-
群感效应(quorum sensing, QS)作为细菌间的通讯方式,调节细胞分化、细胞运动及生物膜形成等多种生理功能[1]。基于N-酰基高丝氨酸内酯(acyl homoserine lactones, AHLs)的QS已被证明可以通过加速胞外聚合物(extracellular polymeric substances, EPS)合成分泌影响生物膜的形成,提高细胞聚集能力,在活性污泥颗粒形成过程中起着重要作用[2-4]。好氧颗粒污泥(aerobic granular sludge, AGS)本质上是混合菌群自固定化微球,由QS介导的微生物代谢活动在AGS功能活性中具有重要作用[5]。尽管AGS的应用已经取得了重大发展,但启动慢、不稳定、易解体等问题依然制约着AGS在废水处理方面的应用[6-8]。污水处理系统是污染物的储存库,复杂的环境因素影响着污水处理系统的稳定运行和污染物去除效率。抗生素耐药基因作为新型污染物,复杂的环境因素导致其在系统微生物群落中传播和扩散,影响污水处理系统的处理效率[9-11]。耐药质粒是污水抗性组中的重要组成部分,废水处理厂中的微生物群落包含大量耐药质粒,编码对几乎所有临床相关抗生素的耐药性[12-15]。ZHAO等[16]研究编码多耐药基因的代表性共轭转移质粒RP4对氨氧化的影响,结果表明,供体菌株携带的RP4质粒可以转移到序批式反应器(sequencing batch reactor,SBR)中的AOB中,RP4质粒转移后,AOB中与氨氧化相关的amoA,amoC,hao,nirK和norB基因表达受到抑制,污水处理厂中抗生素抗性基因存在生态风险。本课题组研究发现,接种携带多重耐药质粒RP4的供体菌E.coli K12( RP4) 后,反应器氨氮去除率从94.7%降低至32.8%[17]。污水生物处理系统在受到耐药菌株冲击负荷后系统处理性能下降,其稳定运行受到影响。因此,在系统受到耐药菌株冲击负荷后,探索耐药基因传播、QS分子变化以及系统稳定和性能维护对污水处理工艺完善具有十分重要的指导意义。
本研究在前期构建稳定的AGS反应器基础上,向反应器内投加携带耐药质粒RK2的供体菌E.coli K12:RK2,监测了反应器的污泥形态、EPS含量以及QS信号分子AHLs的变化,探讨了耐药质粒对系统中AGS稳定性的影响,阐明了EPS含量变化与群体感应之间的关系,揭示了QS在AGS中的调控机制。通过找到QS调控机制维持AGS系统稳定运行的方法,以期为AGS系统稳定运行提供参考。
-
实验构建了2个平行运行的SBR,分别命名为R1和R2。SBR有效容积为5 L,径高比为0.32,体积交换率为60%。以4 h为一个周期,每个周期包含1 min进水、好氧曝气200 min、沉降30 min、排水4 min和静置5 min。由可编程控制器PLC控制全周期的运行,采用潜水泵控制进水,好氧时微孔曝气头输送空气。
接种污泥取自实验室前期稳定运行的好氧颗粒污泥系统,接种污泥浓度(mixed liquor suspended solids, MLSS)为(4 000±100) mg·L−1,进水采用模拟废水,各种成分的质量浓度如下:进水以乙酸钠为唯一进水碳源,控制在440 mg·L−1;以氯化铵为氮源,N-NH4Cl 40 mg·L−1;以磷酸二氢钾为磷源,P-KH2PO4 5 mg·L−1;其他营养盐有50 mg·L−1 MgSO4·7H2O、20 mg·L−1 KCl、20 mg·L−1 CaCl2、0.1 mg·L−1 FeSO4·7H2O、0.1 mg·L−1 CuSO4·5H2O、0.1 mg·L−1 MnSO4。
-
实验投加2种菌株,一种为不带抗性的E.coli K12;另一种带有RK2质粒的供体菌E.coli K12:RK2。在E.coli K12基因组上标记有mCherry红色荧光基因,在587 nm的激发光下发红色荧光,RK2质粒上标记有EGFP绿色荧光基因,在488 nm的激发光下发绿色荧光。 E.coli K12和 E.coli K12:RK2分别于800 mL液体LB培养基中在37 ℃,150 r·min−1条件下培养至适宜浓度,用于培养E.coli K12:RK2的液体培养基中含有质量浓度为50 µg·mL−1的硫酸安普霉素。培养后的菌液以6 000 r·min−1离心5 min,去上清,磷酸盐缓冲液清洗3次后用新鲜人工配水重悬,分别投加E.coli K12于R1、E.coli K12:RK2于R2中,相应的终浓度均为108 CFU·mL−1。
-
根据运行情况,每天取泥水混合样,水质及污泥特性指标测定参考标准方法[18];乙酸钠采用高效液相色谱法;氨氮采用纳氏试剂分光光度法;MLSS采用称量法测定;污泥粒径筛分采用湿筛法,分别使用孔径为0.2 mm和0.9 mm的钢筛将污泥筛分成3个粒径(d)范围:d<0.2 mm、0.2 mm<d<0.9 mm和d>0.9 mm。将粒径小于0.2 mm以下的污泥定义为絮状污泥;粒径在0.2~0.9 mm的颗粒定义为小颗粒污泥;粒径大于0.9 mm的颗粒定义为大颗粒污泥,计算各粒径范围污泥占总数的比例。胞外聚合物提取采用加热法[19];胞外蛋白(extracellular protein, PN)测定采用BCA试剂盒(Beyotime);胞外多糖(exopolysaccharide, PS)测定采用蒽酮-硫酸法[20]。AHLs的提取与测定参考SUN等[21]的方法。
-
取泥水混合物1.5 mL于6 000 r·min−1离心5 min,沉淀用磷酸盐缓冲液洗涤3次,采用土壤基因组提取试剂盒(TIANGEN)提取污泥基因组DNA。采用NanoDrop对DNA浓度及纯度进行分析,其A260 /A280均在1.7~1.9,并使用0.8%琼脂糖凝胶电泳对基因组DNA完整性进行检测,得到完整性和纯度较高的基因组DNA,置于-80 ℃保存。
RK2质粒丰度采用qPCR技术进行定量分析,以RK2质粒与总细菌的比值作为污泥中RK2质粒的丰度。RK2质粒上下游引物分别为P1(5’-CCATTGCCCCTGCCACCTCACTC-3’)和P2(5’- CGACGCGTACCAACTTGCCATCCT-3’);以16S rRNA为内参基因,上下游引物分别为314F(5’-CCTACGGGAGGCAGCAG-3’)和
518R (5’-ATTACCGCGGCTGCTGG-3’)。反应体系为:10 µL SYBR Green PCR Master Mix 2x,1 µL正反向引物,2 µL模板,总体积为20 µL。16S rRNA的反应程序为:预变性95 ℃,10 min;40个循环:变性94 ℃,25 s,退火51 ℃,25 s,延伸72 ℃,25 s。RK2质粒的反应程序为:预变性,95 ℃,10 min;40个循环:变性95 ℃,15 s,退火60 ℃,1 min。根据总细菌和RK2质粒标准品扩增的Ct值绘制标准曲线(R2>0.996),通过标准曲线对样品进行绝对定量分析。
-
分析了反应器运行过程中泥相中C4-HSL和EPS及其相关组分之间的相关性,并进一步验证AHLs与关键EPS组分的关系。验证实验采用外源投加AHLs分子进行批次实验,实验取构建好的好氧末期的AGS于250 mL锥形瓶中,用新鲜配水将污泥浓度调整为(4 000±100) mg·L−1。实验设置6组:G1为对照组;G2和G3分别为添加108 CFU·mL−1的
E.coli K12和E.coli K12:RK2;G4为添加1 mg·L−1的C4-HSL;G5为添加1 mg·L−1的C4-HSL和108 CFU·mL−1的E.coli K12;G6为添加1 mg·L−1的C4-HSL和108 CFU·mL−1的E.coli K12:RK2。实验用流量计控制曝气量,时间为4 h。分析EPS组分和含量变化,实验重复3次。
-
实验数据使用Prism8软件进行处理,实验平行3组,以P<0.05为具有统计学意义的差异。使用单因素方差分析检验数据间的相关性。图表绘制采用Origin2019b进行。
-
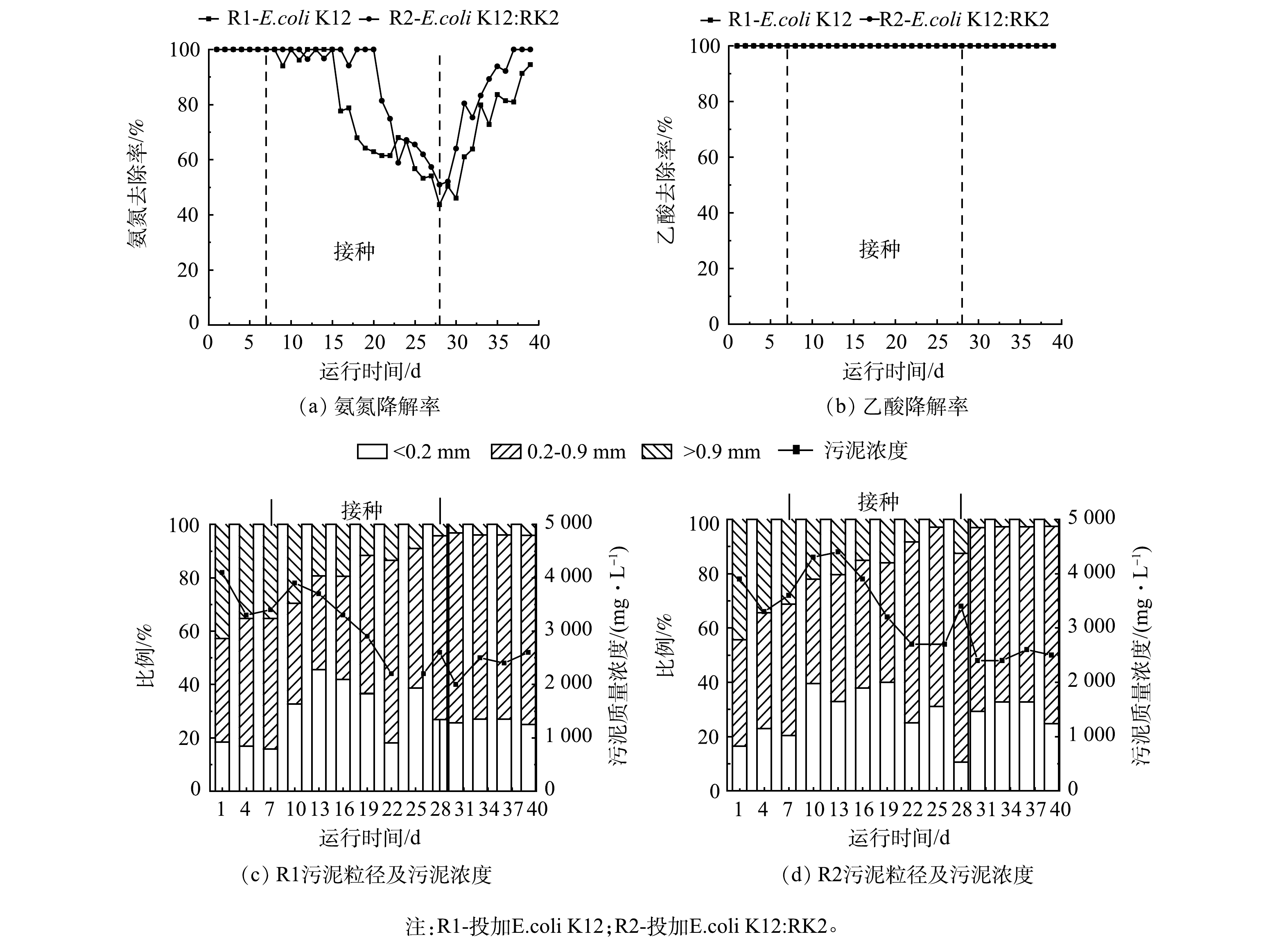
为观察耐药基因对反应器性能的影响,在反应器稳定运行7 d后,向R1和R2反应器内分别投加E.coli K12和携带RK2质粒的供体菌E.coli K12:RK2。如图1(a)和图1(b)所示,投加前2个反应器内NH4+-N和乙酸降解率均为100%。投加后2个反应器中氨氮降解率均有下降,在第28天降到最低,R1和R2中分别为43.65%和50.90%。第28天停止投菌,氨氮降解率逐渐恢复,第39天,R1和R2中氨氮降解率分别稳定在94.50%和100%,2个反应器中氨氮及乙酸降解情况无显著差异。
图1(c)和图1(d)分别为R1和R2中MLSS及粒径变化。结果表明,投菌后MLSS显著降低,反应器运行40 d后,R1、R2中MLSS分别由4 100 mg·L−1和3 900 mg·L−1降低至2 600 mg·L−1和2500 mg·L−1。分析污泥粒径发现,投菌后,2个反应器内大颗粒逐渐解体,絮状污泥增多。反应器运行后期,基本以小颗粒污泥为主,R1和R2中比例分别为73%和71%。絮状污泥呈现逐渐减少最后稳定的趋势,后期小颗粒污泥与投加前出现大幅度的增加,对比2个反应器污泥粒径,至反应器运行结束时,R1和R2中絮状污泥比例分别维持在26%和32%左右,而大颗粒污泥持续减少,由初始的44%和42%分别减少至3%和4%,2个反应器粒径变化趋势一致,说明大肠杆菌投加后对反应器的微生物聚集体形态影响较大。
-
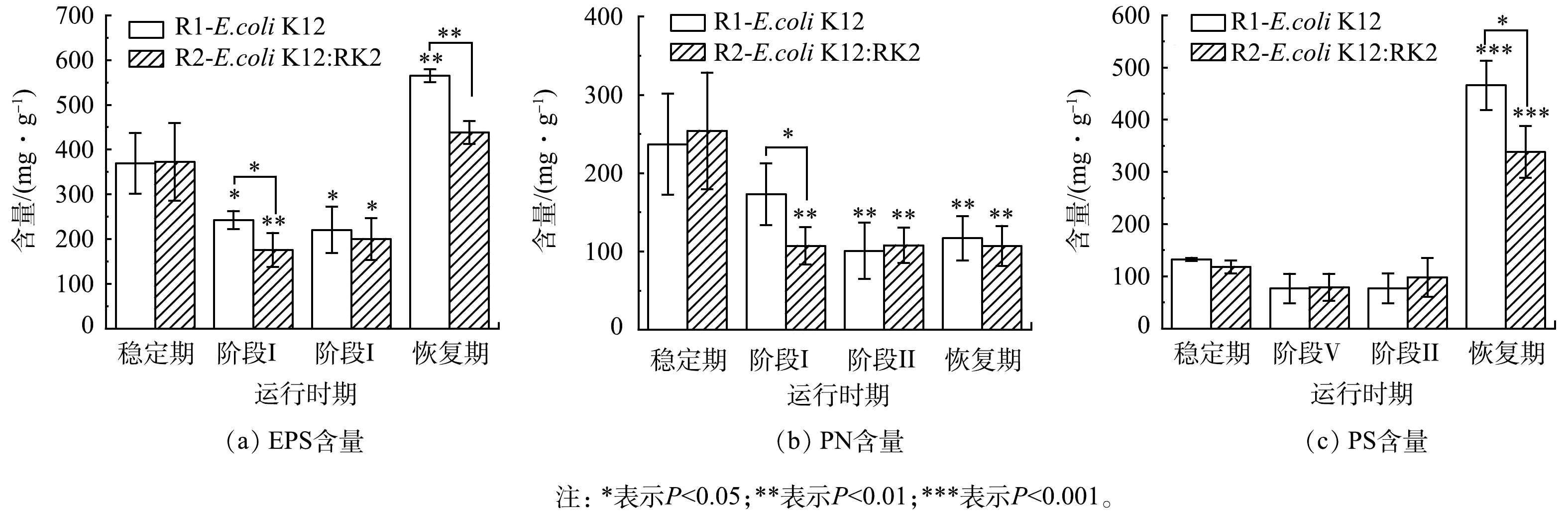
为了观察反应器运行过程中EPS含量及其组分的变化,将实验过程分为四个阶段:稳定期(0~7 d)、投菌阶段Ⅰ(8~17 d)、投菌阶段Ⅱ(18~28 d)和恢复期(29~39 d)。结果发现,投菌后2个反应器中EPS含量都呈先下降再上升的趋势。投菌阶段Ⅰ和阶段Ⅱ中EPS含量相较于稳定期呈现显著下降(P<0.05),阶段ⅠR1和R2中EPS含量分别由(369.28±67.70) mg·g−1和(372.22±86.53) mg·g−1降至(242.47±20.25) mg·g−1和(175.66±37.99) mg·g−1(以MLSS计,下同),该阶段R1和R2中EPS的含量出现显著性差异(P<0.05)。如图2(a)所示,停止投菌后的恢复期,R1和R2中EPS含量与投加前期均显著升高(P<0.01),分别升高至(564.77±14.51) mg·g−1和(438.33±25.43) mg·g−1,且2个反应器之间存在显著性差异,R1中EPS含量高于R2,这表明大肠杆菌进入反应器后抑制了污泥EPS的分泌。EPS是AGS的重要组成部分,对污泥的表面电荷、絮凝及沉降等特性有明显的影响[22-23]。在改变污泥表面理化性质、微生物聚集、造粒及维持颗粒外部结构等方面也具有重要作用[21, 24-26]。EPS 能通过架桥等作用连接和粘附细胞,从而形成颗粒污泥[27]。EPS含量的减少可能是导致污泥颗粒崩解、污泥浓度下降的主要原因。投菌后,对比R1与R2中EPS变化发现,无论是在投菌阶段还是停止投菌后的恢复期,R2中EPS含量始终低于R1,且存在显著性差异,说明携带RK2质粒的供体菌抑制了污泥EPS分泌。
为了进一步深入分析EPS的变化,运行过程中对EPS主要组成成分PN和PS含量的变化进行测定。结果表明,如图2(b)和图2(c)所示,投菌后,PN和PS含量显著低于稳定期,至投菌阶段Ⅱ,R1中PN和PS含量相比于稳定期分别下降了53.24%和41.88%,R2中PN和PS含量分别下降了60.65%和17.31%,且2个反应器中PN与稳定期比较均存在显著性差异(P<0.05)。投菌阶段Ⅰ,R1、R2的PN含量分别为(173.36±39.38) mg·g−1和(107.23±23.73) mg·g−1 ,R1的PN含量显著高于R2,阶段Ⅱ和恢复期PN含量无显著性差异,与EPS的变化趋势相似。说明RK2质粒的存在影响了微生物的PN分泌,且与R1存在不同。PN和PS作为EPS的主要组成部分,在颗粒污泥维持结构以及造粒等方面都发挥了巨大作用。有研究表明,在污泥造粒过程中,PN的增加可以促进污泥颗粒化并产生稳定的生物聚集体[26]。当PN/PS由1.4降至0.9时,污泥相对疏水性也由0.775降至0.500,颗粒无法维持原来结构密实的规则形态而变得松散,进而易于解体[28]。投菌后,系统受到外界冲击后PN的分泌受到抑制,R1和R2中PN含量下降存在差异,随着投菌进行,系统中微生物逐渐适应,PN分泌趋于稳定,说明PN对两个反应器颗粒形态变化起到重要的作用。停止投菌后,EPS含量迅速增加,R1和R2中PS贡献度分别为88%和95%,PS在污泥成粒过程中起到架桥作用,但以PS架桥作用为核心形成的颗粒污泥结构松散,不紧密也不稳定[29],所以此时虽然PS含量增加,但是仍然无法为污泥造粒提供足够的支撑作用,在停止投菌后污泥粒径仍保持相对稳定,且过量的PS存在不利于污泥的絮凝[30]。
-
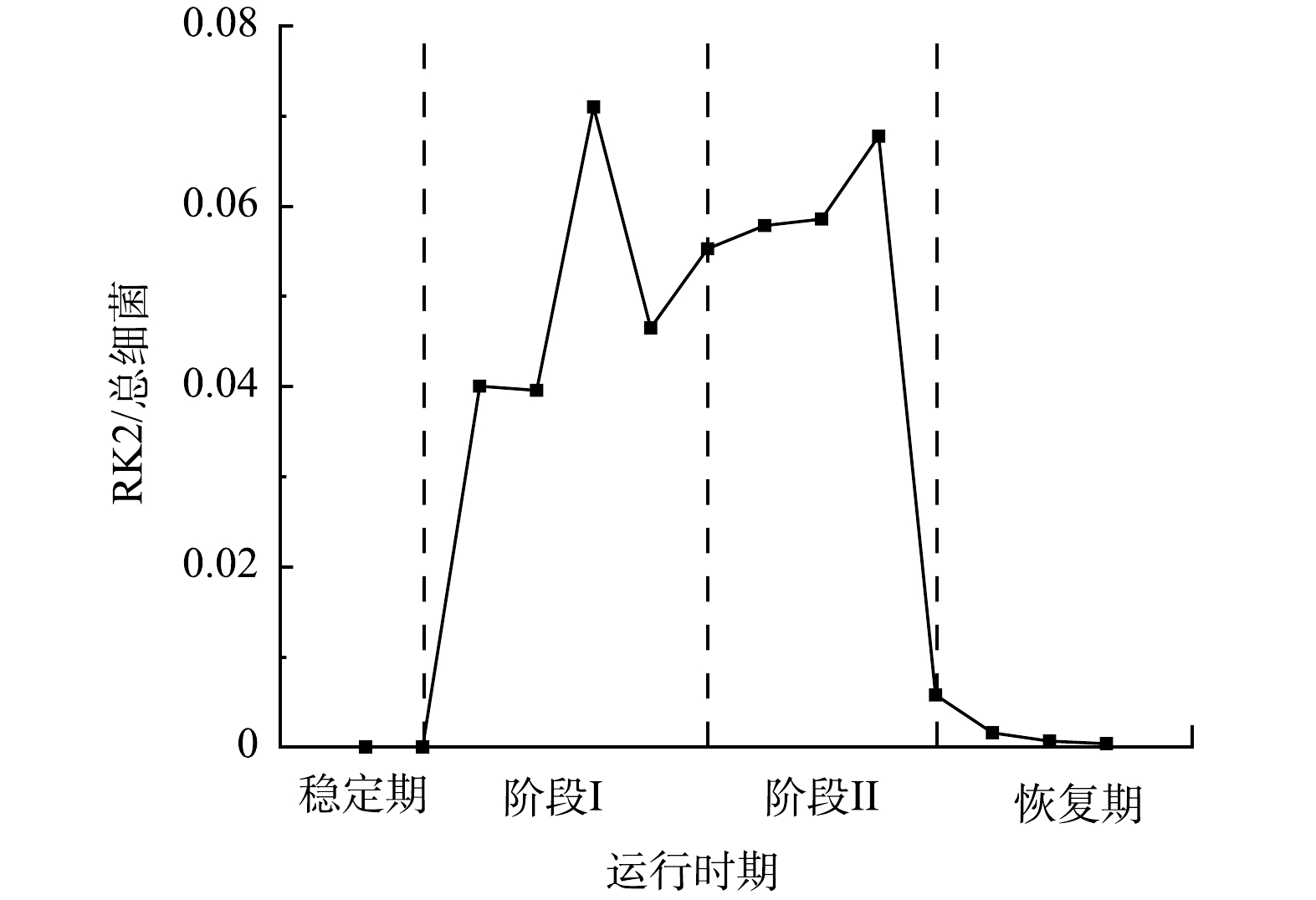
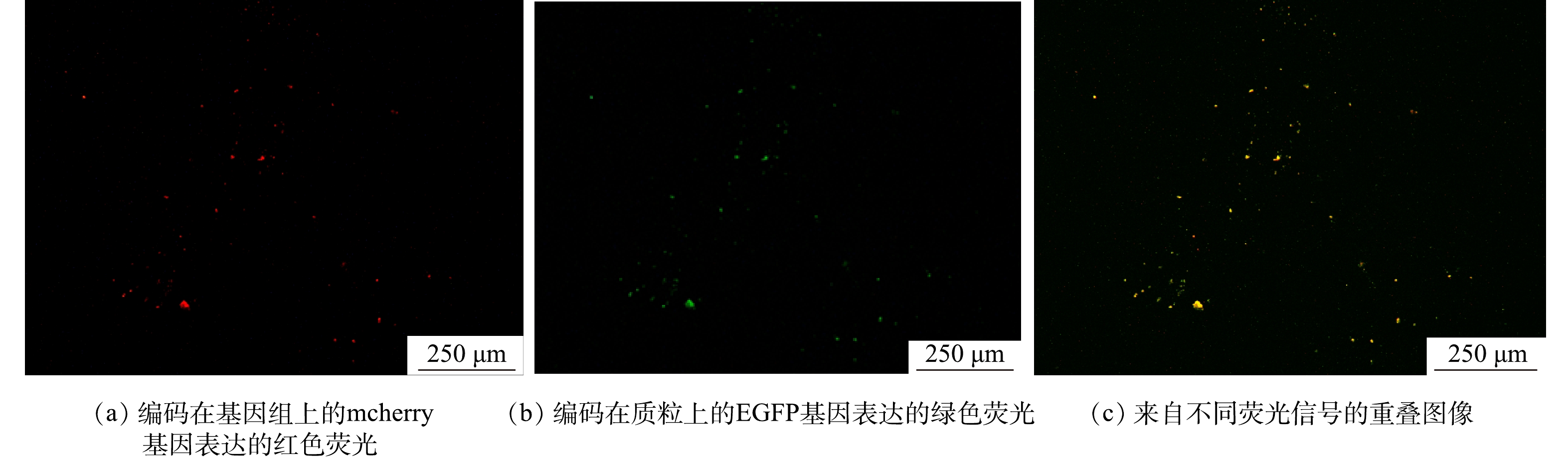
为了分析RK2在EPS分泌中的调控作用,采用qPCR技术检测了R2中RK2质粒的丰度变化,RK2质粒的相对含量为RK2质粒绝对含量与总细菌绝对含量的比值 (简写为RK2/总细菌)。投加E.coli K12:RK2后,反应器中RK2质粒丰度逐渐升高,在投菌阶段污泥中RK2质粒丰度稳定在0.04~0.07(图3);停止投菌后,RK2质粒随反应器的淘洗逐渐丢失,其丰度下降,至恢复期末小于0.001。进一步通过荧光显微镜观察了反应器内RK2质粒的转移,如图4所示,E.coli K12基因组上标记了mCherry红色荧光基因,在587 nm的激发光下发红色荧光,RK2质粒上携带的EGFP绿色荧光蛋白,在488 nm的激发光下发绿色荧光。图4(c)为使用ImageJ软件处理的同一视野下的红绿荧光叠加图,其中橙色荧光为投加的供体菌,红色荧光为丢失了质粒的供体菌,而单独的绿色荧光为发生了RK2接合转移后的受体菌,证明了RK2质粒在反应器发生了接合转移。前期研究发现,向SBR中投加携带RP4质粒的供体菌后,质粒在活性污泥菌群中发生了接合转移,使得反应器内COD、氨氮等污染物的降解效率显著降低[16-17]。本研究中,投菌期间R2中RK2质粒丰度的增加伴随着PN含量的显著降低,与R1中PN的含量存在显著性差异。有研究表明,EPS是细胞抵御外界压力的重要屏障,EPS可通过捕获胞外质粒,降低耐药基因向细胞中的转移效率,在无EPS条件下,E.coli的接合子数量和水平转移效率提高了29倍[31]。R2中EPS含量的减少有利于RK2质粒的穿梭转移,同时RK2质粒转移可能又影响到微生物的EPS代谢,说明RK2质粒的转移影响到EPS中PN和PS含量,进而影响到污泥的形态。
-
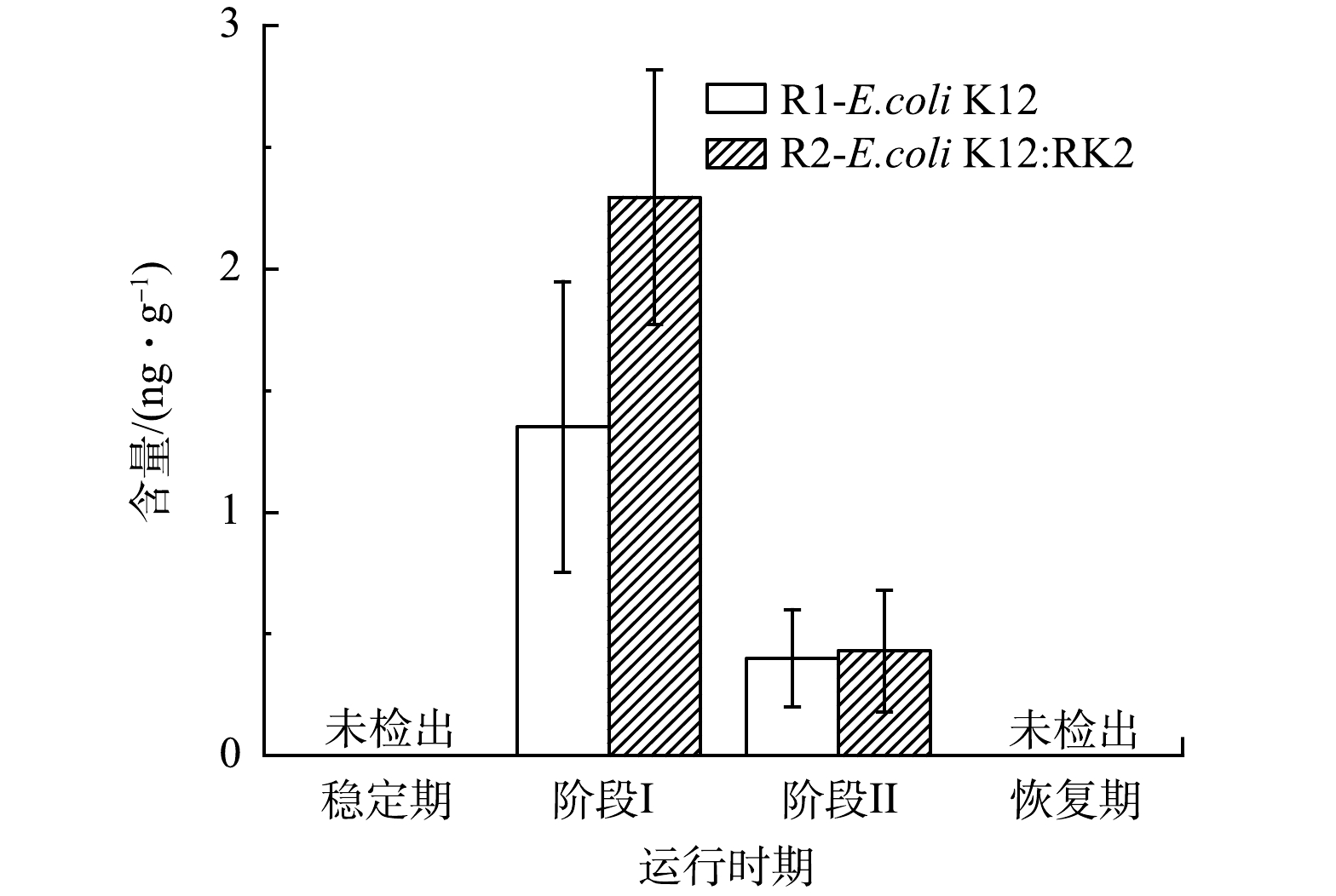
微生物间的通讯交流影响生物体的代谢和系统性能,在反应器的运行过程中,观察了泥相中群感效应分子AHLs水平变化。如图5所示,在R1和R2的污泥相中主要检出了C4-HSL。稳定期系统中未检出C4-HSL,投菌后2个反应器中C4-HSL水平迅速上升,投菌阶段ⅠR1和R2中C4-HSL含量分别上升至(1.35±0.59) ng·g−1和(2.30±0.52) ng·g−1,R2中含量高于R1,而阶段ⅡR1和R2中C4-HSL含量分别降至(0.40±0.20) ng·g−1和(0.40±0.25) ng·g−1,恢复期降至稳定期的水平,表明投加E.coli K12:RK2能够显著增加系统中C4-HSL水平,C4-HSL是投菌阶段主要的群感效应分子。C4-HSL被广泛报道能促进氨氧化菌(AOB)的活动以实现良好的脱氮性能[32-33],GAO等[34]发现,在纳米ZnO暴露期间,C4-HSL与NH4+-N和TN去除效率呈正相关(r>0.5,P<0.05),因此,系统初期氨氮去除未受明显影响可能与C4-HSL的增加密切相关。也有研究发现,C4-HSL可抑制铜绿假单胞菌属生物膜形成,且在AGS形成过程中,C4-HSL的浓度由950 ng·g−1降至520 ng·g−1,相关性分析结果表明,C4- HSL与EPS、PN及PS均呈现极显著负相关趋势,C4-HSL不利于菌胶团的形成[35-36]。本研究中,投菌后C4-HSL含量逐渐增加,同时伴随着颗粒污泥解体,絮状污泥增加,C4-HSL与系统污泥形态变化密切相关。
-
EPS是高密度的菌胶团,容易聚集并附着生长,使得污泥具有良好的抗冲击能力。而群感效应信号分子AHLs被证明能够显著加快生物膜的形成,产生厚而结构化的生物膜[37]。AHLs在多种微生物中存在,并与微生物生物膜的形成以及运动等多种生理活动密切相关。因此,分析了R1与R2中C4-HSL和EPS、PN及PS之间的相关关系。结果如表1所示,C4-HSL与EPS和PS呈负相关趋势;而C4-HSL与PN的相关性R1和R2存在差异,在R1中两者呈现正相关(R=0.300 0),而在R2两者呈现负相关(R=-0.360 8)。AHLs作为微生物分泌的信号分子,直接对分泌EPS的微生物传递信息,开启对环境变化的响应机制,调节生物膜的生长[2, 26, 38]。C4-HSL增加对EPS产生了负面影响,不利于菌胶团的生长,使得污泥结构松散且不利于絮状污泥向颗粒的转化,与前人研究结果一致[36]。PN在R1和R2反应器中的不同,可能是由于RK2质粒在颗粒污泥菌群中转移所致。
-
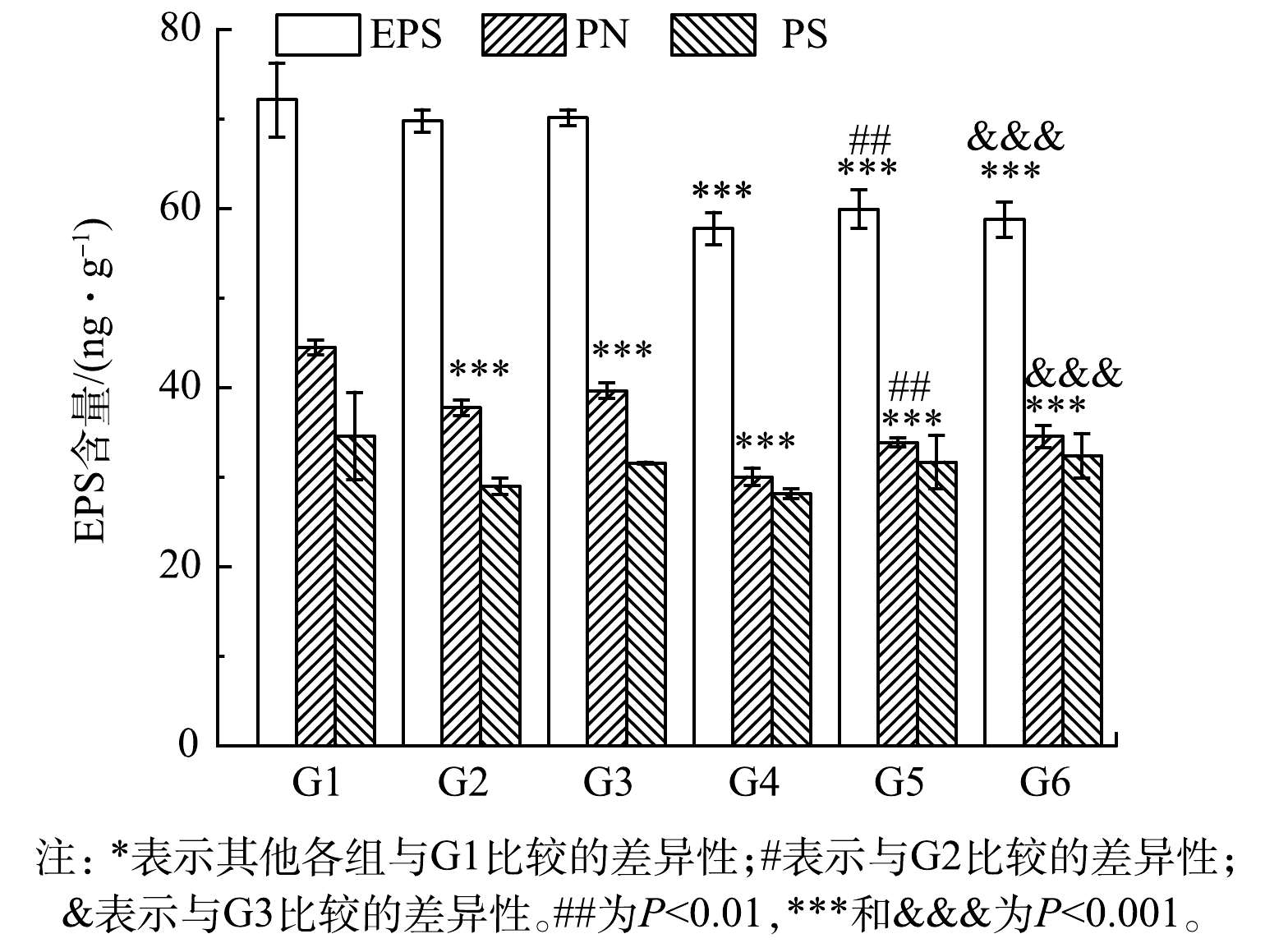
为了验证C4-HSL与关键组分EPS的关系,采用外源添加C4-HSL观察其对EPS的影响作用。结果如图6所示。G1~G6的EPS含量分别为(72.13±4.13)、(69.77±1.26)、(70.14±0.90)、(57.75±1.80)、(59.92±2.13)和(58.75±1.95) mg·g−1。与对照组相比,各实验组EPS含量均降低,PN含量显著降低(P<0.01),而PS含量变化无显著性差异,表明添加大肠杆菌与C4-HSL均对污泥EPS分泌产生了抑制作用。对比G1与G4、G2与G5、G3与G6,添加C4-HSL后总EPS和PN的含量显著降低(P<0.01)。G1~G6各组PN含量分别为(44.49±0.81)、(37.75±0.87)、(39.64±0.87)、(30.02±0.99)、(33.86±0.52)和(34.54±1.2) mg·g−1,与空白对照(G1)相比,各组PN减少在EPS减少中贡献度更大,为80%~85%。这说明高浓度的C4-HSL能够显著抑制污泥中EPS特别是PN的分泌,从EPS组分来看,PN在EPS含量减少中贡献度更大,C4-HSL可能影响了微生物的PN代谢,与前期实验结果一致。所以,C4-HSL能够通过影响EPS含量来影响污泥性能,进而实现对整个污泥系统的调控。
-
1)分别向反应器投加E.coli K12及E.coli K12:RK2后,系统氨氮降解效率下降,反应器内颗粒污泥解体,絮状污泥增多。投菌后EPS分泌受到抑制,投加供体菌组RK2质粒发生了接合转移,在RK2质粒影响下,EPS含量,特别是PN含量比对照组显著减少。
2)投菌后系统主要响应AHLs为C4-HSL,C4-HSL在投菌后出现,并随着投菌过程浓度降低至原水平,其在R2中的含量略高于R1。
3)相关性分析结果表明,C4-HSL与EPS、PS呈负相关,且R1和R2中PN与C4-HSL相关性不同。进一步验证发现,C4-HSL能够抑制好氧颗粒污泥的EPS含量,尤其是PN合成,表明携带耐药质粒RK2胁迫下系统能够通过群感效应分子C4-HSL调控EPS含量来影响颗粒污泥的稳定性。
耐药菌E.coli K12:RK2负荷下群感效应对好氧颗粒污泥稳定性的影响
Effect of quorum sensing on the stability of aerobic granular sludge under drug resistant bacteria E.coli K12:RK2 load
-
摘要: 研究了在携带RK2质粒的供体菌E.coli K12 :RK2负荷下群体感应对好氧颗粒污泥稳定性的影响,同步运行了2个反应器(R1与R2),分别向反应器中添加E.coli K12和携带RK2质粒的供体菌E.coli K12:RK2,考察了其对系统污染物去除效率、污泥形态、EPS以及AHL含量的影响,分析并验证群体感应在维持好氧颗粒污泥稳定中的作用。结果表明,R1和R2中氨氮降解及污泥形态变化趋势一致,系统氨氮降解率下降,反应器中大颗粒污泥解体、絮状污泥增多。投菌作用下,R1和R2中胞外聚合物(EPS)含量显著降低(P<0.05),分别由(369.28±67.70) mg·g−1和(372.22±86.53) mg·g−1(以MLSS计)降至(242.47±20.25) mg·g−1和(175.66±37.99) mg·g−1,且组间存在显著差异(P<0.05)。通过荧光定量PCR及荧光显微镜镜检发现RK2质粒在系统中发生接合转移。C4-HSL为系统的主要响应AHLs,在投菌后出现,R1和R2中含量分别为增加至(1.35±0.59) ng·g−1和(2.30±0.52) ng·g−1,随后2个反应器中C4-HSL浓度均随着投菌进行逐渐降低;相关性分析结果表明,C4-HSL与EPS呈负相关关系;进一步研究发现,C4-HSL能够显著抑制污泥中EPS和胞外蛋白(PN)分泌。以上结果表明在携带RK2质粒的供体菌E.coli K12 :RK2负荷下系统通过群感效应分子C4-HSL调控EPS含量来影响污泥形态,可为提高好氧颗粒污泥系统稳定性提供参考。Abstract: The effect of quorum sensing on the stability of aerobic granular sludge under the load of E. coli K12:RK2 carrying RK2 plasmid was studied. Two reactors (R1 and R2) were run simultaneously, and E.coli K12 and donor bacteria E.coli K12:RK2 carrying RK2 plasmid were added to these two reactors, respectively, and their effects on the pollutant removal efficiency of the reactors, sludge morphology, EPS and AHL content were investigated. The role of quorum sensing in maintaining aerobic granular sludge stability was analyzed and verified. The results showed that the degradation trend of ammonia nitrogen was consistent with that of sludge morphology in R1 and R2, the degradation rate of ammonia nitrogen in the system decreased, and the large particle sludge was disintegrated and flocculent sludge increased in the reactor. Under the action of bacterialization, the content of extracellular polymers in R1 and R2 decreased significantly (P<0.05), from (369.28±67.70) mg·g−1 and (372.22±86.53) mg·g−1 (in terms of MLSS) to (242.47±20.25) mg·g−1 and (175.66±37.99) mg·g−1, respectively, and the significant differences occurred between groups (P <0.05). Fluorescence microscopy and qPCR showed that the conjugation transfer occurred for RK2 plasmids in the system. C4-HSLwas the main response of AHLs in this system. After germination, the contents of C4-HSL increased to (1.35±0.59) ng·g −1 and (2.30±0.52) ng·g−1, respectively, and then the concentration of C4-HSL gradually decreased with the propagation process. The correlation analysis results showed that C4-HSL had a negative correlation with EPS. Further studies have found that C4-HSL could significantly inhibit EPS and PN secretion in sludge. The above results show that under the donor bacteria E. coli K12:RK2 carrying the RK2 plasmid, the system regulated the EPS content to affect the sludge morphology by the group sensing effect molecule C4-HSL, which can provide a reference for improving the stability of aerobic granular sludge system.
-
Key words:
- aerobic granular sludge /
- RK2 plasmid /
- EPS /
- C4-HSL /
- stability
-

-
表 1 R1与R2中C4-HSL与EPS、PN及PS之间的Pearson相关系数及P值
Table 1. Pearson correlation coefficient and P value between C4-HSL and EPS, PN and PS in R1 and R2
组分 R1 R2 Pearson相关系数 P值 Pearson相关系数 P值 EPS −0.648 3 0.351 7 −0.754 8 0.351 7 PN 0.300 0 0.700 1 −0.360 8 0.700 1 PS −0.544 1 0.455 9 −0.522 5 0.477 5 -
[1] DANGCONG P, BERNET N, DELGENES J-P, et al. Aerobic granular sludge—a case report[J]. Water Research, 1999, 33(3): 890-893. doi: 10.1016/S0043-1354(98)00443-6 [2] BANDARA H M H N, LAM O L T, JIN L J, et al. Microbial chemical signaling: a current perspective[J]. Critical Reviews in Microbiology, 2012, 38(3): 217-249. doi: 10.3109/1040841X.2011.652065 [3] ZHANG Z, CAO R, JIN L, et al. The regulation of N-acyl-homoserine lactones (AHLs)-based quorum sensing on EPS secretion via ATP synthetic for the stability of aerobic granular sludge[J]. Science of the Total Environment, 2019, 673: 83-91. doi: 10.1016/j.scitotenv.2019.04.052 [4] ZHANG Z, YU Z, WANG Z, et al. Understanding of aerobic sludge granulation enhanced by sludge retention time in the aspect of quorum sensing[J]. Bioresource Technology, 2019, 272: 226-234. doi: 10.1016/j.biortech.2018.10.027 [5] SARMA S J, TAY J H, CHU A. Finding knowledge gaps in aerobic granulation technology[J]. Trends in Biotechnology, 2017, 35(1): 66-78. doi: 10.1016/j.tibtech.2016.07.003 [6] TAO J, QIN L, LIU X, et al. Effect of granular activated carbon on the aerobic granulation of sludge and its mechanism[J]. Bioresource Technology, 2017, 236: 60-67. doi: 10.1016/j.biortech.2017.03.106 [7] VERAWATY M, PIJUAN M, YUAN Z, et al. Determining the mechanisms for aerobic granulation from mixed seed of floccular and crushed granules in activated sludge wastewater treatment[J]. Water Research, 2012, 46(3): 761-771. doi: 10.1016/j.watres.2011.11.054 [8] LIN H, MA R, HU Y, et al. Reviewing bottlenecks in aerobic granular sludge technology: Slow granulation and low granular stability[J]. Environmental Pollution, 2020, 263: 114638. doi: 10.1016/j.envpol.2020.114638 [9] RIZZO L, MANAIA C, MERLIN C, et al. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review[J]. Science of the Total Environment, 2013, 447: 345-360. doi: 10.1016/j.scitotenv.2013.01.032 [10] LAMBA M, GRAHAM D W, AHAMMAD S Z. Hospital wastewater releases of carbapenem-resistance pathogens and genes in urban india[J]. Environmental Science & Technology, 2017, 51(23): 13906-13912. [11] JAYA DIVAKARAN S, SARA PHILIP J, CHEREDDY P, et al. Insights into the bacterial profiles and resistome structures following the severe 2018 flood in Kerala, South India[J]. Microorganisms, 2019, 7(10): 474. doi: 10.3390/microorganisms7100474 [12] GILBERT J A, ZHANG T, ZHANG X-X, et al. Plasmid metagenome reveals high levels of antibiotic resistance genes and mobile genetic elements in activated sludge[J]. PloS one, 2011, 6(10): e26041. doi: 10.1371/journal.pone.0026041 [13] SENTCHILO V, MAYER A P, GUY L, et al. Community-wide plasmid gene mobilization and selection[J]. Integrated Genomics and Post-Genomics Approaches in Microbial Ecology, 2013, 7(6): 1173-1186. [14] LI A-D, LI L-G, ZHANG T. Exploring antibiotic resistance genes and metal resistance genes in plasmid metagenomes from wastewater treatment plants[J]. Frontiers in Microbiology, 2015, 6: 1025. [15] CHE Y, XIA Y, LIU L, et al. Mobile antibiotic resistome in wastewater treatment plants revealed by Nanopore metagenomic sequencing[J]. Microbiome, 2019, 7(1): 1-13. doi: 10.1186/s40168-018-0604-3 [16] ZHAO C, LI J, LI C, et al. Horizontal transfer of the multidrug resistance plasmid RP4 inhibits ammonia nitrogen removal dominated by ammonia-oxidizing bacteria[J]. Water Research, 2022, 217: 118434. doi: 10.1016/j.watres.2022.118434 [17] ZOU W, XUE B, ZHI W, et al. Effects of antibiotic resistance genes on the performance and stability of different microbial aggregates in a granular sequencing batch reactor[J]. Journal of Hazardous Materials, 2016, 304: 343-351. doi: 10.1016/j.jhazmat.2015.10.057 [18] 魏复盛主编;国家环境保护总局, 水和废水监测分析方法编委会编. 水和废水监测分析方法 第4版 [M]. 北京: 中国环境科学出版社, 2002. [19] MCSWAIN B S, IRVINE R L, HAUSNER M, et al. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge[J]. Applied and Environmental Microbiology, 2005, 71(2): 1051-1057. doi: 10.1128/AEM.71.2.1051-1057.2005 [20] LIANG Z, LI W, YANG S, et al. Extraction and structural characteristics of extracellular polymeric substances (EPS), pellets in autotrophic nitrifying biofilm and activated sludge[J]. Chemosphere, 2010, 81(5): 626-632. doi: 10.1016/j.chemosphere.2010.03.043 [21] SUN Y, HE K, YIN Q, et al. Determination of quorum-sensing signal substances in water and solid phases of activated sludge systems using liquid chromatography–mass spectrometry[J]. Journal of Environmental Sciences, 2018, 69: 85-94. doi: 10.1016/j.jes.2017.04.017 [22] JIANG Y, LIU Y, ZHANG H, et al. Aerobic granular sludge shows enhanced resistances to the long-term toxicity of Cu(II)[J]. Chemosphere, 2020, 253: 126664. doi: 10.1016/j.chemosphere.2020.126664 [23] WANG J, LIU Q, DONG D, et al. In-situ monitoring of the unstable bacterial adhesion process during wastewater biofilm formation: A comprehensive study[J]. Environment International, 2020, 140: 105722. doi: 10.1016/j.envint.2020.105722 [24] ZHANG L, FENG X, ZHU N, et al. Role of extracellular protein in the formation and stability of aerobic granules[J]. Enzyme and Microbial Technology, 2007, 41(5): 551-557. doi: 10.1016/j.enzmictec.2007.05.001 [25] SEVIOUR T, PIJUAN M, NICHOLSON T, et al. Understanding the properties of aerobic sludge granules as hydrogels [J]. 2009, 102(5): 1483-1493. [26] PENG T, WANG Y, WANG J, et al. Effect of different forms and components of EPS on sludge aggregation during granulation process of aerobic granular sludge[J]. Chemosphere, 2022, 303: 135116. doi: 10.1016/j.chemosphere.2022.135116 [27] TIAN S, SU L, LIU Y, et al. Self-targeting, zwitterionic micellar dispersants enhance antibiotic killing of infectious biofilms-An intravital imaging study in mice[J]. Science Advances, 2020, 6(33): eabb1112. doi: 10.1126/sciadv.abb1112 [28] WANG Z, LIU L, YAO J, et al. Effects of extracellular polymeric substances on aerobic granulation in sequencing batch reactors[J]. Chemosphere, 2006, 63(10): 1728-1735. doi: 10.1016/j.chemosphere.2005.09.018 [29] 朱邦辉, 万金保. 好氧颗粒污泥中胞外聚合物作用机理的探讨[J]. 江西科学, 2009, 27(6): 848-854. [30] LIAO B Q, ALLEN D G, DROPPO I G, et al. Surface properties of sludge and their role in bioflocculation and settleability[J]. Water Research, 2001, 35(2): 339-50. doi: 10.1016/S0043-1354(00)00277-3 [31] HU X, KANG F, YANG B, et al. Extracellular polymeric substances acting as a permeable barrier hinder the lateral transfer of antibiotic resistance genes[J]. Frontiers in Microbiology, 2019, 10: 736. doi: 10.3389/fmicb.2019.00736 [32] FENG Z, SUN Y, LI T, et al. Operational pattern affects nitritation, microbial community and quorum sensing in nitrifying wastewater treatment systems[J]. Science of the Total Environment, 2019, 677: 456-465. doi: 10.1016/j.scitotenv.2019.04.371 [33] LIU F, ZHANG Y, LIANG H, et al. Specific quorum sensing molecules of ammonia oxidizers and their role during ammonium metabolism in Zhalong wetland, China[J]. Science of the Total Environment, 2019, 666: 1106-1113. doi: 10.1016/j.scitotenv.2019.02.261 [34] GAO H, YE J, ZHAO R, et al. Pluripotency of endogenous AHL-mediated quorum sensing in adaptation and recovery of biological nitrogen removal system under ZnO nanoparticle long-term exposure[J]. Science of the Total Environment, 2022, 842: 156911. doi: 10.1016/j.scitotenv.2022.156911 [35] WANG W, ZANG Y, WANG C, et al. Effects of four kinds of oxide nanoparticles on proteins in extracellular polymeric substances of sludge[J]. BioMed Research International, 2020, 2020: 1-13. [36] 支丽玲, 马鑫欣, 刘奇欣, 等. 好氧颗粒污泥形成过程中群感效应的作用研究[J]. 中国环境科学, 2020, 40(5): 2148-2156. doi: 10.19674/j.cnki.issn1000-6923.2020.0245 [37] XIONG F, ZHAO X, WEN D, et al. Effects of N-acyl-homoserine lactones-based quorum sensing on biofilm formation, sludge characteristics, and bacterial community during the start-up of bioaugmented reactors[J]. Science of the Total Environment, 2020, 735: 139449. doi: 10.1016/j.scitotenv.2020.139449 [38] KAMATH A, SHUKLA A, PATEL D. Quorum sensing and quorum quenching: Two sides of the same coin[J]. Physiological and Molecular Plant Pathology, 2023, 123: 101927. doi: 10.1016/j.pmpp.2022.101927 -



 下载:
下载: