年代以来逐渐成为科学研究的热点. MNPs具有独特的物理化学特性,近年来在多个领域广泛应用,据估计MNPs年产量接近100万吨[1]. 随着MNPs的大量涌现和广泛应用,关于MNPs的环境行为及其生态毒性引起了公众的高度关注. 2003年Science[2]和Nature[3]杂志相继发表文章探讨纳米颗粒存在的安全问题以及对环境和人类健康的影响,从此,MNPs对环境及生物的毒性效应研究逐渐成为国内外的研究热点.
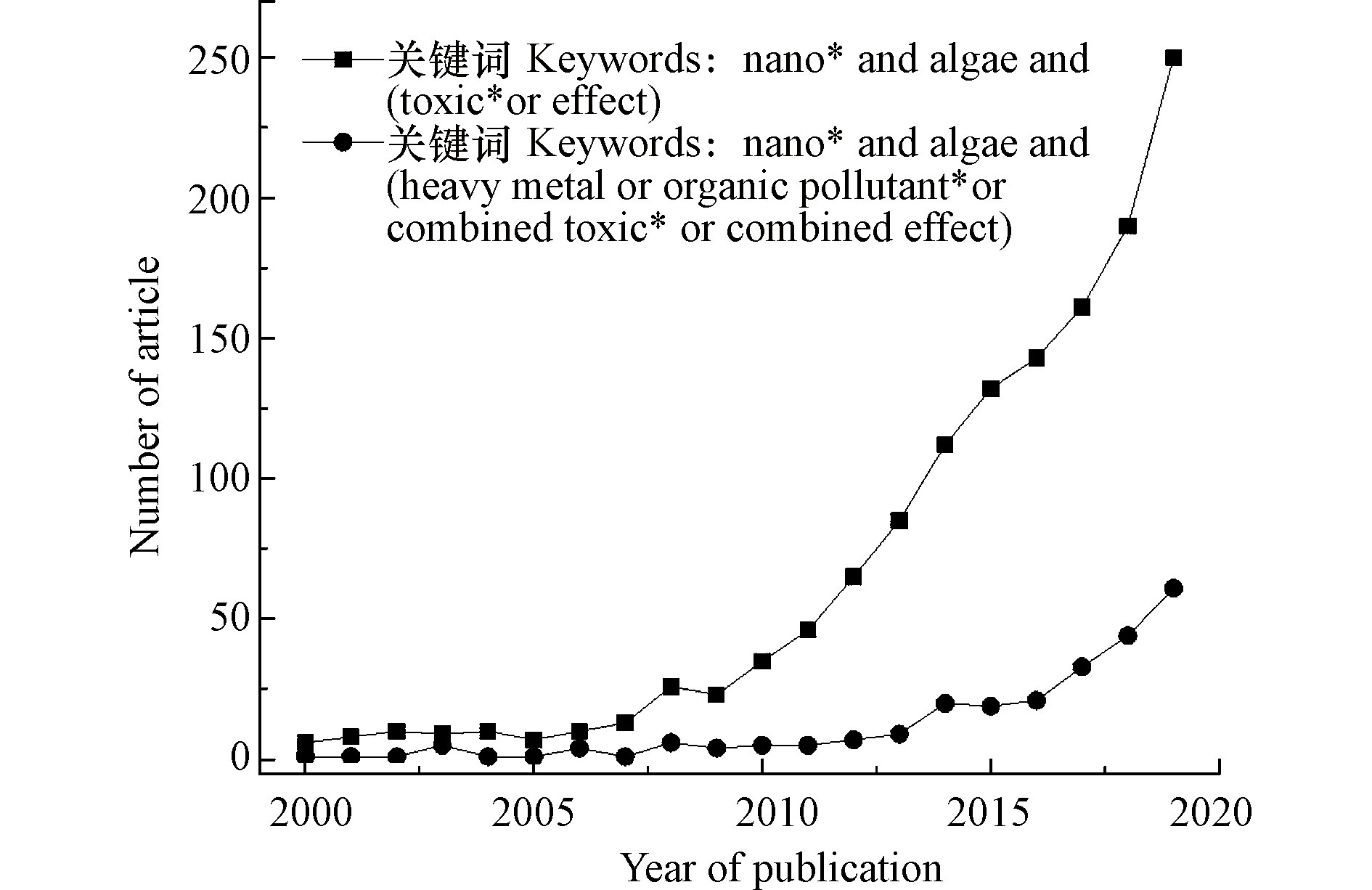
随着MNPs生产和使用的增加,其在环境中的释放量也越来越多,尤其对于水环境,水环境是MNPs的主要汇集地. 在水环境中MNPs会经历各种物理化学反应,且会受到各种因素的影响(如MNPs的物化特性和环境条件).MNPs暴露于水环境中,势必会与水生生物接触,进而被水生生物吸收,并对其产生系列影响. 藻类是水环境中的主要生产者,由于其在MNPs存在时的EC50(在规定暴露时间内产生50%生长抑制率的浓度)值低,即对MNPs敏感性高,而被作为研究MNPs生态影响的典型模型生物之一[4-5]. MNPs被藻类吸收后会随着食物链传递,最终暴露于人类,因此研究MNPs对藻类的毒性效应及效应机制具有重要意义. 近年来MNPs对藻类的毒性研究得到了迅速发展,特别是从2010年以来,相关研究的发文量直线提升,表明MNPs安全性问题特别是藻毒性受到公众的普遍关注(图1).
从图1可以看出,目前已广泛研究单一MNPs对藻类的毒性效应,而尚未充分开展MNPs与环境中共存污染物共同暴露对藻类的复合效应相关研究. 真实环境中,生物往往同时暴露于多种污染物,因此对不同污染物质及多种MNPs共同暴露进行风险评估至关重要[6]. 因此,本文重点介绍了MNPs与环境中共存污染物对藻类的复合效应. 随着组学技术的发展,MNPs对藻类毒性效应的机理研究进入了一个新阶段,蛋白质组学[7]、基因组学[8]、代谢组学[9]、转录组学[10]等组学技术是进一步了解MNPs毒理学的关键,但是目前关于MNPs对藻类的毒性机理的认识并不全面. 此外,对MNPs在环境中的长期动态变化的认知也不够深入. 因此,应该发挥多组学的优势,与传统的生物终端指标结合,探索MNPs的毒性机理,以进一步认识MNPs的安全性问题. 本文系统地综述了MNPs的环境行为、MNPs毒性的影响因素、不同MNPs对藻类的毒性效应和毒性机制、MNPs与环境中共存污染物的联合效应,并对目前相关研究中存在的问题进行了总结及就今后的研究方向进行了展望,希望能有助于理解MNPs在水环境中的风险,促进纳米技术的可持续发展.
全文HTML
-
按照美国试验与材料协会 (the American Society for Testing and Materials) 的定义,纳米颗粒(nanoparticles,NPs)为至少在一个维度上小于100 nm的颗粒[11]. NPs因其纳米粒径而具有独特的小尺寸效应、表面效应、宏观量子隧道效应、量子尺寸效应等,并在日用品、农业、光电、生物、医学、环境、能源等多个领域得到广泛应用[12-15]. NPs是天然或人造的小颗粒,按来源可分为天然纳米颗粒(natural nanoparticles,NNPs)和人工纳米颗粒(manufactured nanoparticles,MNPs),按化学组成可分为金属基NPs(包括Ag、Zn、Cu等零价金属NPs和TiO2、ZnO、CeO2等金属氧化物NPs)、碳基NPs(如石墨烯、富勒烯和碳纳米管)、量子点(quantum dots,QDs)和有机聚合物等其他纳米颗粒. 根据纳米技术消费品目录,32个国家或机构的622家公司中含有MNPs的产品共有1814种,其中金属及金属氧化物纳米颗粒(如Ag、TiO2、SiO2和ZnO)是占比最大(37%)的纳米材料[16]. 据预测到2022年,全球纳米材料市场值将从2015年的147亿美元增长到550亿美元,从2017年到2024年纳米材料市场的年增长率预计为17%[17].
-
随着MNPs的大量使用,其会通过大气沉降、地表径流等途径在生产、运输、使用及处理处置等过程中释放到环境介质土壤和水中. 相比于土壤,MNPs在水环境中具有更强的迁移能力,影响范围也更广,且水环境是MNPs进入其他环境介质的连接点,是MNPs的主要汇集地[18]. 因此,探究MNPs在水环境中的行为至关重要. 目前,对MNPs的环境行为的研究也集中于水环境. MNPs的环境行为是了解其对环境健康影响的基础,且是对其进行风险评估的关键. 了解MNPs的源、汇及转化途径,能够进一步认识MNPs对环境及生物体的特定危害,如MNPs在水生环境食物网中的生物蓄积能力.
MNPs释放出来要经历多种转化过程,主要取决于MNPs的固有性质(颗粒尺寸、表面性质、颗粒浓度和类型)和水化学条件(天然有机质(natural organic matters,NOM)、pH、温度和离子强度). MNPs的水环境行为主要为物理(聚集、团聚和沉积)、化学(光化学反应、溶解和氧化还原反应)或生物(生物降解和生物修饰)转化. MNPs在水环境中的行为根据纳米颗粒的性质和环境条件(例如pH,离子强度以及NOM)的不同而不同[19]. 转化前后的MNPs都有可能被水生生物吸收,并在其体内积累. 因此,MNPs在水环境中的存在会对水生生物产生影响,特别是水生微生物、藻类和水生植物、水生无脊椎动物和鱼类,并最终影响人类健康. 总之,随着MNPs的广泛生产和使用,会使其暴露于环境,特别是水环境中. 即使90%的MNPs在进入水体后10—100 h会通过沉积、团聚等过程从水环境中去除[20],但仅通过短时间的暴露,MNPs就会与水生生物相互作用,从而被水生生物吸收,并会通过食物链暴露于人类[21]. 虽然目前已经知道MNPs在水环境中的转化取决于MNPs的性质和环境因素,但是这两个因素的可变性和复杂性使得进一步理解和预测MNPs的环境行为极具挑战性.
1.1. 人工纳米颗粒概述
1.2. 人工纳米颗粒的环境行为
-
MNPs在水环境中的毒性受到多种因素的影响,主要有纳米颗粒的物理化学特性和环境因素.
-
人工纳米颗粒的理化特性在调节MNPs的环境行为方面起着关键的作用,会影响MNPs的毒性机制[22]. 纳米毒理学研究中一项重要的工作就是表征纳米颗粒的物理化学特性. 了解纳米颗粒的物理化学性质对了解纳米颗粒的环境行为、归宿及与其他共存污染物的相互作用至关重要. MNPs的物理化学特性(例如颗粒的粒径、表面性质与溶解度)会影响MNPs的毒性.
粒径对MNPs的性能及生态毒性具有很大的影响,粒径的改变不仅会使表面积与体积比及表面活性随之改变,而且会影响MNPs在生物表面上的附着效率和沉积效率. Angel 等发现CeO2 NPs比CeO2微米颗粒(micron particles,MPs)的毒性更大[23],Carlson等也发现15 nm AgNPs与55 nm AgNPs相比,使吞噬细胞产生更多的活性氧(reactive oxygen species,ROS)[24]. 20—80 nm的AgNPs的毒性效应主要由其溶解释放的Ag+引起,而与Ag+相比,10 nm的AgNPs更容易被生物体利用,毒性更大[25]. MNPs进入藻类是一个复杂的过程,涉及与细胞壁和细胞膜的相互作用. 尺寸较小的MNPs可以穿透细胞壁,而较大的颗粒会受到限制[26]. 粒径在30—50 nm的MNPs与细胞膜受体有效地相互作用,从而被内在化,而其他粒径的MNPs被摄取减少[27]. 总体来说,纳米颗粒尺寸越小,生物毒性越大. 表面电荷控制MNPs的稳定性,进而控制MNPs的团聚和毒性[22]. MNPs的表面涂层(如PbS NPs和SiO2 NPs表面的聚合物和Al2O3涂层)会降低颗粒之间的相互作用,提高颗粒的稳定性,降低其对藻细胞的毒性效应[28]. 金属NPs的溶解也是影响其毒性效应的关键因素,如AgNPs、CuO NPs、CaO NPs和ZnO NPs[29-32]. 此外,MNPs的浓度、光化学特性及结晶结构等都会影响其生态毒性.
-
MNPs在水体中的环境行为会受到水化学条件(pH、离子强度和天然有机物)、光照、风速、水流等环境因素的影响[33]. 其中水化学条件是影响MNPs悬浮的主要因素. 水环境中的pH值会影响MNPs表面的电荷分布[34]. MNPs的稳定性取决于pH与零电势点(pHzpc)的差值,二者差值较大时,MNPs表面电荷增多,不易团聚,体系较稳定,反之亦然. 如TiO2 NPs的等电点与自然水体的pH接近,因此其在水环境中具有极强的团聚性能[35]. MNPs在水环境中会形成双电层,水中的离子会产生吸附电中和或压缩双电层作用,从而促进MNPs的团聚. 由于二价离子压缩双电层的作用比单价离子更强,因此其更有利于MNPs的团聚[36].
天然有机物(NOM)在水生系统中起着至关重要的作用,探索MNPs与NOM之间的相互作用对确定MNPs在水生环境中的命运和毒性具有重要意义. NOM主要是通过改变MNPs与生物体细胞间的静电作用,来清除MNPs产生的ROS,进而影响MNPs的毒性. MNPs的表面积、表面特性及疏水性会对MNPs与NOM之间的静电作用产生显著的影响. 如带负电的NOM可以将带正电的TiO2 NPs表面电荷由正变为负,而导致MNPs的生物利用度降低[37]. 现有的关于在NOM存在下MNPs(CeO2 NPs和AgNPs)毒性的研究发现NOM减轻了MNPs对藻类的不利影响,并通过吸附到MNPs的表面来增加MNPs的稳定性[38-39]. NOM可以充当保护涂层,通过化学键来加强MNPs与NOM之间的联系,将MNPs与外界隔离,从而减弱MNPs的毒性. 藻类分泌的胞外聚合物(extracellular polymeric substances,EPS)是NOM中的一种,其主要由多糖和蛋白质组成. EPS中的多糖可以将Ag+转化为AgNPs[40],而ESP中的蛋白质可以附着在MNPs表面,形成稳定的MNPs的蛋白质复合物[41]. 总体来说,在NOM存在下,通过静电作用和化学键结合,可以有效地抑制MNPs在藻类细胞的内在化能力. 但也有研究报道了相反的结果,如在NOM存在下,多壁碳纳米管对硅藻细胞分裂的抑制作用明显增强[42].
除上述水化学条件外,水硬度、温度等也会影响MNPs的毒性效应. Nolte等[43]发现水硬度具有减少MNPs溶解及减轻MNPs毒性的潜力. Goswami等[44]通过研究发现升高温度可以加快AgNPs的溶解速度. 综上,环境因素可以通过改变MNPs的悬浮性、表面性质等进一步影响MNPs的环境行为,从而影响MNPs的毒性.
2.1. 人工纳米颗粒的物理化学特性
2.2. 环境因素
-
水环境是MNPs的重要汇集地之一,当前关于MNPs的毒理学研究主要关注MNPs对水生生物的毒性效应. 而藻类是水环境中构成食物链的基础,参与水环境中的养分循环,且对MNPs表现出高敏感性,因此MNPs对藻类的生态毒性引起了广泛的关注. 多种淡水藻类(如小球藻、斜生栅藻和铜绿微囊藻)和海藻成为MNPs生态毒性研究中的受试生物[45].
MNPs对藻类毒性也主要分为两个方面: 一是直接作用,MNPs直接破坏细胞膜或引起藻类结构的改变,造成氧化应激、DNA损伤、蛋白质激活或失活; 二是间接作用,如在细胞外释放毒物(如金属离子或活性氧),然后毒物触发MNPs的毒性[46-47]. 目前,国内外关于MNPs对藻类的毒性效应研究主要从以下两个方面进行: 一是通过实验室模拟控制变量,观察确定条件下MNPs对藻类的毒性效应; 二是原位分析,即在特定的环境条件下探究外界因素对MNPs毒性效应的影响. 总体来说,MNPs对藻类的毒性不仅是MNPs特异性的,也是藻类特异性的. 即不同的MNPs对藻类的影响不同,同种MNPs对不同藻类的毒性也不同. 高浓度的MNPs抑制藻类的生长,而低浓度的MNPs则刺激藻类的性能. 此外,金属MNPs是目前研究最为普遍的MNPs,其对藻类的毒性主要由纳米颗粒特性(如TiO2 NPs和CeO2 NPs)和溶解离子(如CuNPs和AgNPs)决定. 表1总结了典型MNPs对藻类的毒性效应.
-
纳米颗粒具有表面积大、表面活性高等物理化学特性,在环境中易与其他污染物质(如重金属污染物、有机污染物、表面活性剂、无机配体和天然有机质)相互作用,产生复合效应[68]. MNPs对共存污染物的吸附会对污染物的毒性产生影响,吸附能力强的MNPs也可能成为共存污染物在环境中迁移转化的载体. Yang等[69]发现,TiO2 NPs对Cr2+的吸附量与TiO2 NPs的浓度呈正比,TiO2 NPs的浓度分别为1、3、10、30、100 mg·L−1时,Cd2+的吸附量分别为0.16、0.33、0.70、0.90、0.93 mg·L−1. 大量研究表明,碳基纳米颗粒(如石墨烯、富勒烯和碳纳米管)会显著提高有机污染物(如芳香族污染物菲和布洛芬)在生物体内的积累量,诱导氧化应激反应,从而增大其毒性效应[70-73]. 而TiO2 NPs和Al2O3 NPs等金属氧化物纳米颗粒会降低重金属离子对藻类的毒性[74-75]. 不同MNPs对不同污染物生物效应的影响都不尽不同. Chen等[76]发现TiO2 NPs可以减少Cu的生物利用度,从而降低Cu对铜绿微囊藻的毒性; 而Tang等[77]则发现TiO2 NPs和Zn对鱼腥藻光合作用和存活能力都具有协同作用. 同样地,Tang等[78]研究表明,低浓度的GO会增强Cd2+的毒性. 不同的MNPs对重金属的藻毒性影响不同,这种毒性影响不是绝对的. 多项研究表明,这可能取决于MNPs的浓度,且MNPs与环境中共存污染物的复合毒性并不等于二者的简单加和. 例如,在较低的TiO2 NPs浓度下,Zn2+的毒性可能增加,而当TiO2 NPs浓度增加至一定值时,毒性会降低. 同样,关于Cd2+与GO系统组合毒性的研究也得出了相同的结果. 不同的表面活性剂与MNPs间会产生不同的相互作用. 十二烷基苯磺酸钠(SDBS)和叔辛基苯氧基聚乙烯乙氧基乙醇(TX-100)通过促进MWCNTs的细胞内化,引发更高的氧化应激,从而提高MWCNTs的毒性[79]. 十六烷基三甲基氯化铵(CTAC)抑制ZnO溶解为Zn2+,减少Zn2+在藻细胞内的蓄积,产生拮抗作用[80]. 而SDBS促进Zn2+溶出,增加ZnO NPs的毒性[81]. 无机配体主要通过络合金属纳米颗粒释放的金属离子,改变纳米颗粒的毒性. 例如,ZnO NPs能与PO43-反应生成影响细胞活性的磷酸锌,细胞培养液中存在PO43-时,会提高ZnO NPs对成纤维细胞的致死率[82]. 总体来说,MNPs可通过增加共存污染物的生物利用度、促进有机污染物的代谢、改变重金属的形态和改变细胞膜的功能和结构等方式来增强共存污染物的毒性,也可通过吸附污染物、竞争细胞膜上的结合位点、调节生物体内的酶活性等途径来减轻共存污染物的毒性.
另一方面,共存污染物也可能通过改变MNPs表面性质和性能来影响MNPs的毒性. 例如,在Zn2+与AuNPs组合系统中,AuNPs的毒性减弱了,主要是因为Zn2+诱导了AuNPs的聚集,进而显著降低了MNPs的毒性[83]. 不同污染物质通过不同的途径影响MNPs的毒性,如重金属主要通过吸附在MNPs的表面,表面活性剂主要通过改善MNPs的分散性,而无机配体主要通过络合金属NPs释放出来的游离离子来改变MNPs的毒性. 共存污染物影响MNPs的毒性主要通过以下几种方式:(1)吸附在MNPs的表面,从而改变MNPs的悬浮性和颗粒表面的电负性;(2)改变生物体细胞膜的完整性和流动性,从而促进MNPs的内在化,增加MNPs的毒性;(3)改变生物体对MNPs的耐受性,从而减轻MNPs对生物体的影响;(4)通过清除或增加MNPs产生的ROS,从而减轻或增加毒性;(5)共存污染物还有可能通过改变MNPs的官能团或者影响MNPs的胞吐过程等来改变MNPs的毒性.
总的来说,一方面,MNPs能够成为污染物质的载体,改变污染物质的毒性效应,且NPs具有极强的吸附能力,能够吸附污染物质,从而使其生物利用度降低. 另一方面,共存污染物也能够通过修饰MNPs的表面性质来改变MNPs的性能,进而对MNPs的毒性产生影响. 由于MNPs与共存污染物会相互影响双方在环境中的毒性,而在自然环境中,生物不仅暴露于一种污染物,通常情况下会受到多种污染物的混合影响. 因此,探究MNPs与其他污染物共同暴露的生态效应至关重要.
-
MNPs不仅会与环境中共存的其他污染物质相互作用,不同MNPs之间也会相互影响,具有联合毒性效应. 目前,大量研究关注于单一MNPs对藻类的毒性效应,而对于多种MNPs共同暴露的研究尚不充分. 而自然界是一个复杂的体系,藻类往往会同时暴露于多种MNPs,因此对多种MNPs共同暴露的毒性效应研究具有重要的现实意义. Ko等[59]通过对30种二元纳米颗粒混合物组合对藻类叶绿素含量的影响进行评估,再与单个MNPs的毒性进行比较,发现有67%的二元纳米颗粒混合物表现出加成作用,而拮抗作用和协同作用各占16.5%.Li等[84]发现ZnO NPs与CuO NPs的联合毒性远远大于二者单独作用的单纯加和毒性. 另外,即使是加成作用,每种MNPs对毒性的贡献也不同. 如Ye等[85]研究了ZnO NPs和CuO NPs二元系统对淡水斜生藻的生态毒性,浓度—响应的分析结果表明,CuO NPs对二元系统组合毒性的贡献大于ZnO NPs,该研究还表明溶解的金属离子不是二元系统组合毒性的主要来源. 他们的另外一项关于ZnO NPs和GO混合物对不同营养水平的生物毒性的研究也同样表明,在MNPs二元系统中,溶解的金属离子不能决定二元组合系统对不同营养水平的生物的综合毒性[86].
尽管目前关于多种MNPs的联合毒性的研究十分有限,但当前的研究揭示了单一MNPs与其他MNPs存在时可能发生的各种毒性机制. 主要包括: MNPs与生物之间的接触减少、通过吸附MNPs释放的金属离子,而使其生物利用度降低以及MNPs的光催化活性. 如Tong等[87]研究了ZnO NPs和TiO2 NPs单独存在和同时存在时的生态毒性,结果发现1 mg·L−1的ZnO NPs就可以消除100 mg·L−1的TiO2 NPs的破坏作用,主要是因为ZnO NPs减少了细胞与TiO2的接触,从而减少了TiO2的毒性. 同时,TiO2 NPs会吸附Zn2+来减轻ZnO NPs的毒性. MNPs的光催化活性对MNPs的毒性也产生了较大的影响. 在黑暗中,TiO2 NPs通过Ag+的表面吸附减弱了AgNPs的毒性[88];但在光照下,由于MNPs的光催化活性会产生更多的ROS,二者对生物体的毒性表现出协同作用[89]. 在关于MNPs组合系统的联合毒性研究中,大多研究发现会出现明显的异物聚集. 如Zhang等[90]发现CuNPs与CNT之间形成聚集体,会使CuNPs对微型藻类的毒性减弱; HemNPs和AgNPs发生杂聚会降低AgNPs的毒性[91]. 而Huang等[92]关于AgNPs和赤铁矿(HemNPs)及AgNPs和PsNPs对两种淡水藻类的联合毒性研究中,发现这几种MNPs呈良好的分散状态,没有出现杂聚现象; 另外一项研究也发现HemNPs与TiO2 NPs之间也不会形成聚集体[93].
相比于二元系统,三元MNPs混合物的生态毒性更加的复杂. Liu等[94]研究了TiO2 NPs、SiO2 NPs和ZrO2 NPs对单细胞藻类的毒性,通过二元和三元组合的比较研究,结果表明,三元组合体系显著提高了藻类线粒体的膜电位及细胞内活性氧的含量. 另外,抗氧化酶(超氧化物歧化酶(SOD)和过氧化氢酶(CAT))、脂质过氧化产物及小分子代谢产物在三元组合体系下都增加了. 且三元组合体系可引发明显的氧化损伤,比单一及二元系统表现出更强烈的氧化应激. 多元组合系统毒性增加的原因可能是MNPs种类和数量的增加,不同MNPs同时存在会产生相互影响. 自然环境是一个复杂的系统,包含不同类型的MNPs,单一类型MNPs的毒性研究结果可能无法反映其对环境的真实影响. 因此,对不同MNPs组合系统的联合毒性研究具有至关重要的现实意义,更能够了解MNPs在环境中的生态效应. 而目前关于不同MNPs组合系统的生态效应及其机制研究尚不全面,特别是组合系统的毒性机制还有待于进一步研究.
4.1. 人工纳米颗粒与其他污染物的复合效应
4.2. 不同人工纳米颗粒共同暴露的复合效应
-
大量研究表明MNPs对藻类具有毒性效应,借助生物终端指标(活性氧、膜损伤、抗氧化酶和叶绿素等)和组学指标(包括蛋白质组、基因组、转录组和代谢组),能够有效地研究MNPs对藻类的毒性效应.
-
MNPs暴露对藻类的毒性机制主要包括氧化应激、光催化效应、遮光效应、物理损伤及内化效应. 目前,MNPs毒性的研究重点主要分为两个方面. 一是间接影响,具体取决于它们的物理和化学特性,包括遮光作用、聚集行为、溶解度等; 二是直接影响,MNPs直接引起藻类细胞膜、结构和分子的改变. MNPs对藻类的毒性效应主要受物理作用和氧化应激的影响[95]. 积累的MNPs在细胞表面聚集,不仅减少了光合作用的可用光,而且会阻止养分的吸收. MNPs暴露于微藻会刺激微藻细胞内过度积累ROS,从而导致氧化应激的发生. MNPs存在条件下,ROS的水平提高了90%,最终导致藻细胞发生氧化应激[96]. 大量研究表明,氧化应激是MNPs对藻类的主要毒性机制[97]. MNPs诱导的ROS积累与MNPs的剂量及藻类种类有关. 研究表明,在200—500 mg·L−1的MNPs下诱导的ROS水平最高达207%,且MNPs暴露后,铜绿微囊藻是最易感的藻类[96].
MNPs的吸收和内化是一个复杂的过程,目前尚不清楚其吸收和内化的具体机制. MNPs可能通过不同的途径内化,一方面会受到细胞膜和细胞壁的作用,另一方面可能通过内吞作用进入细胞. MNPs的存在会刺激磷脂代谢且激活相关的酶,而特定区域磷脂的减少是膜穿孔的驱动力,因为这些磷脂是细胞膜的组成部分,它们的缺乏会破坏细胞膜的完整性[98]. 如AgNPs下调铜绿微囊藻中的磷脂代谢,导致磷脂酰相关化合物(如磷脂酰胆碱、磷脂酰乙醇胺和磷脂酰甘油)减少,从而影响细胞膜的结构[99]. 一些研究发现MNPs内在化之后细胞膜的结构并未发生改变,这表明MNPs可能通过其他的途径(如通过运输载体蛋白和离子通道)进入细胞[100]. 目前,关于水生环境中MNPs的生物利用度和吸收机制的实验证据还远远不够,需进一步完善.
光合作用是藻类存活和生长必不可少的过程,叶绿素和光系统2(PSII)可用来表示藻类的生长和健康状况[101]. MNPs暴露后,叶绿素a和b的含量降低,PSII反应也受到抑制[102]. 其中叶绿素含量的变化受MNPs表面修饰、MNPs类型、MNPs剂量和藻类种类的影响. 而PSII反应主要依赖于MNPs的剂量. 暴露于MNPs后,叶绿素含量下降可能是由于ROS在叶绿体中的积累导致了色素-蛋白质复合物的脂蛋白比例变化[48,58]. 叶绿素含量降低会导致光化学反应的能量传导中断,从而阻碍藻类的光合作用,使藻细胞的密度降低. 而Li等的研究则表明,AuNPs(5 nm)可以被藻类内在化,提高类胡萝卜素的含量. 主要原因是AuNPs提高了PSII的电子传递速率,且使ROS的产生量增多,从而增强了类胡萝卜素的光合作用[103]. 为应对MNPs的毒性,藻类细胞会启动防御系统,以保护生物体免受ROS干扰. 常见的清除ROS的主要抗氧化酶有超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)、谷胱甘肽过氧化物酶(GPX)和谷胱甘肽转移酶(GST). 在MNPs暴露后,抗氧化酶的活性增强,从而降低MNPs对藻类的毒性. 如小球藻可以通过诱导抗氧化酶SOD和POD的产生,从而有效地降低AgNPs的毒性[104]. 但是当ROS的产生量超过它们的清除能力,防御能力不足,导致某些酶失活,会生成丙二醛(MDA)并发生脂质过氧化反应. 而脂质过氧化作用会导致细胞膜通透性增加,细胞膜的选择性、流动性和完整性降低,甚至完全丧失[105]. 总的来说,MNPs的暴露会影响藻类的光合作用及损害藻类的细胞膜,进而可能会进一步影响水生生态系统中营养级别更高的生物体的生长.
-
对MNPs毒性的研究,除了上述常规的生物终端指标外,组学技术(蛋白质组学、基因组学、转录组学和代谢组学)是进一步了解MNPs毒性机制的关键. 在蛋白质组学方面,转录和翻译的相关蛋白(如核糖体蛋白和延伸因子)的下调表明MNPs会阻碍蛋白质的翻译和折叠[104]. Zhang等[62]通过蛋白质组学研究发现带负电的AgNPs能够特异性地调节与线粒体功能相关的蛋白质,从而破坏了几种相关的代谢途径,如与氧化磷酸化、氨基酸合成及能量代谢有关的代谢途径. 在基因组学方面,与细胞分裂相关的电子传输链(如cox3、nad5和psaB)、吸光蛋白(LHCA3,5,8、LHCB4,5)、二酰基甘油酰基转移酶和PSII反应蛋白(D1)下调[8,106-107]. 在光化学合成过程中,光能向光合电子的转化率下降,电子传输的速率随之下降,从而降低了NADPH和ATP的合成速率,最终会限制细胞的分裂. AgNPs会抑制铜绿假单胞菌(PAO1)的生长,诱导氧化应激使细胞膜损伤,且抑制PAO1中与群体感应及金属外排相关的基因的表达,但会促进甘油、氨基酸、红霉素等的过量生成[9]. 在转录组学方面,通过转录组分析,发现AgNPs使参与运输、碱基代谢和能力产生的相关基因上调,而参与运输和定位的基因下调[10]. 应用全基因组测序和转录组发现,莱茵衣藻中与光合作用(PSBP1)、钙转运(CHLREDRAFT_189266、CHLREDRAFT_187187、CHLREDRAFT_191203、CSE1)及谷胱甘肽代谢(CHLREDRAFT_167073)相关的基因会影响镉耐受性[108]. 在代谢组学方面,MNPs暴露减少了碳固定途径的代谢产物,并且抑制了氨基酸、核苷酸和脂肪酸的合成,从而抑制了藻类的代谢功能[109]. 另外,小球藻的代谢谱显示,在碳纳米管存在下,小球藻的代谢产物对ROS的产生具有促进作用[110]. 代谢组学和蛋白质组学组合分析表明,抑制碳水化合物、脂肪酸和氨基酸的代谢有利于ROS的产生[7]. 通过代谢组学研究发现,MNPs暴露于小球藻会使其代谢途径发生变化,如与CuO MPs和Cu2+相比,CuO NPs暴露引起如下代谢途径变化: 叶绿素中间产物积累、膜脂重塑、谷胱甘肽代谢紊乱及渗透调节物质的积累[60]. 且MNPs暴露对脂肪酸氧化具有特异的破坏模式,藻细胞的膜流动性可能由于颗粒的附着而特异性地降低. 目前,代谢组学的研究使得在MNPs存在下能够更早的发现ROS.Hu等人认为代谢产物可能会成为快速识别和检测ROS的生物标志[51].
蛋白质组学、基因组学、转录组学和代谢组学结果表明,MNPs暴露主要抑制与细胞增殖、光合作用及脂质生物合成有关的基因表达. 然而,当前并没有系统化的组学研究,并不能根据不同组学方面得到的结果来整体评估细胞的状态. 因此,进一步理解生物标志物及其信号传导途径对于控制MNPs的藻毒性至关重要. 此外,组学分析表明MNPs暴露时,微型藻类具有修复机制. 如0.01—0.1 mg·L−1的AgNPs促进了小球藻的生长[97]. 5 mg·L−1的Fe2O3 NPs不仅促进了绿藻的生长,而且提高了不饱和脂肪酸及脂质的含量[111]. 由此说明,低剂量的MNPs可能会促进藻类的生长. 鉴于MNPs暴露对藻类影响的复杂性和多样性,我们有必要进一步深入的研究,以更准确、更全面的了解MNPs对藻类的毒性效应及其毒性机制.
5.1. 生物终端毒性指标
5.2. 组学指标
-
随着MNPs的广泛应用,其在环境中的迁移转化及其生态效应方面的研究成为当务之急. 近年来,MNPs的相关研究已取得了巨大进步,如在MNPs的转化、生物利用度和毒性机理方面都有明显的发展. 但由于纳米颗粒的多样性和生态系统的复杂性,MNPs的致毒机理及MNPs与其他污染物的相互影响等方面还不清楚. 相关研究建议从以下4个方面进一步开展.
(1)目前的相关研究大都是在超环境水平下进行的,研究中MNPs的暴露浓度很高,且暴露时间短,多为急性实验,还没有确切的关于低浓度MNPs长期暴露下可能影响的描述,在不断变化的复杂环境中,低浓度MNPs的分布及迁移转化机理的研究仍是一个挑战.
(2)自然环境是个复杂的系统,MNPs与共存污染物(如重金属和有机污染物)相互作用,会影响它们的环境行为和毒性. 另外,不同的MNPs共同暴露也会改变它们对藻类的毒性效应,多元MNPs组合系统对藻类的毒性大多为加成作用,小部分为协同作用和拮抗作用. 为了更好地了解真实环境中MNPs对藻类的毒性效应,应加强多元MNPs组合体系对藻类毒性效应及毒性机制的研究. 且随着更复杂的新型复合纳米材料和产品的开发与使用,纳米技术有待于进一步发展和创新.
(3)氧化应激被认为是MNPs对藻类的主要毒性机制,而毒性作用效果主要取决于MNPs的类型和剂量. 通过生物终端指标和组学指标可以有效地解释MNPs的毒性机制. 但是,关于MNPs对藻类的毒性机制目前还没有系统全面的解释,有待于进一步研究. 且在MNPs暴露期间藻类会发生自我修复,有必要了解这种响应机制,从而更深入的理解MNPs与藻类之间的相互作用.
(4) MNPs在水环境中的迁移转化会影响MNPs的生态效应,应建立一套MNPs在环境中迁移转化的模型,通过模型研究MNPs的环境行为,进而分析MNPs对不同生物体的毒性情况. 另外,需要完善及标准化MNPs的毒理学测定和研究方法,相关检测仪器的缺乏会使很多研究无法开展,开发相关的检测仪器有利于更好地认识MNPs的环境行为及生态效应,促进MNPs的毒理学研究及MNPs可持续发展.



 下载:
下载:

