-
抗生素在生产和使用过程中会产生大量含抗生素废水[1],制药废水是抗生素的最大来源,通常含抗生素浓度高、盐分高、毒性大,其处理是水处理领域中的一项难题[2]。磺胺甲恶唑(Sulfamethoxazole, SMZ)是一类典型的磺胺类抗生素,其在水体中相对稳定,不易被降解[3]。根据一项针对中国七大典型河流水域抗生素赋存的研究,SMZ的检出浓度最高[4],而且有研究表明人的尿液中可检出高达10 mg·L−1的SMZ[5]。SMZ对动植物以及人体健康均会造成危害,因此,研发利用高效的处理技术迫在眉睫。
许多研究表明,高级氧化技术对抗生素废水具有较好去除效果。其中电芬顿(electro-Fenton, EF)技术仅消耗O2和电能,绿色清洁、倍受关注[6-8]。EF技术可通过两电子氧还原反应(2e− ORR)原位生成H2O2,随后H2O2进一步被活化生成活性氧物种(reactive oxygen species, ROS),其可进一步高效去除水中抗生素[9-11]。基于铁离子催化的均相EF技术需在酸性条件下才能有效运行,反应前后需要调节pH,为拓宽EF技术的pH适用范围,开发了基于固相催化剂的非均相EF技术[12-14]。然而受阴极催化剂过渡金属氧化还原电对循环速率慢、稳定性差等限制[15],非均相EF技术对抗生素的降解效率亟待提升,开发高效稳定的阴极催化剂是目前非均相EF技术的主要应用瓶颈。
近年来,金属有机框架材料(metal organic framework, MOFs)是催化领域的研究热点,其不仅具有发达的孔隙结构,且拓扑结构能保障金属位点的均匀分散[16-19]。为了进一步促进金属氧化还原电对在催化反应中的循环速率,构建更多活性位点,研究通过组合不同种类的金属开发出系列双金属MOFs,显著提高了MOFs的催化活性[20-21]。然而MOFs作为EF阴极催化剂时导电性能欠佳[22-23],且其有机配体容易被EF反应中生成的ROS氧化,造成催化剂多孔结构坍塌及失活[24],因此,同步提高电学性能和化学稳定性是关键需求。
本研究以双金属MOFs—FeCo-ZIF为前体,以三聚氰胺(melamine, MA)为碳源和氮源,将两者共混煅烧制备了氮掺杂碳纳米管封装铁钴合金阴极催化剂(N-CNT@FeCo),考察了MA对FeCo-ZIF衍生催化剂EF性能的影响规律,探究了体系中的主要活性物种和催化机理,考察了溶液初始pH、应用电位、共存阴离子及重复循环次数对SMZ降解效果的影响,以评价N-CNT@FeCo作为EF阴极催化剂的基础应用性能。
-
实验材料:六水合硝酸钴(Co(NO3)2·6H2O)、无水硫酸钠(Na2SO4)、无水乙醇(C2H6O)均为分析纯并购于南京化学试剂股份有限公司;七水合硫酸亚铁(FeSO4·7H2O)、氢氧化钠(NaOH)、浓硫酸(H2SO4)、浓硝酸(HNO3)均为分析纯并购于国药集团化学试剂有限公司;三聚氰胺(C3H6N6)为分析纯并购于上海麦克林生化科技股份有限公司;磺胺甲恶唑(C10H11N3O3S)、N,N-二甲基甲酰胺(C3H7NO, N,N-Dimethylformamide, DMF)、2-甲基咪唑(C4H6N2)、甲醇(CH3OH, Methanol, MeOH)、叔丁醇(C4H10O, Tert-Butanol, TBA)、糠醇(C5H6O2, Furfuryl alcohol, FFA)、对苯醌(C6H4O2, 1,4-Benzoquinone, p-BQ)、磷酸二氢钾(KH2PO4)、草酸钛钾(K2TiO(C2O4)2)均为分析纯并购于上海阿拉丁生化科技股份有限公司;5,5-二甲基-1-吡咯啉-N-氧化物(C6H11NO, 5,5-Dimethyl-1-pyrroline N-oxide, DMPO)购于和光纯药工业株式会社;4-氨基-2,2,6,6-四甲基哌啶(C9H20N2, 4-Amino-2,2,6,6-tetramethylpiperidine, TEMP)购于希恩思生化科技有限公司;碳布(W1S1009)购于台湾碳能科技有限公司;Nafion溶液(5 wt%)购于美国杜邦科技有限公司。
实验仪器:管式炉(TL1200,南京博蕴通仪器科技有限公司);电化学工作站(CHI760E,上海辰华仪器有限公司);高效液相色谱仪(LC-2040C,日本岛津公司,high performance liquid chromatography,HPLC);电感耦合等离子光谱发生仪(iCAP 8000,美国赛默飞世尔科技公司,inductive coupled plasma emission spectrometer,ICP);总有机碳分析仪(Multi N/C 3100,德国耶拿分析仪器公司,total organic carbon,TOC)扫描电子显微镜(S4800,日本日立公司,scanning electron microscope,SEM);透射电子显微镜(Tecnai G2 F30,美国FEI公司,transmission electron microscope,TEM);X射线衍射仪(D8 ADVANCE,德国布鲁克公司,X-ray diffractometer,XRD);X射线光电子能谱仪(K-Alpha,美国赛默飞世尔科技公司,X-ray photoelectron spectroscopy,XPS)。
-
1) N-CNT@FeCo的制备:通过典型水热法制备FeCo-ZIF[25],以FeCo-ZIF为前驱体,将FeCo-ZIF和MA按照质量比为1:M混合均匀后置于坩埚中并加盖放入管式炉内,使用氩气作为保护气体(300 mL·min−1),以5 ℃·min−1升温至800 ℃,煅烧2 h,待冷却至室温得到煅烧后的产物,在此条件下制备的催化剂命名为N-CNT@FeCo-M,其中N-CNT@FeCo-100简写为N-CNT@FeCo。此外,在不添加MA的条件下制备的催化剂命名为FeCo-N。
2)修饰阴极的制备:称取6.0 mg催化剂加入到100 μL去离子水、300 μL无水乙醇和5 μL Nafion溶液的混合液中,所配溶液超声30 min使催化剂分散均匀,然后将其滴涂到直径约为4 cm的碳布上,在室温下自然干燥得到负载催化剂的碳布修饰阴极。
-
采用XRD对催化剂的晶体结构进行表征。采用SEM和TEM对催化剂的微观形貌进行分析。采用XPS对催化剂进行元素价态分析。利用循环伏安法(cyclic voltammetry, CV)、电化学阻抗测试(electrochemical impedance spectroscopy, EIS)和旋转盘电极测试(rotating disk electrode, RDE)研究材料的电化学性能。
-
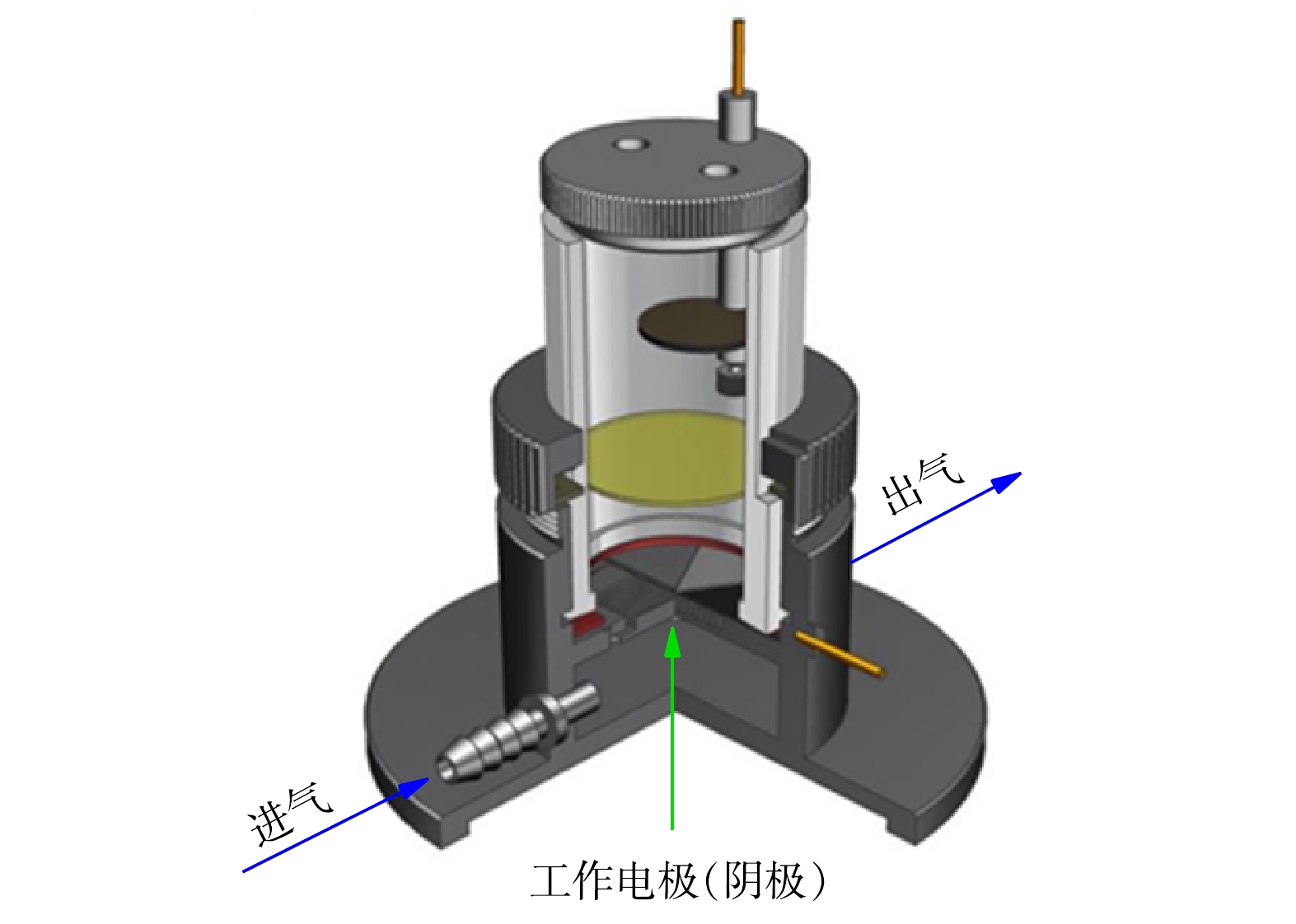
非均相EF降解实验:非均相EF反应在气体扩散反应器中(图1)进行,以均匀负载催化剂的碳布为工作电极,2.0 cm2的铂片和饱和甘汞电极分别为对电极和参比电极,反应通过CHI760E电化学工作站控制,氧气从进气口进入,为保证催化剂不易从碳布上被气流吹落,在装置另一侧设计了出气口。除非另作说明,降解实验均在−0.5 V的恒电位模式下进行。配置含20 mg·L−1 SMZ和50 mmol·L−1的Na2SO4的溶液为模拟制药废水,溶液pH使用NaOH和H2SO4调节,若未作说明,实验在初始pH下进行(pH = 5.2)。降解实验开始前,工作电极浸没在电解液中30 min达到吸附平衡。
SMZ浓度测定:SMZ浓度通过HPLC进行测定,样品通过C18色谱柱进行分离,在柱温30 ℃的条件下,用二极管阵列检测器进行检测,流动相为磷酸二氢钾/乙腈(60:40)混合液,流速为1 mL·min−1,检测波长为270 nm。金属浸出量测定:反应60 min取适量水样经聚四氟乙烯滤膜过滤后,使用ICP测定溶液中铁离子和钴离子浓度。H2O2浓度测定:将样品加入到去离子水、H2SO4与K2TiO(C2O4)2混合溶液中,摇匀后静置10 min显色,在波长386 nm处测定其吸光度[26]。
通过准一级动力学方程对非均相EF降解SMZ的过程进行拟合,SMZ降解速率常数根据式(1)计算。
式中:C0为SMZ初始质量浓度,mg·L−1;t为反应时间,min;C为反应t时的SMZ质量浓度,mg·L−1;k为降解速率常数,min−1。
-
如图2(a)所示,FeCo-ZIF的形貌为表面粗糙的球状微粒,粒径约为300~500 nm,而添加MA后FeCo-ZIF的煅烧产物形貌产生显著变化。如图2(b)所示,FeCo-ZIF的球状结构转变为N-CNT@FeCo相互缠绕的碳纳米管(carbon nanotubes, CNT)结构。
由图3(a)可以看出,N-CNT@FeCo由CNT封装深色的纳米颗粒组成,CNT的管径约为10~120 nm。在图3(b)中能明显观察到对应Fe0.3Co0.7合金中(110)晶面的晶格间距(0.201 nm)[27],结合XRD谱图(图3(c))中45.1°和65.5°处的Fe0.3Co0.7合金特征峰(PDF#50-0795)以及44.0°、51.2°和76.8°处的Fe0.28Co0.72合金特征峰(PDF#51-0740),证实深色纳米颗粒为铁钴合金。此外,在图3(b)观察到0.340 nm的晶格间距对应石墨碳的(002)晶面,XRD谱图中26.5°处也出现了明显的石墨碳特征峰(PDF#41-1487)。这种高结晶度的石墨碳具有优异的导电性能,有利于内层合金纳米颗粒在EF体系中ORR与类芬顿反应的电荷传输。
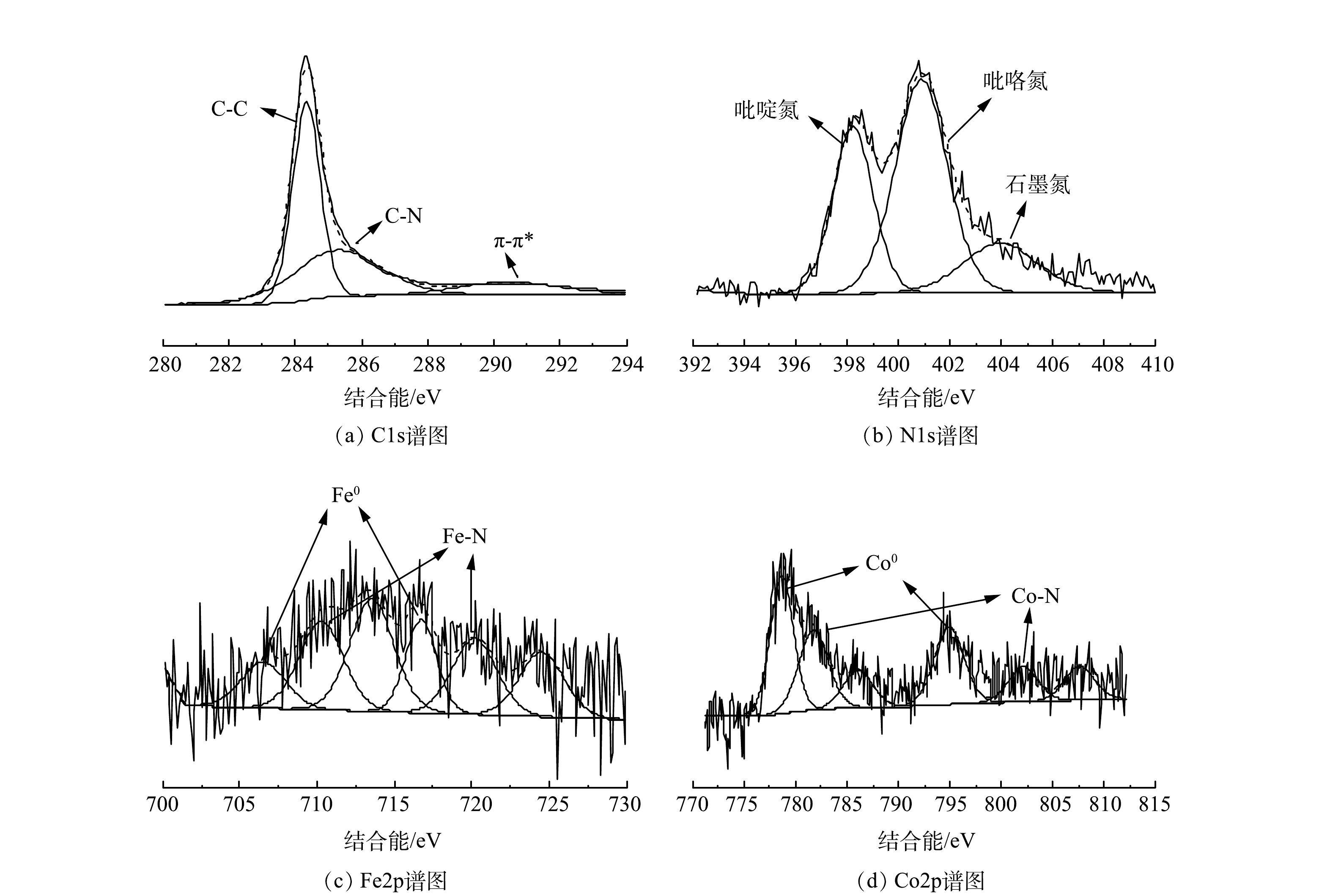
利用XPS分析了材料表面各元素组成及价态。由图4(a)的C1s精细谱中可以看出,位于284.3、285.3和290.4 eV处的3个峰,分别对应石墨碳、C-N和π-π*。这说明碳材料的石墨化程度较高,与TEM以及XRD分析结果一致[28]。在图4(b) 的N1s精细谱中位于398.2、400.9和403.9 eV处的特征峰分别对应吡啶氮、吡咯氮和石墨氮[29]。证明氮在CNT上的成功掺杂,这有利于提高催化剂对2e− ORR的选择性[30-31]。图4(c)和图4(d)中出现的Fe0特征峰(706.5 eV和716.7 eV处)及Co0特征峰(778.7 eV和794.9 eV)则进一步证明了铁钴合金的存在[32]。Co2p精细谱在781.8 eV和802.8 eV处还出现了对应Co-N的特征峰[33],表明铁钴合金可能通过金属-氮(M-N)结合键与外层N-CNT相连。
-
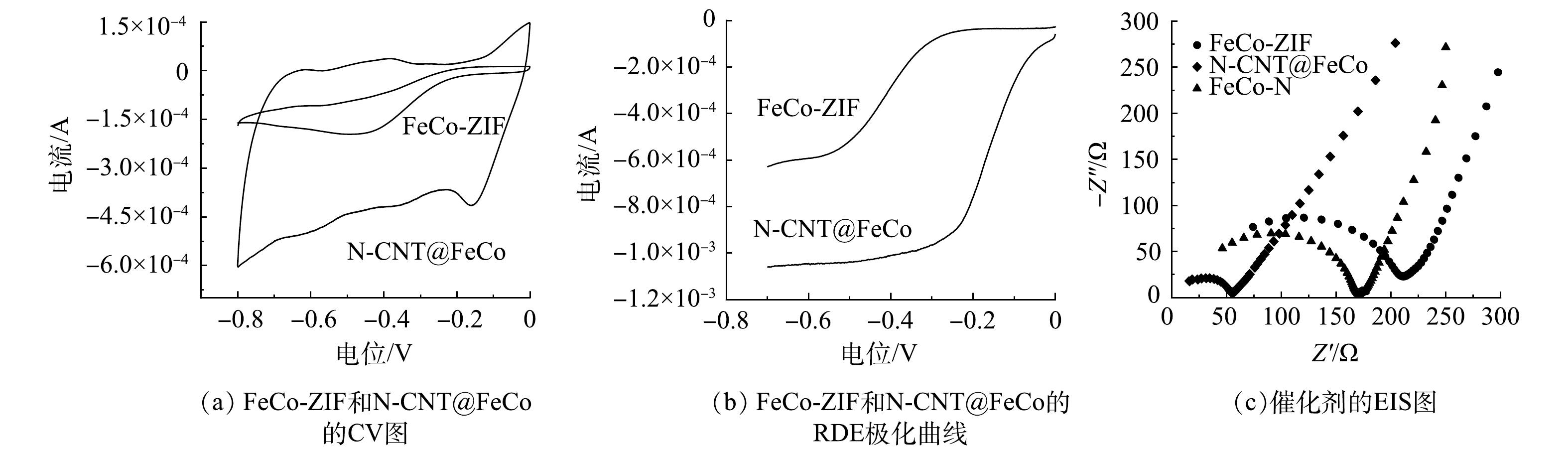
如图5(a)和图5(b)所示,N-CNT@FeCo对ORR的响应电流较FeCo-ZIF明显提升,ORR起始电位更正。表明N-CNT@FeCo的ORR活性高于FeCo-ZIF。如图5(c)所示,催化剂的EIS图均由一段高频区的圆弧和一条低频区的斜线组成。高频区中圆弧的直径代表电荷转移阻抗,直径越小则电荷传递速率越高,低频区斜线的斜率代表电解质和电极间的物质扩散[34-35]。煅烧后的N-CNT@FeCo圆弧直径明显小于FeCo-ZIF,表现出更低的电荷转移阻力(60.7 Ω<187.9 Ω),这也导致了更强的电化学活性,与CV测试结果一致,以上结果表明煅烧处理提高了催化剂的电子传递速率。
-
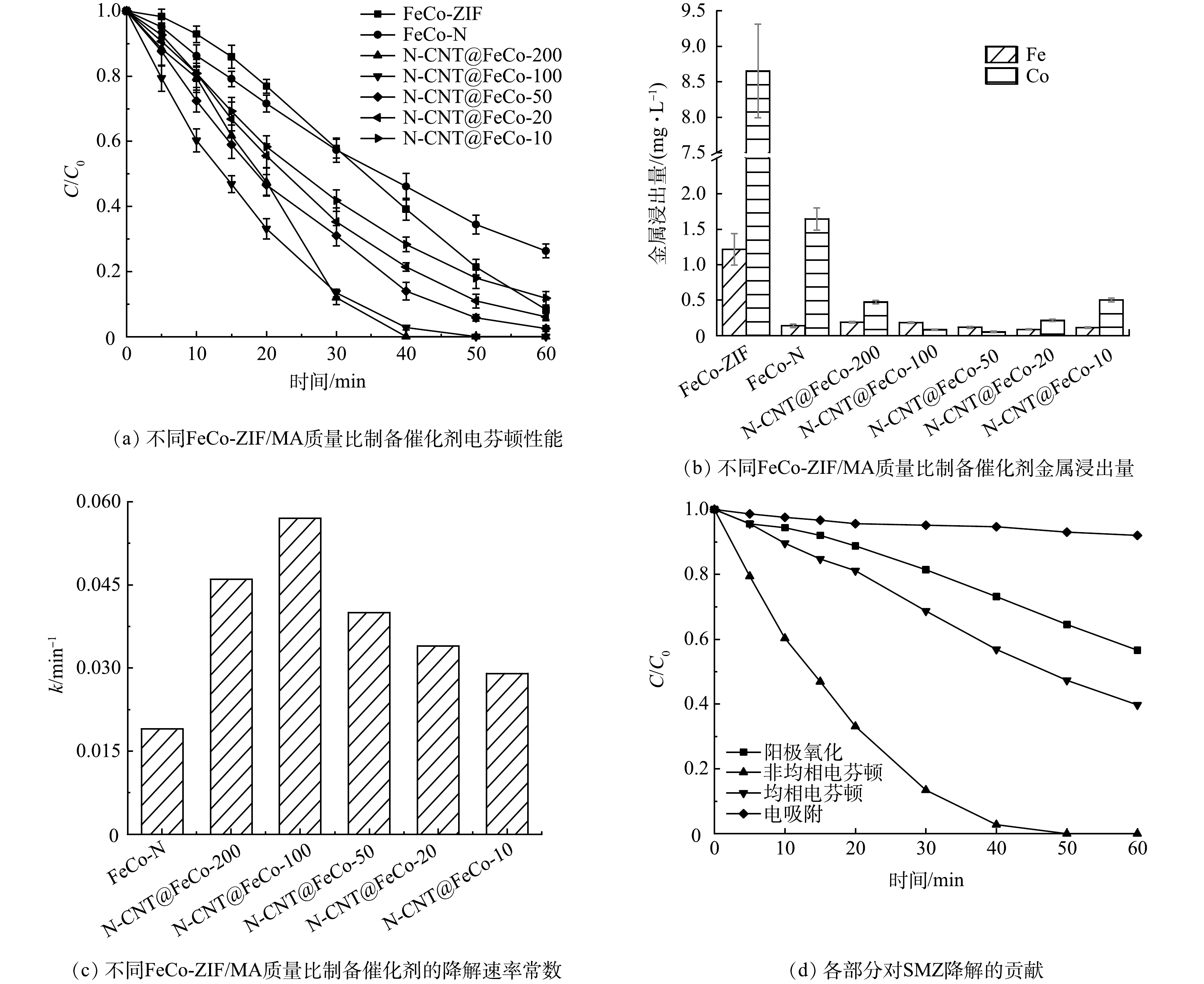
如图6(a)和图6(b)所示,FeCo-ZIF直接应用于EF降解SMZ,虽然60 min对SMZ的去除率可达91.7%,但铁离子和钴离子的浸出量高达1.22 mg·L−1和8.65 mg·L−1,均相EF的作用不容忽视。由FeCo-ZIF直接煅烧得到的FeCo-N在EF反应中催化氧化SMZ的活性及稳定性低,在60 min对SMZ的去除率仅为73.7%,且钴离子的浸出量仍有1.65 mg·L−1。图2(c)中看到,FeCo-N微球表面密集覆盖了大量金属团簇,在EF反应中直接暴露在强氧化环境下,导致材料稳定性较差。而FeCo-ZIF在添加不同比例的MA煅烧后得到的N-CNT@FeCo-M,反应60 min时对SMZ的去除率提升至88.2%~100%,钴的浸出量下降至0.05~0.50 mg·L−1,性能最佳的N-CNT@FeCo-100的k值高达0.057 min−1,约为FeCo-N的3倍(图6(c)),60 min可实现SMZ的完全降解,TOC去除率为45.3%。
EIS测试结果(图5(c))表明,N-CNT@FeCo-100(60.7 Ω)的电荷转移阻力远小于FeCo-N(145.9 Ω)。这可能得益于添加MA后衍生出的N-CNT能加速电荷传输[36-37]。结合N-CNT@FeCo的表征分析,MA作为碳源和氮源衍生出的N-CNT能通过促进内部铁钴合金之间的电子转移,提高EF催化活性,同时N-CNT作为金属纳米颗粒的保护层,还能有效提高内层合金在强氧化环境下的化学稳定性。综上所述,优选FeCo-ZIF与MA质量比为1:100进行后续研究。
SMZ的降解通常是电吸附、阳极氧化、均相EF以及非均相EF过程共同作用的结果,因此评估了反应40 min时各部分的贡献率,结果如图6(d)所示。电吸附、阳极氧化、均相EF以及非均相EF过程对SMZ降解的贡献率依次为5.5%、22.1%、16.7%和55.7%,说明非均相EF对SMZ的降解起主要作用。
-
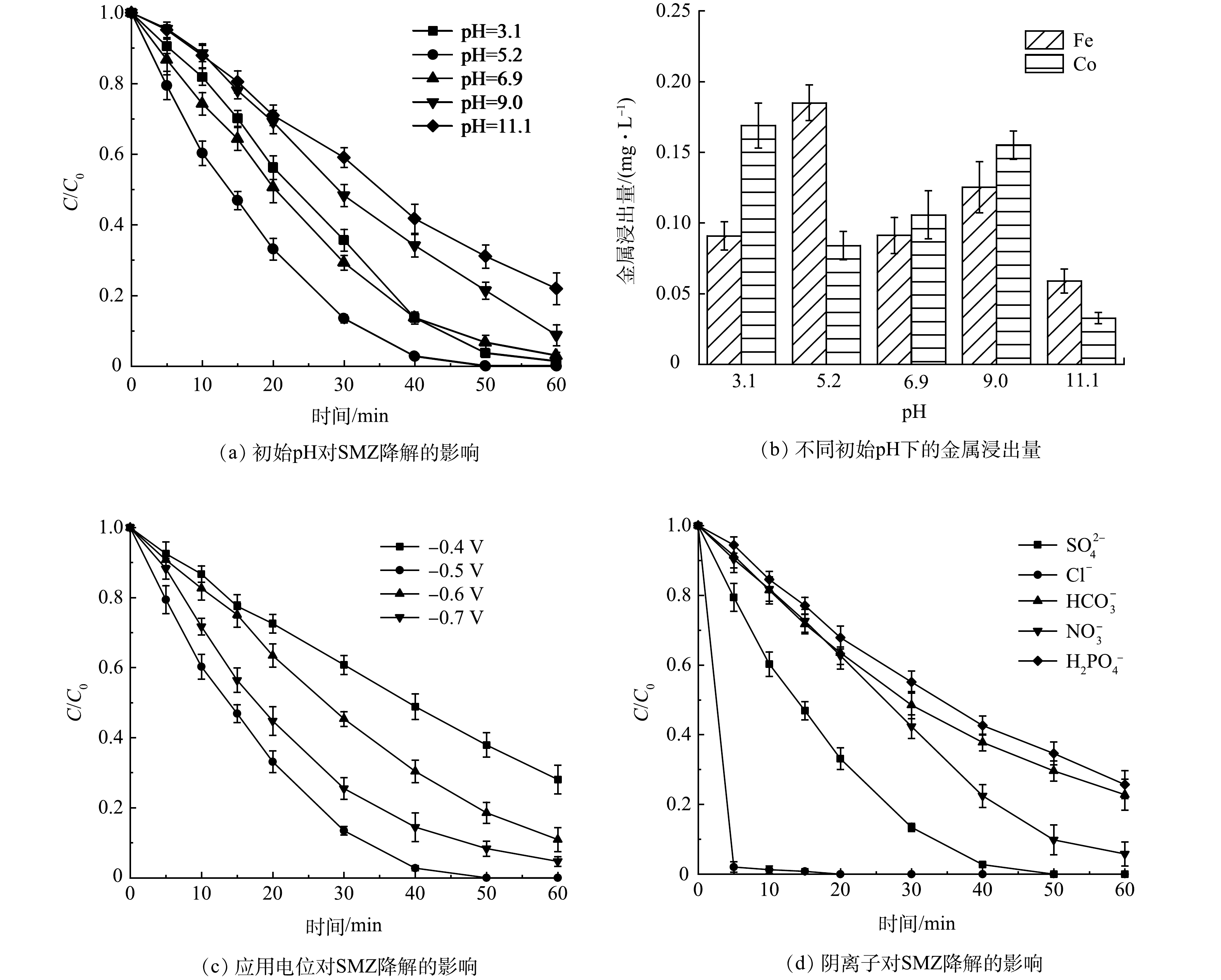
1) pH的影响。不同初始pH下SMZ的降解效率变化如图7(a)所示。EF性能随着初始pH的增大先升高后下降,无需调节pH时(pH=5.2)降解性能最佳,40 min对SMZ的去除率即可达到97.3%。由图7(b)可知,即使在pH=3.1时,催化剂仍保持高稳定性,铁和钴的浸出量分别仅有0.09 mg·L−1和0.17 mg·L−1。综上所述,后续实验选择在pH=5.2下进行。
2)应用电位的影响。如图7(c)所示,当应用电位为-0.5 V时,在60 min时SMZ的去除率为100%。将应用电位调节至-0.6 V时,SMZ的去除率下降了11.0%。这是由析氢反应以及H2O2被进一步还原为H2O所致,系列副反应抑制了SMZ降解。选择应用电位为-0.5 V进行后续实验。
3)阴离子的影响。由图7(d)可见,Cl−能够促进SMZ的降解。这是因为Cl−在阳极生成强氧化性的HClO[38]。而其余3种阴离子抑制了SMZ的降解,抑制作用从强到弱依次为H2PO4−≈HCO3−>NO3−,因为3种阴离子均会淬灭·OH生成氧化性更弱的自由基[39]。
-
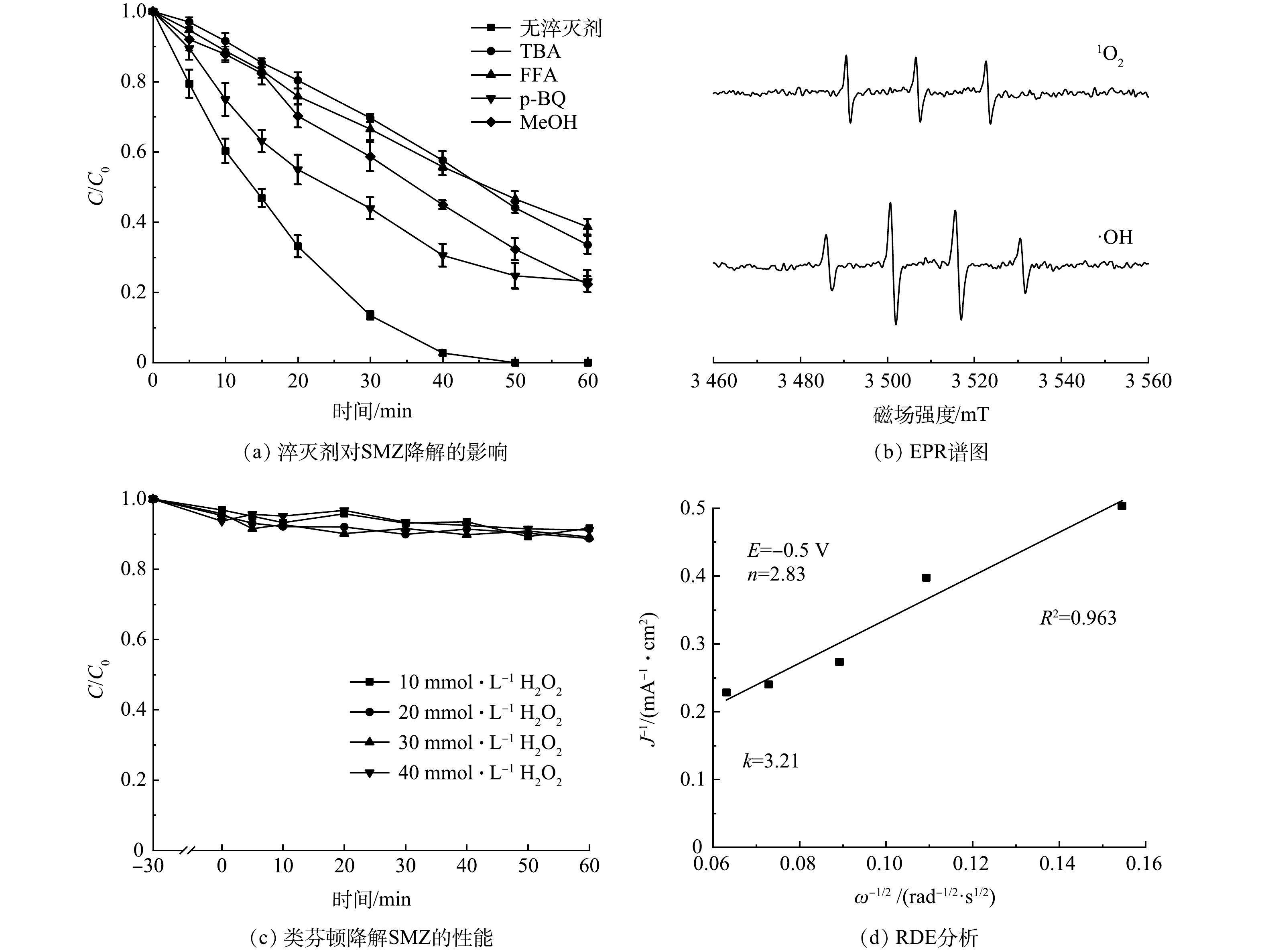
使用TBA(100 mmol·L−1)、FFA(5 mmol·L−1)、p-BQ(5 mmol·L−1)、MeOH(50 mmol·L−1)分别作为·OH、1O2、·O2−、·SO4−的淬灭剂[40],在此条件下TBA、FFA和MeOH对·OH的淬灭能力维持在同一水平。如图8(a)所示,TBA的加入对SMZ降解产生明显抑制,而加入FFA的抑制效果(38.7%)与TBA(33.6%)几乎一致。这说明·OH对污染物的降解起到了至关重要的作用,1O2虽然能通过链式反应生成,但其贡献较小。MeOH对SMZ降解的抑制作用弱于TBA,说明·SO4−的贡献可以忽略不计。淬灭·O2−不但会抑制H2O2的生成,同时也会抑制Fe(Ⅲ)还原为Fe(Ⅱ),降低了·OH的生成速率,进而削弱了SMZ的降解[41-42]。图8(b)为电子顺磁共振(EPR)测试结果。可以看出,基于N-CNT@FeCo的EF体系中出现了DMPO-OH和TEMP-1O2的特征峰,证实·OH和1O2的存在[24]。综上所述,污染物的降解是以·OH为主导,1O2和·O2−共同参与的过程。
此外,还考察了N-CNT@FeCo在类芬顿体系中直接活化H2O2的能力,发现N-CNT@FeCo(图8(c))无法有效地活化H2O2生成·OH降解水中的SMZ。这是因为碳材料自身活化H2O2的能力较差。同时铁钴合金被较厚的碳层(图2(a))包裹,在碳层层数较厚(> 3层)的情况下电子难以从铁钴合金中传递到碳材料表面[43],因此N-CNT@FeCo的非均相芬顿活性差,以上结果证实了电场不可或缺的作用。
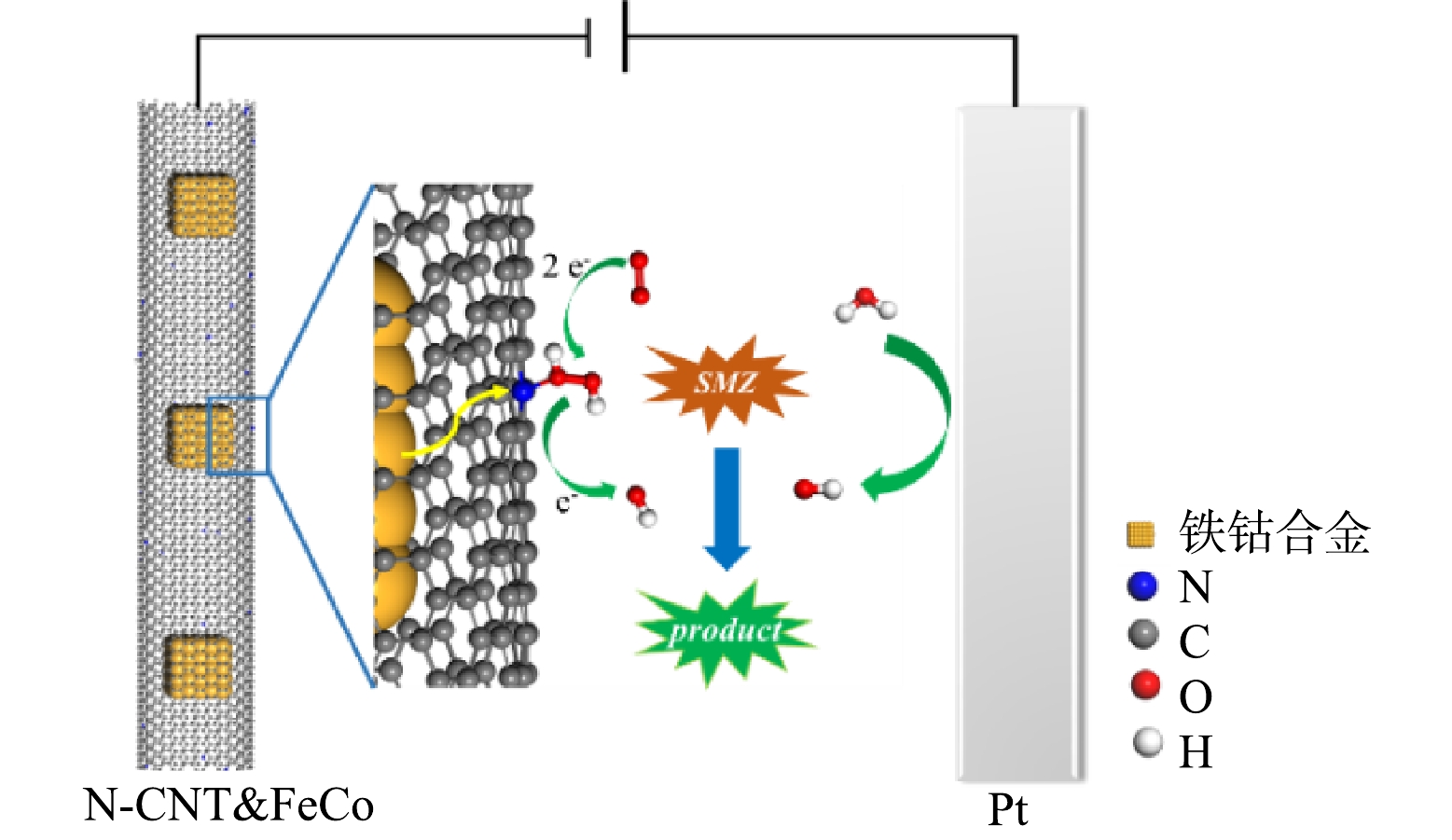
N-CNT已经被证实是一种有效的2e− ORR催化剂,其中石墨氮是生成H2O2的主要活性位点,基于N-CNT@FeCo的EF技术可能的机理如图9所示。虽然被N-CNT封装的铁钴合金不直接参与H2O2的活化,但在电场作用下其具有强给电子能力,使N-CNT表面生成的H2O2在解吸前就得到电子被还原为·OH,O2通过如式(2)所示的3电子途径直接生成·OH[44],因此,溶液中H2O2浓度维持在较低水平(< 5.0 mg·L−1)。RDE测试结果如图8(d)所示,在−0.5 V的应用电位下N-CNT@FeCo的ORR电子转移数为2.83,近似于3的电子转移数也证明O2可能通过式(2)生成·OH,这种3电子氧还原途径能有效地避免传统EF中过渡金属氧化还原电对循环速率慢的问题。
-
N-CNT@FeCo的循环回用性能如表1所示,经过5次循环后60 min时SMZ的去除率仍有96.0%,且铁、钴离子最高浸出质量浓度分别仅为0.31 mg·L−1和0.30 mg·L−1,表明N-CNT@FeCo具有良好的稳定性能。
-
1)以MA为碳源和氮源,在铁钴合金外衍生的N-CNT可同时提高催化剂的活性和稳定性。在FeCo-ZIF与MA质量比为1:100条件下制备的N-CNT@FeCo具有最佳的催化性能,相较于FeCo-N,在60 min对SMZ的去除率提升了26.3%,而金属浸出量降低了84.9%。
2)在初始pH为5.2,应用电位为-0.5 V的最佳运行条件下,N-CNT@FeCo的k值可达0.057 min−1。H2PO4−、HCO3−和NO3−会抑制SMZ的降解,而Cl−会促进污染物的降解。N-CNT@FeCo循环回用5次,60 min对SMZ的去除率仍有96.0%。
3)·OH是主要的活性物种,1O2和·O2−也参与了SMZ的降解;结合H2O2累积浓度检测、非均相芬顿实验以及RDE检测,推测基于N-CNT@FeCo的EF体系中O2是通过3电子还原生成·OH。
4)反应40 min时电吸附、阳极氧化、均相EF以及非均相EF对SMZ降解的贡献率依次为5.5%、22.1%、16.7%和55.7%。
封装型双金属阴极催化剂强化电芬顿技术高效去除磺胺甲恶唑
Highly efficient removal of sulfamethoxazole by encapsulated bimetallic cathode catalyst enhanced electro-Fenton technology
-
摘要: 传统非均相电芬顿(EF)技术主要面临活性氧物种生成速率慢、催化剂稳定性差等不足。将FeCo-ZIF和三聚氰胺(MA)共混(质量比为1:100)煅烧,成功制备出氮掺杂碳纳米管封装铁钴合金阴极催化剂(N-CNT@FeCo),可强化EF高效去除水中磺胺甲恶唑(SMZ,初始质量浓度为20 mg·L−1),在近中性条件下50 min内即可完全去除,降解速率常数可达0.057 min−1,是单独煅烧FeCo-ZIF制备的裸露型双金属催化剂FeCo-N的3倍,且前者的金属浸出总量(0.27 mg·L−1)仅为后者(1.79 mg·L−1)的15.1%。循环回用5次后,60 min内N-CNT@FeCo对SMZ的去除率仍可达到96.0%。扫描电子显微镜表征与电化学阻抗测试结果表明,由MA诱导生成的N-CNT,不仅通过封装结构有效限制了内部铁钴合金受强氧化性环境腐蚀破坏,而且显著加速了内部铁钴合金的电子传递速率,N-CNT@FeCo的独特封装结构使其兼具高催化活性和高稳定性。本研究为高效稳定的阴极催化剂提供了稳定、可控、易放大的封装策略。Abstract: The conventional non-homogeneous electro-Fenton (EF) technology mainly faces the deficiencies of slow generation rate of active oxygen species and poor catalyst stability. A nitrogen-doped carbon nanotube-encapsulated iron-cobalt alloy cathode catalyst (N-CNT@FeCo) was successfully prepared by calcination of FeCo-ZIF and melamine (MA) in a co-blend with mass ratio of 1:100, which could enhance EF to efficiently remove sulfamethoxazole (SMZ, initial concentration set as 20 mg·L−1) from water, and SMZ could be completely removed within 50 min under near-neutral conditions with degradation. The degradation rate constant was up to 0.057 min−1, which was two times higher than that of the bare bimetallic catalyst FeCo-N prepared by calcination of FeCo-ZIF alone, and the total metal leaching from the former (0.27 mg·L−1) was only 15.1% of that from the latter (1.79 mg·L−1). 96.0% of SMZ removal was still achieved at 60 min when N-CNT@FeCo was recycled after five times. Scanning electron microscopy analysis and electrochemical impedance test results showed that the N-CNT induced by MA not only effectively limited the corrosion damage of the internal iron-cobalt alloy by the strong oxidizing environment through the encapsulation structure, but also significantly accelerated the electron transfer rate of the internal iron-cobalt alloy, and the unique encapsulation structure of N-CNT@FeCo made it both highly catalytic activity and highly stable. This study provides a stable, tunable and easy to enlarge encapsulation strategy for the preparation of efficient and stable cathode catalysts.
-
Key words:
- electro-Fenton /
- encapsulate /
- bimetallic /
- iron-cobalt alloy /
- sulfamethoxazole
-

-
表 1 N-CNT@FeCo的循环回用性能
Table 1. Cyclic reuse performance of N-CNT@FeCo
循环次数 去除率/% 浸出量/(mg·L-1) Fe Co 第1次 100 0.19 0.08 第2次 100 0.27 0.10 第3次 96.6 0.31 0.30 第4次 96.0 0.24 0.27 第5次 96.0 0.13 0.28 -
[1] 孟庆玲, 欧晓霞, 张梦然, 等. 抗生素污染废水处理技术研究进展[J]. 绿色科技, 2021, 23(2): 81-83. doi: 10.3969/j.issn.1674-9944.2021.02.029 [2] 齐亚兵, 张思敬, 孟晓荣, 等. 抗生素废水处理技术现状及研究进展[J]. 应用化工, 2021, 50(9): 2587-2593. doi: 10.3969/j.issn.1671-3206.2021.09.054 [3] CARVALHO I T, SANTOS L. Antibiotics in the aquatic environments: A review of the European scenario[J]. Environment International, 2016, 94: 736-757. doi: 10.1016/j.envint.2016.06.025 [4] 赵富强, 高会, 张克玉, 等. 中国典型河流水域抗生素的赋存状况及风险评估研究[J]. 环境污染与防治, 2021, 43(1): 94-102. doi: 10.15985/j.cnki.1001-3865.2021.01.018 [5] ZHANG J J, LIU X J, ZHU Y T, et al. Antibiotic exposure across three generations from Chinese families and cumulative health risk[J]. Ecotoxicology and Environmental Safety, 2020, 191: 110237. doi: 10.1016/j.ecoenv.2020.110237 [6] QIU S Y, WANG Y, WAN J Q, et al. Enhanced electro-Fenton catalytic performance with in-situ grown Ce/Fe@NPC-GF as self-standing cathode: Fabrication, influence factors and mechanism[J]. Chemosphere, 2021, 273: 130269. doi: 10.1016/j.chemosphere.2021.130269 [7] DU X, FU W, SU P, et al. Trace FeCu@PC derived from MOFs for ultraefficient heterogeneous electro-Fenton process: Enhanced electron transfer and bimetallic synergy[J]. ACS ES& T Engineering, 2021, 1(9): 1311-1322. [8] CHENG S, ZHENG H, SHEN C, et al. Hierarchical iron phosphides composite confined in ultrathin carbon layer as effective heterogeneous electro-Fenton catalyst with prominent stability and catalytic activity[J]. Advanced Functional Materials, 2021, 31(48): 2106311. doi: 10.1002/adfm.202106311 [9] 王奇, 潘家荣, 梅朋森, 等. 电Fenton及光电Fenton法废水处理技术研究进展[J]. 三峡大学学报(自然科学版), 2008(2): 89-94. [10] LIU X C, HE C S, SHEN Z Y, et al. Mechanistic study of Fe(III) chelate reduction in a neutral electro-Fenton process[J]. Applied Catalysis B:Environmental, 2020, 278: 119347. doi: 10.1016/j.apcatb.2020.119347 [11] WANG Y Z, ZHANG H M, LI B K, et al. γ-FeOOH graphene polyacrylamide carbonized aerogel as air-cathode in electro-Fenton process for enhanced degradation of sulfamethoxazole[J]. Chemical Engineering Journal, 2019, 359: 914-923. doi: 10.1016/j.cej.2018.11.096 [12] GANIYU S O, ZHOU M H, MARTÍNEZ-HUITLE C A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment[J]. Applied Catalysis B:Environmental, 2018, 235: 103-129. doi: 10.1016/j.apcatb.2018.04.044 [13] ZHANG J J, QIU S, FENG H P, et al. Efficient degradation of tetracycline using core–shell Fe@Fe2O3-CeO2 composite as novel heterogeneous electro-Fenton catalyst[J]. Chemical Engineering Journal, 2022, 428: 131403. doi: 10.1016/j.cej.2021.131403 [14] ZHAO K, QUAN X, CHEN S, et al. Enhanced electro-Fenton performance by fluorine-doped porous carbon for removal of organic pollutants in wastewater[J]. Chemical Engineering Journal, 2018, 354: 606-615. doi: 10.1016/j.cej.2018.08.051 [15] YE Z H, PADILLA J A, XURIGUERA E, et al. A highly stable metal–organic framework-engineered FeS2/C nanocatalyst for heterogeneous electro-Fenton treatment: Validation in wastewater at mild pH[J]. Environmental Science & Technology, 2020, 54(7): 4664-4674. [16] YANG T Y, YU D Y, WANG D, et al. Accelerating Fe(Ⅲ)/Fe(Ⅱ) cycle via Fe(Ⅱ) substitution for enhancing Fenton-like performance of Fe-MOFs[J]. Applied Catalysis B:Environmental, 2021, 286: 119859. doi: 10.1016/j.apcatb.2020.119859 [17] YIN Y, REN Y, LU J H, et al. The nature and catalytic reactivity of UiO-66 supported Fe3O4 nanoparticles provide new insights into Fe-Zr dual active centers in Fenton-like reactions[J]. Applied Catalysis B:Environmental, 2021, 286: 119943. doi: 10.1016/j.apcatb.2021.119943 [18] YUAN R R, QIU J L, YUE C L, et al. Self-assembled hierarchical and bifunctional MIL-88A(Fe)@ZnIn2S4 heterostructure as a reusable sunlight-driven photocatalyst for highly efficient water purification[J]. Chemical Engineering Journal, 2020, 401: 126020. doi: 10.1016/j.cej.2020.126020 [19] ZHOU L, LI N, OWENS G, et al. Simultaneous removal of mixed contaminants, copper and norfloxacin, from aqueous solution by ZIF-8[J]. Chemical Engineering Journal, 2019, 362: 628-637. doi: 10.1016/j.cej.2019.01.068 [20] LI H X, ZHANG J, YAO Y Z, et al. Nanoporous bimetallic metal-organic framework (FeCo-BDC) as a novel catalyst for efficient removal of organic contaminants[J]. Environmental Pollution, 2019, 255: 113337. doi: 10.1016/j.envpol.2019.113337 [21] LI H X, YANG Z X, LU S, et al. Nano-porous bimetallic CuCo-MOF-74 with coordinatively unsaturated metal sites for peroxymonosulfate activation to eliminate organic pollutants: Performance and mechanism[J]. Chemosphere, 2021, 273: 129643. doi: 10.1016/j.chemosphere.2021.129643 [22] DU J, LI F, SUN L C. Metal–organic frameworks and their derivatives as electrocatalysts for the oxygen evolution reaction[J]. Chemical Society Reviews, 2021, 50(4): 2663-2695. doi: 10.1039/D0CS01191F [23] SUN L, CAMPBELL M G, DINCĂ M. Electrically conductive porous metal–organic frameworks[J]. Angewandte Chemie International Edition, 2016, 55(11): 3566-3579. doi: 10.1002/anie.201506219 [24] CHENG S, SHEN C, ZHENG H, et al. OCNTs encapsulating Fe-Co PBA as efficient chainmail-like electrocatalyst for enhanced heterogeneous electro-Fenton reaction[J]. Applied Catalysis B:Environmental, 2020, 269: 118785. doi: 10.1016/j.apcatb.2020.118785 [25] MENG J S, NIU C J, XU L H, et al. General oriented formation of carbon nanotubes from metal–organic frameworks[J]. Journal of the American Chemical Society, 2017, 139(24): 8212-8221. doi: 10.1021/jacs.7b01942 [26] 陆平. 草酸钛钾分光光度法测定Fenton高级氧化系统中的过氧化氢[J]. 建筑工程技术与设计, 2014(8): 582-582,517. doi: 10.3969/j.issn.2095-6630.2014.08.553 [27] LI Y S, FENG Y, LI L, et al. PBA@PPy derived N-doped mesoporous carbon nanocages embedded with FeCo alloy nanoparticles for enhanced performance of oxygen reduction reaction[J]. Journal of Alloys and Compounds, 2020, 823: 153892. doi: 10.1016/j.jallcom.2020.153892 [28] AGO H, KUGLER T, CACIALLI F, et al. Work functions and surface functional groups of multiwall carbon nanotubes[J]. The Journal of Physical Chemistry B, 1999, 103(38): 8116-8121. doi: 10.1021/jp991659y [29] LIU Q T, LIU X F, ZHENG L R, et al. The solid-phase synthesis of an Fe-N-C electrocatalyst for high-power proton-exchange membrane fuel cells[J]. Angewandte Chemie International Edition, 2018, 57(5): 1204-1208. doi: 10.1002/anie.201709597 [30] SU P, ZHOU M H, LU X Y, et al. Electrochemical catalytic mechanism of N-doped graphene for enhanced H2O2 yield and in-situ degradation of organic pollutant[J]. Applied Catalysis B:Environmental, 2019, 245: 583-595. doi: 10.1016/j.apcatb.2018.12.075 [31] HAIDER M R, JIANG W L, HAN J L, et al. In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants[J]. Applied Catalysis B:Environmental, 2019, 256: 117774. doi: 10.1016/j.apcatb.2019.117774 [32] LIU X, WANG L, YU P, et al. A stable bifunctional catalyst for rechargeable zinc-air batteries: Iron-cobalt nanoparticles embedded in a nitrogen-doped 3D carbon matrix[J]. Angewandte Chemie International Edition, 2018, 57(49): 16166-16170. doi: 10.1002/anie.201809009 [33] LIANG H W, WEI W, WU Z S, et al. Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction[J]. Journal of the American Chemical Society, 2013, 135(43): 16002-5. doi: 10.1021/ja407552k [34] FAN L S, WU H X, WU X, et al. Fe-MOF derived jujube pit like Fe3O4/C composite as sulfur host for lithium-sulfur battery[J]. Electrochimica Acta, 2019, 295: 444-451. doi: 10.1016/j.electacta.2018.08.107 [35] SU P, ZHOU M H, REN G B, et al. A carbon nanotube-confined iron modified cathode with prominent stability and activity for heterogeneous electro-Fenton reactions[J]. Journal of Materials Chemistry A, 2019, 7(42): 24408-24419. doi: 10.1039/C9TA07491K [36] QIN Y X, ZHANG L Z, AN T C. Hydrothermal carbon-mediated Fenton-like reaction mechanism in the degradation of alachlor: Direct electron transfer from hydrothermal carbon to Fe(III)[J]. ACS Applied Materials & Interfaces, 2017, 9(20): 17115-17124. [37] YOO S H, JANG D, JOH H-I, et al. Iron oxide/porous carbon as a heterogeneous Fenton catalyst for fast decomposition of hydrogen peroxide and efficient removal of methylene blue[J]. Journal of Materials Chemistry A, 2017, 5(2): 748-755. doi: 10.1039/C6TA07457J [38] RAO X F, SHAO X L, XU J, et al. Efficient nitrate removal from water using selected cathodes and Ti/PbO2 anode: Experimental study and mechanism verification[J]. Separation and Purification Technology, 2019, 216: 158-165. doi: 10.1016/j.seppur.2019.02.009 [39] WANG J L, WANG S Z. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism[J]. Chemical Engineering Journal, 2020, 401: 126158. doi: 10.1016/j.cej.2020.126158 [40] LIU Z, DING H J, ZHAO C, et al. Electrochemical activation of peroxymonosulfate with ACF cathode: Kinetics, influencing factors, mechanism, and application potential[J]. Water Research, 2019, 159: 111-121. doi: 10.1016/j.watres.2019.04.052 [41] CAO P K, QUAN X, ZHAO K, et al. Selective electrochemical H2O2 generation and activation on a bifunctional catalyst for heterogeneous electro-Fenton catalysis[J]. Journal of Hazardous Materials, 2020, 382: 121102. doi: 10.1016/j.jhazmat.2019.121102 [42] YANG Z C, QIAN J S, YU A Q, et al. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement[J]. Proceedings of the National Academy of Sciences, 2019, 116(14): 6659-6664. doi: 10.1073/pnas.1819382116 [43] DENG J, YU L, DENG D H, et al. Highly active reduction of oxygen on a FeCo alloy catalyst encapsulated in pod-like carbon nanotubes with fewer walls[J]. Journal of Materials Chemistry A, 2013, 1(47): 14868-14873. doi: 10.1039/c3ta13759g [44] XIAO F, WANG Z N, FAN J Q, et al. Selective electrocatalytic reduction of oxygen to hydroxyl radicals via 3-electron pathway with FeCo alloy encapsulated carbon aerogel for fast and complete removing pollutants[J]. Angewandte Chemie International Edition, 2021, 60(18): 10375-10383. doi: 10.1002/anie.202101804 -



 下载:
下载: