-
挥发性有机化合物(volatile organic compounds,VOCs)是一类重要大气污染物,与大气颗粒物、SO2、NOx同样为研究热点。世界卫生组织将饱和蒸汽压超过133.32 Pa、常压沸点为50~260 ℃的有机物定义为VOCs[1-2]。目前,VOCs处理技术包括吸附[3]、冷凝、热焚烧、催化燃烧、光催化[4]、等离子体催化降解技术[5]等。催化燃烧技术具有处理效率高、能耗低、无二次污染、适用范围广等优点,在VOCs治理领域具有广阔应用前景[6-10]。
研制高效稳定的催化剂是催化燃烧技术发展的核心[11-12]。常见的催化剂有贵金属和非贵金属氧化物催化剂[13-14]。常见的负载制备贵金属催化剂,一般以Pd、Pt、Rh、Pb等为活性组分,对于多种VOCs的催化燃烧具有较高活性[15-16]。钌(Ru) 作为铂系金属元素中最后被发现的金属元素,其活性较高,化学稳定性较强,低温催化效果较好,且Ru的价格相较于其他贵金属更为低廉。Ru与Au、Pt、Pd的催化活性差别不大,且在对乙酸乙酯、丁烷、丙烯等VOCs的催化活性较强,产物基本为CO2和H2O[17-18]。由于贵金属本身价格较昂贵、易挥发且高温下容易烧结,工业上常用比表面积高的载体来提高贵金属催化剂活性组分与反应物分子的接触面积,从而提高催化剂的活性及稳定性[19]。而沸石分子筛是一类具有孔道结构的优良材料,具有丰富的微孔、较大的比表面积、优异的热稳定性和较多的酸位点,可为活性组分提供有效表面和适宜孔结构,以降低活性组分的团聚,并增强催化剂的机械强度,为适宜的催化剂载体材料[20-23]。CeO2具有立方萤石结构[24],Ce3+和Ce4+间转换表现出独特的储氧能力和表面还原性,能有效提高负载型贵金属催化剂的催化活性及稳定性[25-27]。金属相互作用对催化剂活性具有促进作用[28-29]。王玉亭等[30]用Ce改性V2O5/TiO2催化剂发现投加浓度质量分数为10%的Ce可令催化剂在低浓度邻二甲苯的条件下有较好的氧化效率,T90%为380 ℃;SCIRÈ等[31]发现,Au/CeO2催化剂催化燃烧甲苯比CeO2催化剂具有更好的催化活性,由于Au的存在能减弱催化剂表面的Ce—O键强度,提高了催化剂表面晶格氧的流动性和活性,促进了催化剂在VOCs燃烧的活性。因此,以氧化铈与贵金属作为活性组分的催化剂,因其优异的氧转移机制及金属相互作用而备受研究者青睐。
本研究选取贵金属Ru和稀土金属Ce作为活性组分,将其负载到分子筛上制备成RuCe/ZSM-5催化剂,考察钌铈催化剂催化燃烧甲苯的性能,并通过改变Ru的负载量,调整实验条件及工况,得到活性优、选择性好、稳定性高的钌铈双金属催化剂,以期为贵金属催化技术在VOCs治理领域的应用提供参考。
-
RuCe/ZSM-5催化剂采用等体积浸渍法制备,具体方法为:取适量Ce(NO3)3溶液与适量亚硝酰硝酸钌(Ⅲ)溶液混合均匀;将混合前驱体溶液倒入经过450 ℃焙烧预处理1 h的ZSM-5分子筛中,加入适量的高纯水直到溶液刚好完全覆盖样品;室温充分搅拌3 h,置于80 ℃烘箱内12 h蒸干混合物;于500 ℃马弗炉中焙烧3 h,研磨筛分至40~60目保存使用。根据亚硝酰硝酸钌(Ⅲ)溶液添加量的不同制备了一系列不同Ru含量负载的Ru(a)/CeO2(20)-ZSM-5催化剂。催化剂表示为Ru(a)/CZ,a为Ru元素的负载量(%),实验中a取0.5、1.0和1.5。
-
采用X射线衍射仪(XRD,Bruker D8 ADVANCE,CuKα辐射,40 kV,120 mA)测试催化剂的晶体结构,扫描速度2θ=5 º·min−1,扫描范围4~85 °。获得数据使用Jade 6.5软件进行分析处理。样品的比表面积和孔径分布使用Micromeritics ASAP 2620全自动物理吸附仪测定。采用蔡司仪器的Gemini 300分析材料表面形貌,加速电压为30 kV。测试前需对样品进行真空喷金处理120 s以增强导电性。
-
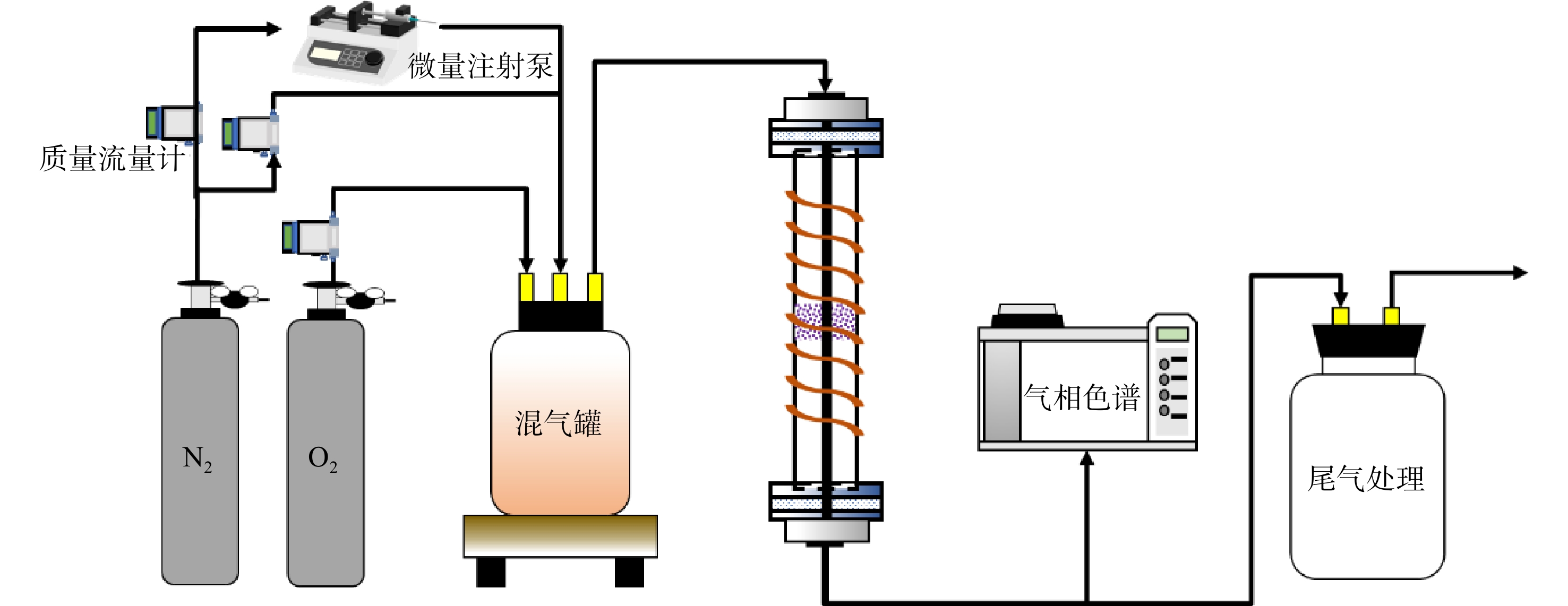
采用实验室自主搭建的固定床-色谱测试装置(图1)对制备的催化剂活性进行评价。装置主要包括气体管路、固定床反应器、流量控制和气体检测4个部分。反应物甲苯(液相)采用微量注射泵注射进入汽化室汽化后在混合室与载气充分混合进入固定床反应器进行燃烧。水蒸气同样利用微量注射泵将液态水加热汽化后与其他气路的气体进行混合。催化反应发生在常压状态下,在中间装有石英筛板的石英管(内径为9 mm)的固定床反应器上进行。催化剂的装填量约为100 mg。由于催化剂为较为致密的粉末,且催化反应为强放热反应,为保证催化剂通气顺畅及受热均匀,催化剂与石英砂按1:4的质量比混合均匀后装填至石英管中。催化反应后的气体进入配备有甲烷转化炉和FID检测器的Thermo Fisher Scientific Trace 1300气相色谱仪进行在线连续检测。用甲苯转化率为50%、90%时的温度T50%、T90%来反映催化剂的反应活性。
-
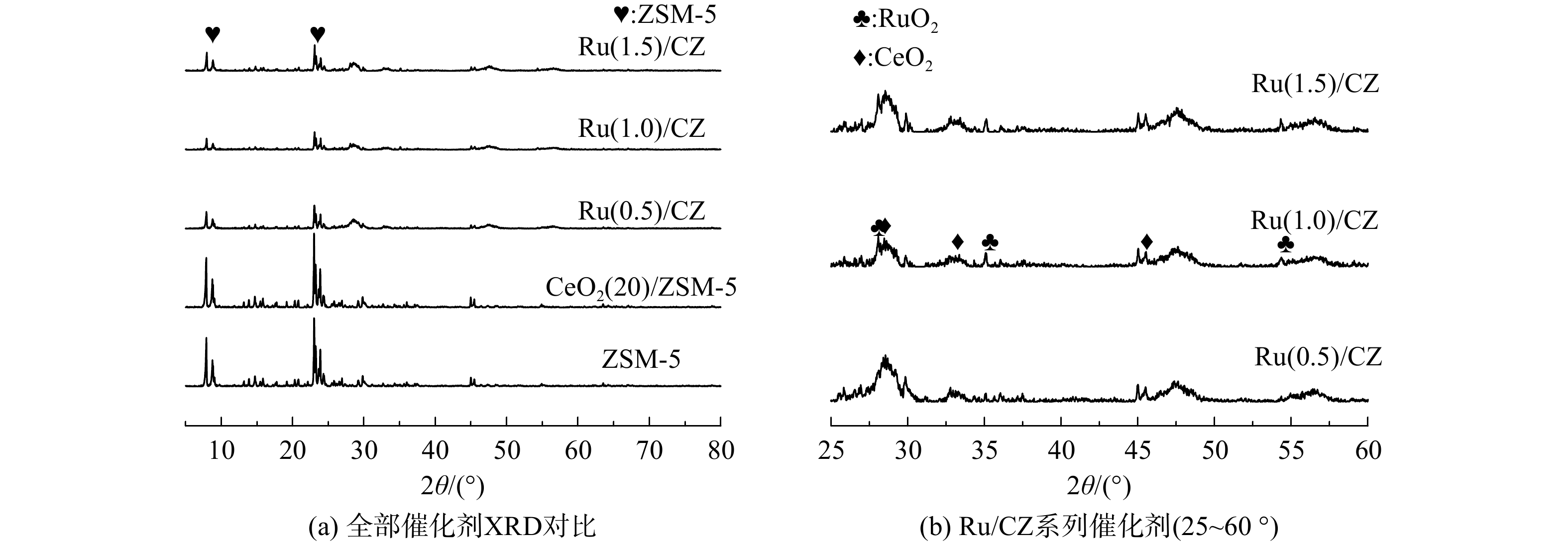
ZSM-5分子筛、CeO2(20)/ZSM-5催化剂和Ru/CZ系列催化剂XRD表征结果如图2所示。Ru和Ce的引入并未改变ZSM-5分子筛的结构,ZSM-5分子筛的特征峰衍射强度仍较高,但引入Ru的Ru/CZ催化剂ZSM-5分子筛衍射峰强度下降。猜测可能是由于Ru的引入降低了ZSM-5分子筛的结晶度,但催化剂载体ZSM-5结构并无明显变化。由于催化剂元素种类较多,不能清晰地分辨负载有Ru元素的Ru/CZ系列催化剂中Ru和Ce元素的XRD衍射峰,故将Ru/CZ系列催化剂3条谱线在25~60 °这一部分放大来观察,见图2(b)。在2θ为28.0 °、35.0 °、54.2 °处,出现了分别对应RuO2(110)、(101)、(211)晶面的衍射峰。在2θ为28.6 °、33.3 °、47.5 °处,出现了分别对应CeO2(111)、(200)、(220)晶面的衍射峰。对于Ru/CZ系列催化剂,随着Ru负载量的增大,代表RuO2物种3个晶面的衍射峰强度均略有增加。
采用扫描电镜对ZSM-5、Ce(20)/ZSM-5、Ru/CZ系列催化剂进行形貌观察,SEM图像见图3。ZSM-5分子筛为块状结构,上面的白色亮点为活性组分RuO2和CeO2,颗粒直径大概为30~50 nm。对比图3(a)~(b)与图4(c)~(e)发现,Ru元素的引入并未明显破坏分子筛本身结构,而部分活性组分颗粒出现团聚现象。这说明钌元素负载在CeO2(20)/ZSM-5材料上而制备的Ru/CZ系列催化剂是有效的。
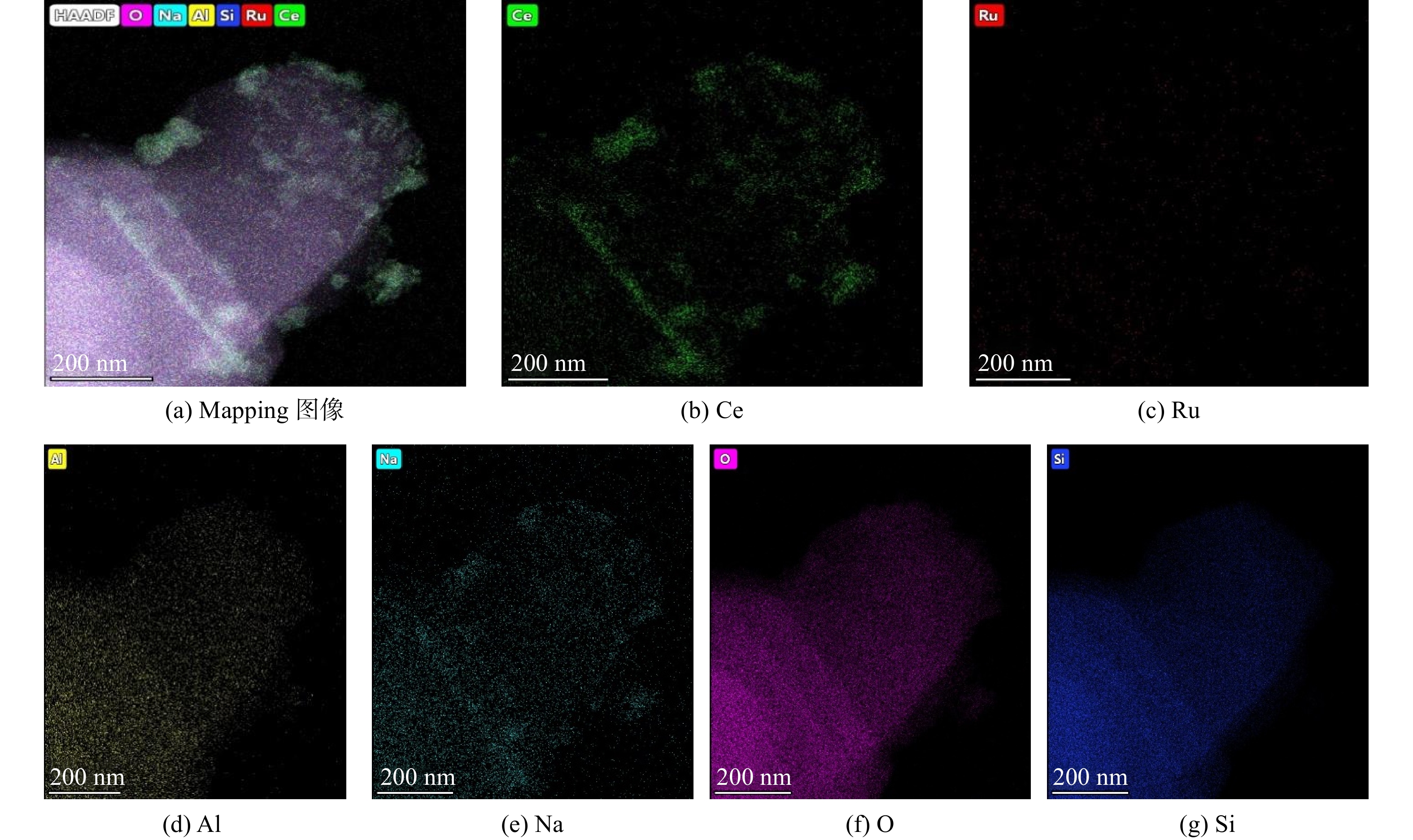
图4为Ru(1)/CZ催化剂的HAADF-STEM图像,展示了Ru(1.0)/CZ催化剂所有元素的分布情况。图4(b)为Ce元素在ZSM-5分子筛上的分布情况,说明Ce元素在整个催化剂表面均有分布,但以小团聚的状态分布,故团聚处斑点的亮度较明显。图4(c)展示了Ru元素的分布情况,表明Ru物种颗粒的尺寸较小,且在分子筛上分布均匀。因此,可推测图3中观察到的ZSM-5分子筛上成形的晶簇主要是由CeO2颗粒导致的。图4(d)~(g)分别为ZSM-5分子筛组成元素Al、Na、O和Si的分布情况。由于ZSM-5分子筛作为载体含量较多,其组成元素含量也较多,全部均匀呈现在mapping图中。
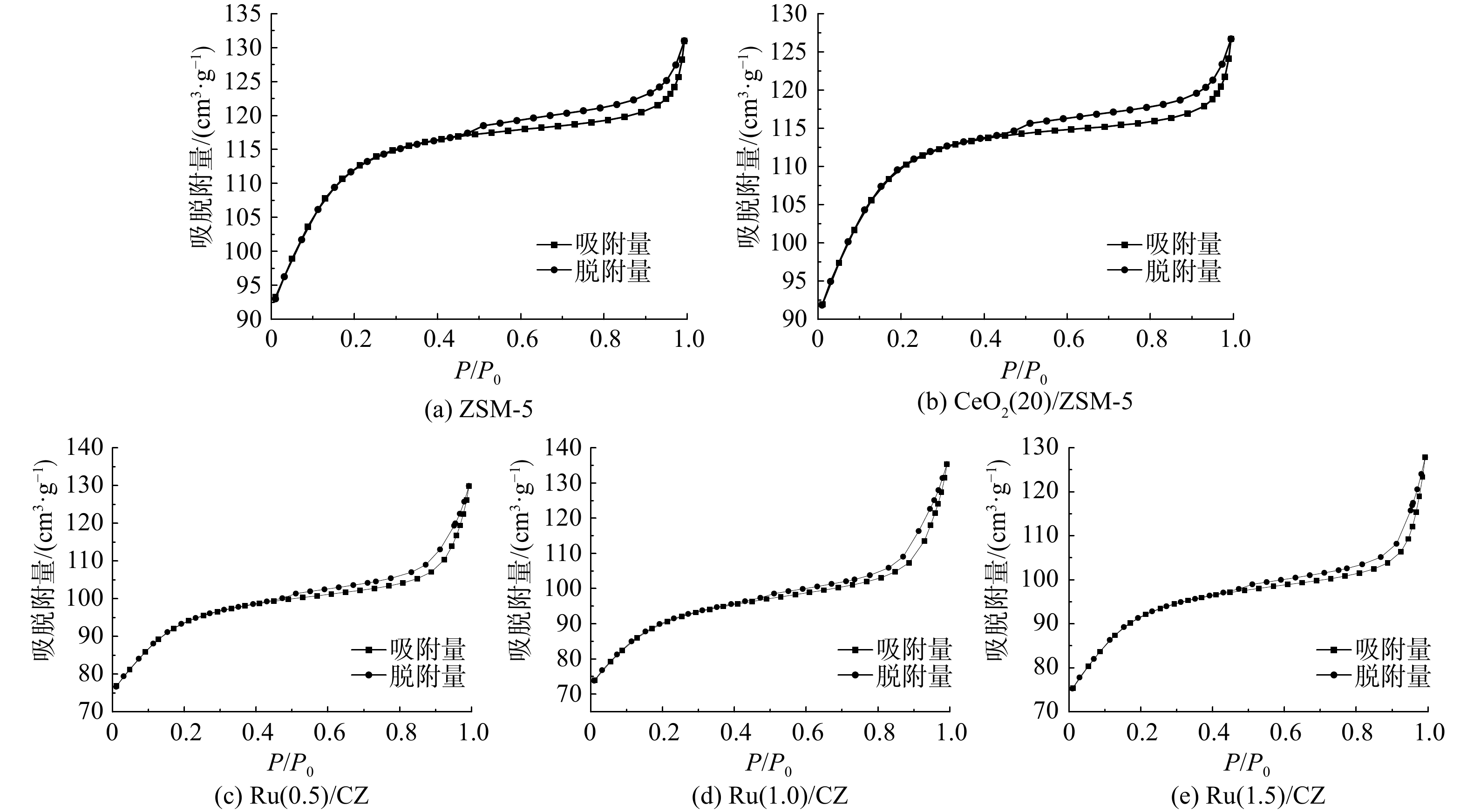
图5为不同Ru负载量的Ru/CZ催化剂的氮气吸附/脱附等温线和孔径分布曲线。表1为ZSM-5分子筛、CeO2(20)/ZSM-5和Ru/CZ系列催化剂的物理结构参数。CeO2(20)/ZSM-5催化剂中加入Ru元素后催化剂的吸附等温线呈现标准Ⅱ型。在P/P0<0.2时,Ru/CZ系列催化剂的吸附量陡然上升,说明吸附过程中存在微孔吸附。随着相对压力的变大,N2吸附量升高,表明样品受到压力影响,表面开始出现多层吸附。在相对压力(P/P0)约为0.48时,吸/脱附等温线H4型回滞环开始出现。这说明催化剂中存在层状结构的孔道并发生了毛细冷凝现象,即Ru/CZ系列催化剂中存在着介孔结构。Ru/CZ系列催化剂中绝大多数颗粒孔径为0~2 nm,平均孔径约为5 nm,与表1的孔径分布结果一致。3种不同Ru负载量的催化剂中,Ru(0.5)/CZ催化剂比表面积最大,为339.733 m2∙g−1;Ru(1.5)/CZ次之,为332.675 m2∙g−1;Ru(1.0)/CZ最小,为326.942 m2∙g−1。与空白ZSM-5分子筛对比可发现,Ru和Ce元素的负载会降低ZSM-5基底材料的比表面积,并堵塞材料的孔道结构。然而,比表面积下降得并不多,Ru/CZ系列催化剂仍具有较高的比表面积。这说明钌元素的加入并不会很大程度上影响材料的吸附性能。钌元素的负载使得催化剂材料的孔体积和孔径变大、比表面积变小,猜测可能是由于孔坍塌多个颗粒烧结形成一个颗粒。孔容孔径的增大可能会使得催化剂暴露更多活性位点,有利于增加反应物与催化剂的接触机会,且孔体积增大可能会使催化剂抗积碳和抗结焦能力变强。将Ru/CZ系列催化剂的物理结构参数互相比较可发现,Ru(1.0)/CZ催化剂的比表面积最小,但孔体积和平均孔径最大,3种催化剂的各项参数并没有随钌负载量的增加而发生线性变化。因此,通过BET表征并不能推测出Ru/CZ系列催化剂的催化活性大小。
-
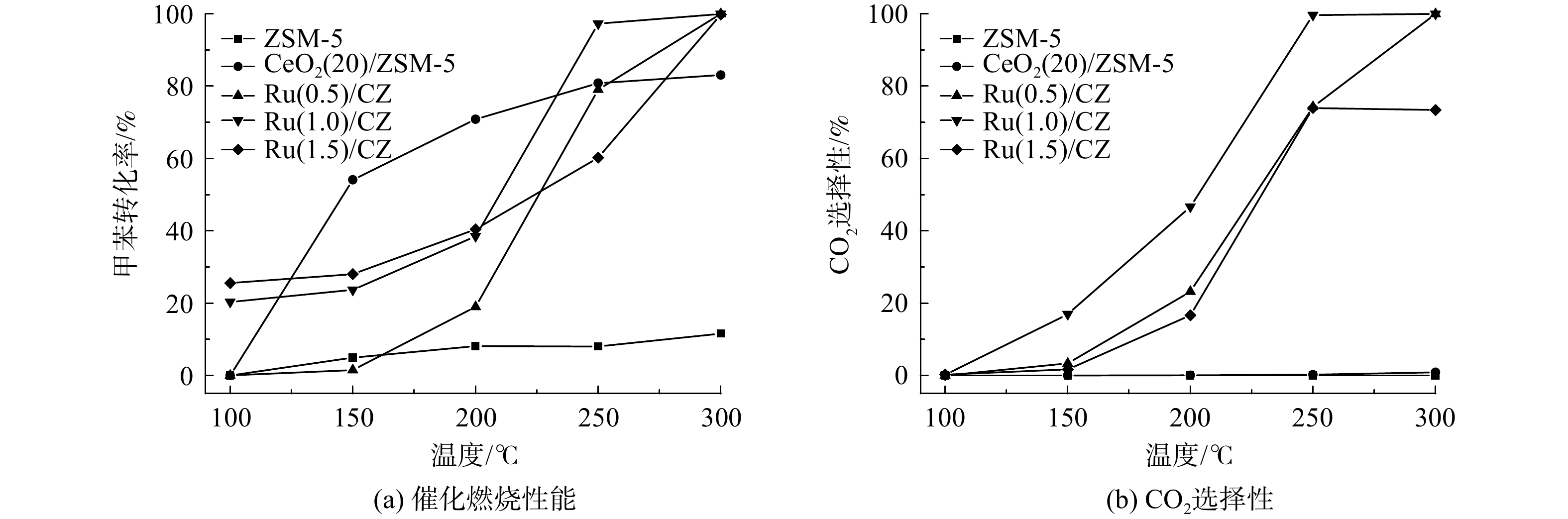
ZSM-5、CeO2(20)/ZSM-5和Ru/CZ系列催化剂的甲苯催化燃烧性能及CO2选择性如图6所示。催化剂的催化活性均随着反应温度的升高而有所提升,且Ru的负载显著提升了催化剂对甲苯的转化率,没有钌负载的CeO2(20)/ZSM-5催化剂在300 ℃时的最终转化率仅为82%。相比之下,有钌负载的Ru/CZ系列催化剂的转化率在同样的300 ℃下已达到100%。与此同时,结合图6和表2可知,Ru(0.5)/CZ催化剂、Ru(1.0)/CZ催化剂和Ru(1.5)/CZ催化剂的T50%值分别为225 ℃、210 ℃和245 ℃,T90%值分别为260 ℃、240 ℃和283 ℃。这表明Ru(1.0)/CZ催化剂在3者中具有最高的低温催化活性,可在240 ℃的低温条件下时就完全转化甲苯。虽然CeO2(20)/ZSM-5催化剂的整体催化转化甲苯效率不高且变化不大,但在低于225 ℃时,其催化甲苯的转化率要高于Ru/CZ系列催化剂,且由表1可知,钌负载后的催化剂比表面积降低,低于CeO2(20)/ZSM-5催化剂。在低温条件下,催化剂对甲苯的脱除效果中物理吸附占主导作用,高比表面积更有利于催化剂对甲苯的吸附。
另外,催化剂的CO2选择性均随反应温度的升高而升高。负载Ru的Ru/CZ催化剂最终催化燃烧甲苯的CO2选择性均可达到80%以上,而CeO2(20)/ZSM-5催化剂的CO2选择性随温度变化不大,且始终不足1%。这表明钌负载可提升CeO2(20)/ZSM-5催化剂催化甲苯使其降解成高浓度CO2的能力。对比Ru(0.5)/CZ催化剂、Ru(1.0)/CZ催化剂和Ru(1.5)/CZ催化剂的活性评价曲线发现:Ru(0.5)/CZ催化剂和Ru(1.0)/CZ催化剂均可将甲苯完全转化成CO2;Ru(1.0)/CZ催化剂在反应温度为240℃时CO2的选择性即可达到90%以上;Ru(0.5)/CZ催化剂在反应温度为300℃时CO2的选择性为100%;而Ru(1.5)/CZ催化剂在250℃和300℃时CO2的选择性均不到80%且相差不大。这说明Ru(1.0)/CZ催化剂CO2的选择性最优,Ru(0.5)/CZ催化剂其次,Ru(1.5)/CZ催化剂在3者中最差。
-
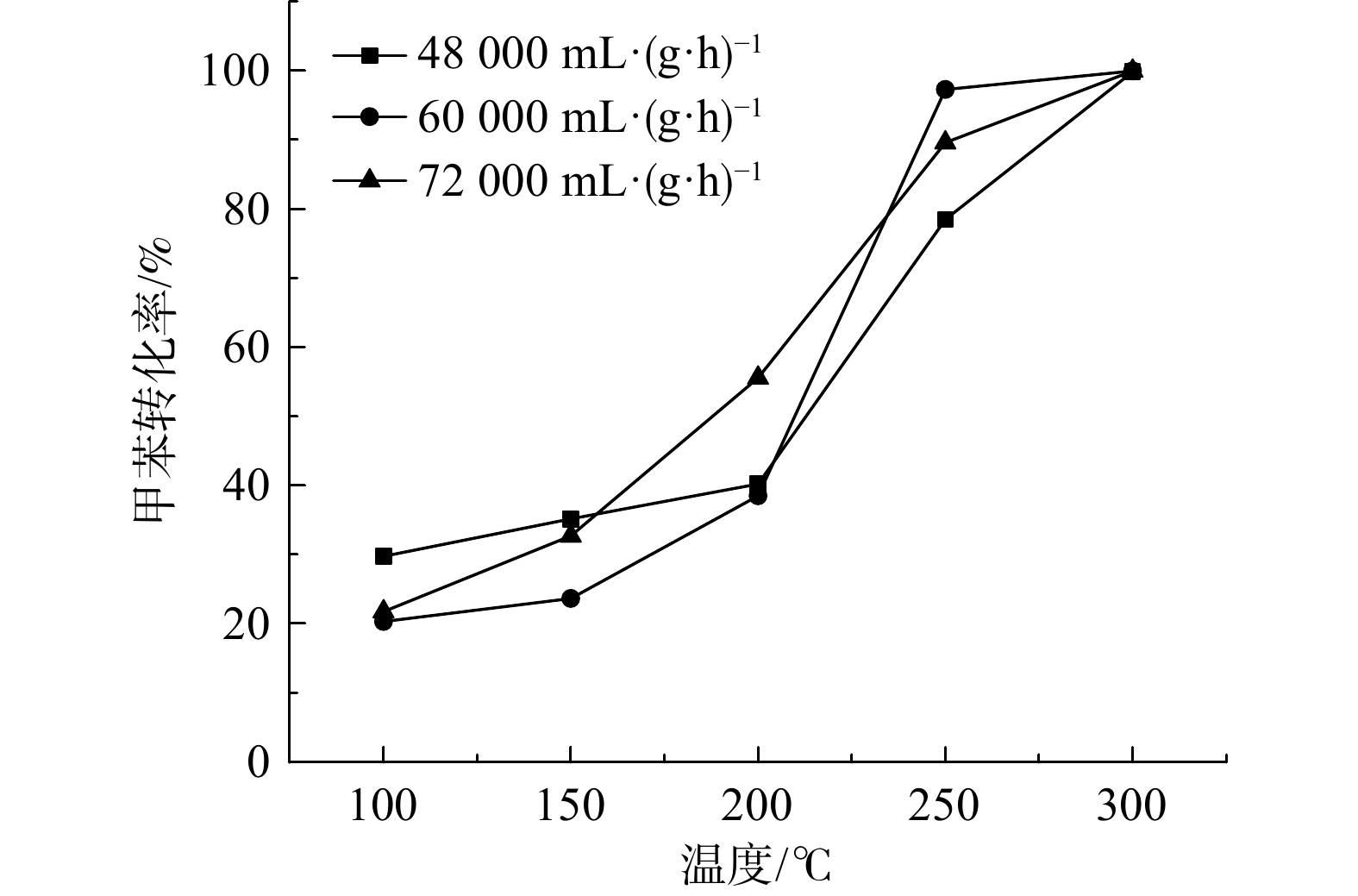
空速对甲苯催化氧化性能的影响如图7所示。在空速条件为60 000 mL·(g·h)−1时,Ru(1.0)/CZ催化剂可在反应温度为250 ℃时完全转化甲苯,而在48 000和72 000 mL·(g·h)−1空速条件下,Ru(1.0)/CZ催化剂要到300 ℃才可将甲苯100%转化。与此同时,反应温度在200 ℃以内、60 000 mL·(g·h)−1空速工况条件下,Ru(1.0)/CZ催化剂催化甲苯的转化率要低于48 000 mL·(g·h)−1空速条件下的转化率;在230 ℃以内60 000 mL·(g·h)−1空速工况下,甲苯的转化率始终低于72 000 mL·(g·h)−1空速条件下的转化率。以完全转化甲苯时的反应温度高低来衡量反应空速对催化剂催化性能的影响,60 000 mL·(g·h)−1的空速条件为最适宜条件。因此,较低空速48 000 mL·(g·h)−1和较高空速72 000 mL·(g·h)−1均不利于催化剂在较低温度下实现甲苯的完全转化。这可能是由于随着空速的增大,气体流速会增大,当反应温度上升时,反应器中气体状态更接近湍流,使得气流和样品接触更充分,反应效率变高。但反应速率同时受限于石英管的型制,又会一定程度上阻碍湍流的形成,空速过高会导致停留时间变短,平均反应效率变低。
-
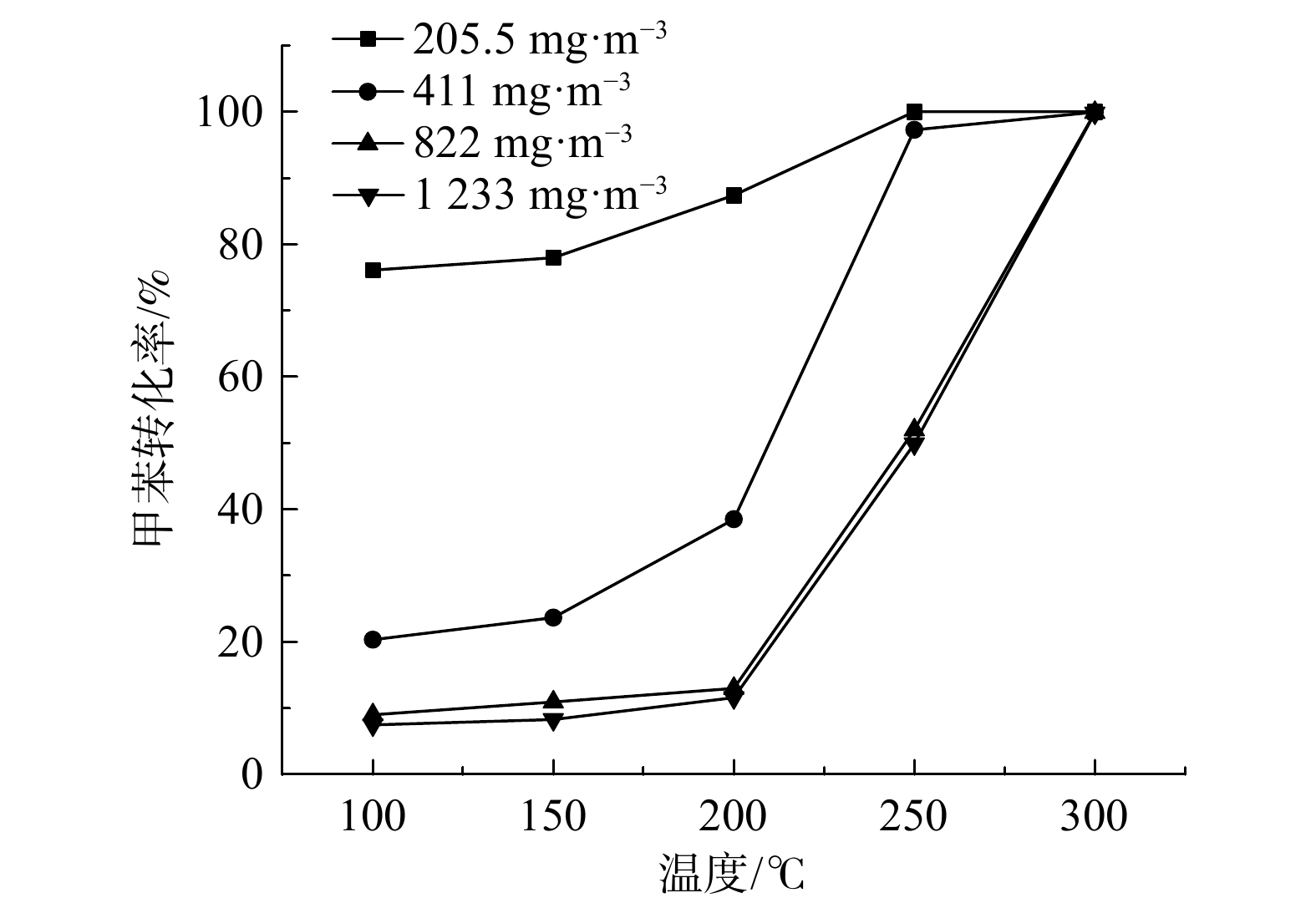
不同甲苯浓度下,Ru(1.0)/CZ催化剂催化燃烧甲苯性能如图8所示,Ru(1.0)/CZ催化剂催化燃烧甲苯的T50%和T90%如表3所示。在甲苯质量浓度为205.5~1 233 mg·m−3时,催化剂的活性随着甲苯质量浓度的升高而降低,转化率曲线逐步向高温移动。这表明在更高温度下才能发生更多的甲苯分子转化。4种不同甲苯质量浓度条件下的T90%分别为210 ℃、240 ℃、280 ℃和281 ℃。Ru(1.0)/CZ催化剂仅在210 ℃即可完全转化质量浓度为205.5 mg·m−3的甲苯,且此时催化剂催化氧化甲苯的T50%为125 ℃。而其余3种甲苯浓度T50%值均高于200 ℃,说明在进口甲苯质量浓度较低的情况下,Ru(1.0)/CZ催化剂表现出更优异的低温催化性能。另外,进口甲苯质量浓度从411 mg·m−3升至1 233 mg·m−3与205.5 mg·m−3升至411 mg·m−3的活性曲线相比,变化幅度较小。这可能是由于催化剂在反应器中的装填量为固定的,随着甲苯质量浓度的增大,甲苯分子数量增多,大量的甲苯分子吸附在催化剂表面还未来得及反应,就占据了部分活性位点,使得竞争吸附明显增强,即单位体积内Ru(1.0)/CZ催化剂表面的活性位点的数目一定,单位时间内相对能催化氧化的甲苯分子数减小,从而导致催化剂的活性降低。
-
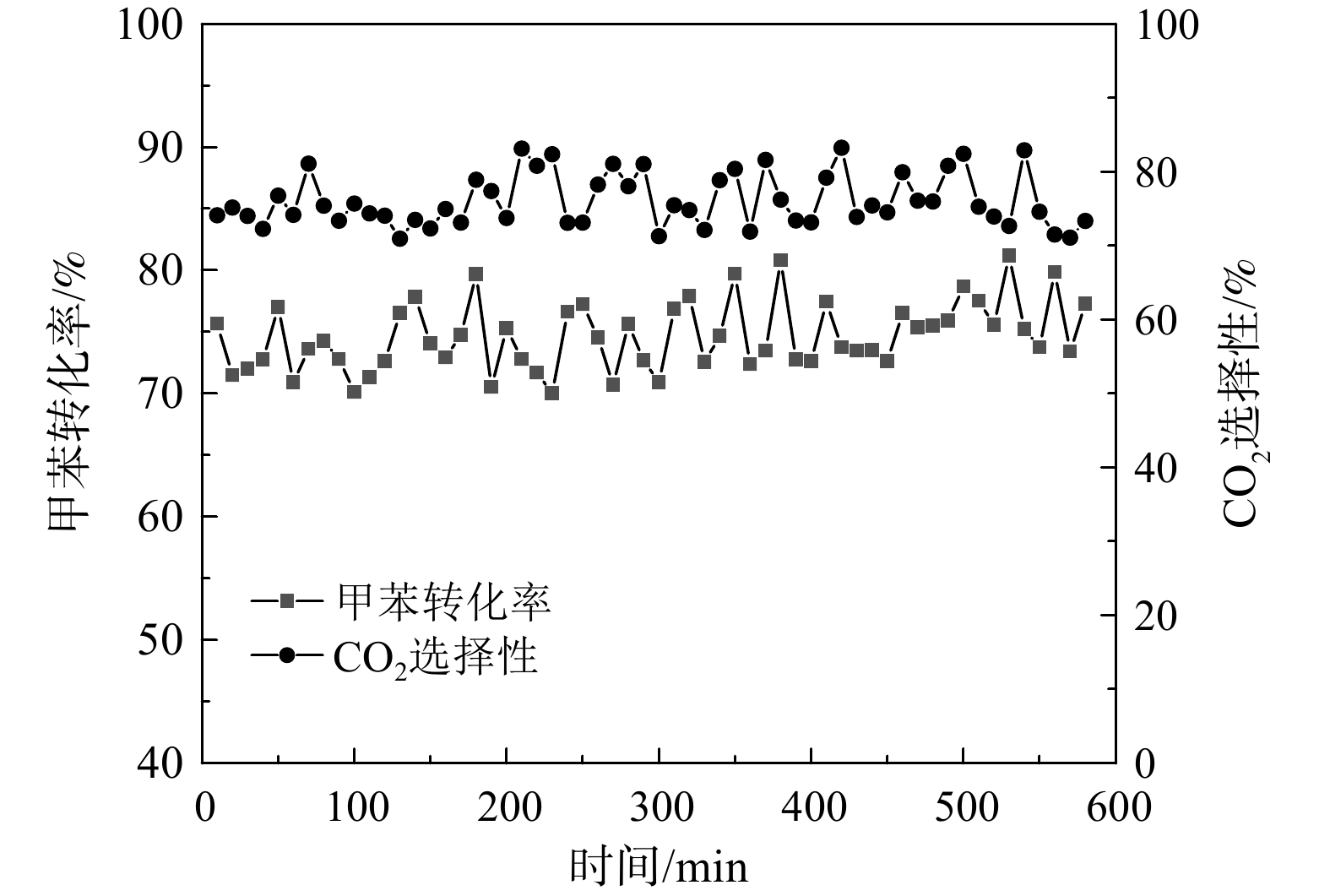
根据前述不同钌负载量对Ru/CZ催化剂催化燃烧甲苯的活性和CO2稳定性的研究结果,Ru(1.0)/CZ催化剂催化燃烧甲苯的活性最强,可在较低的240 ℃条件下完全转化甲苯,且CO2选择性较强,其催化燃烧甲苯不会有大分子副产物产生。因此,为进一步观察Ru(1.0)/CZ催化剂长时间反应条件下的稳定性和CO2选择性是否有改变,选取230 ℃的反应温度条件对催化剂进行活性稳定性评价,其测试结果如图9所示。Ru(1.0)/CZ催化剂催化燃烧甲苯稳定性研究的反应条件为:温度230 ℃,甲苯质量浓度为411 mg·m−3,质量空速为60 000 mL·(g·h)−1。在600 min内,Ru(1.0)/CZ催化剂的甲苯转化率为70%~80%,CO2选择性为70%~82%。转化率和CO2选择性的波动在仪器检测误差范围内。这说明该催化剂的稳定性良好,长时间运行没有失活表现。Ru(1.0)/CZ催化剂在230 ℃反应温度时的测试结果与图6对照一致。这说明Ru(1.0)/CZ催化剂催化燃烧甲苯活性评价的结果具有可重复性。
-
1) Ru的负载可提升CeO2(20)/ZSM-5催化剂催化甲苯使其降解成高浓度CO2的能力。
2) Ru(1.0)/CZ催化剂转换90%甲苯的温度低至210 ℃,并在较大空速和甲苯浓度范围内对甲苯具有优越的低温催化性能。
3) Ru(1.0)/CZ催化剂具有良好的稳定性及CO2选择性,长时间运行没有失活表现,具有在VOC处理领域开展工业化应用的潜力。
负载型钌铈催化剂的甲苯催化氧化性能
Catalytic oxidation of toluene by supported ruthenium-cerium catalyst
-
摘要: 以ZSM-5分子筛为载体,采用浸渍法制备了RuCe/ZSM-5催化剂,研究了催化剂在不同Ru负载量、空速、反应温度、甲苯浓度下对甲苯的催化氧化性能,并探讨了催化剂的CO2选择性及稳定性。结果表明:Ru的负载可提升CeO2(20)/ZSM-5催化剂催化甲苯使其降解成高浓度CO2的能力;Ru(1.0)/CZ催化剂表现出优异的低温催化性能、CO2选择性及稳定性,在210 ℃条件时即可转化90%的甲苯,且CO2选择性达到90%以上。本研究制备的催化剂在较大空速及甲苯浓度范围内对甲苯具有优越的低温催化性能,未来可应用于VOC的工业化处理应用中。
-
关键词:
- RuCe/ZSM-5 /
- 挥发性有机物 /
- 甲苯 /
- 催化氧化
Abstract: RuCe/ZSM-5 catalysts were prepared by impregnation method with ZSM-5 molecular sieve as support. The catalytic oxidation performances of the catalyst of toluene were studied under different Ru loadings, space velocities, reaction temperatures, toluene concentrations. The CO2 selectivity and stability were also discussed. It was indicated that Ru doping could improve the catalytic ability of CeO2(20)/ZSM-5 for toluene degradation into high concentration of CO2. The Ru(1.0)/CZ catalyst exhibited excellent low-temperature catalytic performance, CO2 selectivity and stability. 90% toluence can be converted at 210℃, and the CO2 selectivity reached more than 90%. The catalyst prepared in this study showed superior catalytic oxidation performance to toluene at low temperature in a large space velocity and and toluene concentration range, and can be potentially used in processing VOC industrial applications.-
Key words:
- RuCe/ZSM-5 /
- volatile organic compounds /
- toluene /
- catalytic oxidation
-

-
表 1 ZSM-5分子筛、CeO2(20)/ZSM-5和Ru/CZ系列催化剂的物理结构参数
Table 1. Physical structure parameters of ZSM-5 , CeO2(20)/ZSM-5, Ru(0.5)/CZ, Ru(1.0)/CZ, Ru(1.5)/CZ
样品名称 比表面积/(m2·g−1) 孔体积/(cm3·g−1) 平均孔径/nm ZSM-5 409.578 0.061 1 3.226 5 CeO2(20)/ZSM-5 404.820 0.051 8 3.165 5 Ru(0.5)/CZ 339.733 0.098 2 4.506 5 Ru(1.0)/CZ 326.942 0.103 0 5.175 2 Ru(1.5)/CZ 332.675 0.088 6 4.621 5 表 2 不同催化剂催化燃烧甲苯的T50%和T90%
Table 2. The T50% and T90% of toluene by catalytic combustion of different catalysts
催化剂名称 T50%/℃ T90%/℃ ZSM-5 — — CeO2(20)/ZSM-5 136 - Ru(0.5)/CZ 225 260 Ru(1.0)/CZ 210 240 Ru(1.5)/CZ 245 283 注:T50%、T90%分别代表甲苯转化率为50%、90%时的温度。 表 3 不同甲苯浓度下Ru(1.0)/CZ催化剂催化燃烧甲苯的T50%和T90%
Table 3. The T50% and T90% of toluene by catalytic combustion of Ru(1.0)/CZ catalyst with different toluene concentrations
甲苯浓度/(mg·m−3) T50%/℃ T90%/℃ 205.5 125 210 411 210 240 822 250 280 1 233 252 281 -
[1] GUO Y, WEN M, LI G, et al. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review[J]. Applied Catalysis B:Environmental, 2021, 281: 119447. doi: 10.1016/j.apcatb.2020.119447 [2] YANG C, MIAO G, PI Y, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review[J]. Chemical Engineering Journal, 2019, 370: 1128-1153. doi: 10.1016/j.cej.2019.03.232 [3] ZHANG X, GAO B, CREAMER A E, et al. Adsorption of VOCs onto engineered carbon materials: A review[J]. Journal of Hazardous Materials, 2017, 338: 102-123. doi: 10.1016/j.jhazmat.2017.05.013 [4] LI J, YU E, CAI S, et al. Noble metal free, CeO2/LaMnO3 hybrid achieving efficient photo-thermal catalytic decomposition of volatile organic compounds under IR light[J]. Applied Catalysis B:Environmental, 2019, 240: 141-152. doi: 10.1016/j.apcatb.2018.08.069 [5] WANG H, CHEN S, WANG Z, et al. A novel hybrid Bi2MoO6-MnO2 catalysts with the superior plasma induced pseudo photocatalytic-catalytic performance for ethyl acetate degradation[J]. Applied Catalysis B:Environmental, 2019, 254: 339-350. doi: 10.1016/j.apcatb.2019.05.018 [6] WANG F, DAI H, DENG J, et al. Manganese Oxides with rod-, wire-, tube-, and flower-Like Morphologies: Highly effective catalysts for the removal of Toluene[J]. Environmental Science & Technology, 2012, 46(7): 4034-4041. [7] IKHLAQ A, KASPRZYK-HORDERN B. Catalytic ozonation of chlorinated VOCs on ZSM-5 zeolites and alumina: Formation of chlorides[J]. Applied Catalysis B:Environmental, 2017, 200: 274-282. doi: 10.1016/j.apcatb.2016.07.019 [8] LUO S, GAO L, WEI Z, et al. Kinetic and mechanistic aspects of hydroxyl radical-mediated degradation of naproxen and reaction intermediates[J]. Water Research, 2018, 137: 233-241. doi: 10.1016/j.watres.2018.03.002 [9] LUO S, WEI Z, SPINNEY R, et al. UV direct photolysis of sulfamethoxazole and ibuprofen: An experimental and modelling study[J]. Journal of Hazardous Materials, 2018, 343: 132-139. doi: 10.1016/j.jhazmat.2017.09.019 [10] LIU L, LI J, ZHANG H, et al. In situ fabrication of highly active γ-MnO2/SmMnO3 catalyst for deep catalytic oxidation of gaseous benzene, ethylbenzene, toluene, and o-xylene[J]. Journal of Hazardous Materials, 2019, 362: 178-186. doi: 10.1016/j.jhazmat.2018.09.012 [11] FENG S, LIU J, GAO B. Synergistic mechanism of Cu-Mn-Ce oxides in mesoporous ceramic base catalyst for VOCs microwave catalytic combustion[J]. Chemical Engineering Journal, 2022, 429: 132302. doi: 10.1016/j.cej.2021.132302 [12] ALI S, WU X, ZUHRA Z, et al. Cu-Mn-Ce mixed oxides catalysts for soot oxidation and their mechanistic chemistry[J]. Applied Surface Science, 2020, 512: 145602. doi: 10.1016/j.apsusc.2020.145602 [13] JIANG Y, GAO J, ZHANG Q, et al. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template[J]. Chemical Engineering Journal, 2019, 371: 78-87. doi: 10.1016/j.cej.2019.03.233 [14] LU H, KONG X, HUANG H, et al. Cu-Mn-Ce ternary mixed-oxide catalysts for catalytic combustion of toluene[J]. Journal of Environmental Sciences, 2015, 32: 102-107. doi: 10.1016/j.jes.2014.11.015 [15] GAO P, WANG A, WANG X, et al. Synthesis of highly ordered Ir-containing mesoporous carbon materials by organic-organic self-assembly[J]. Chemistry of Materials, 2008, 20(5): 1881-1888. doi: 10.1021/cm702815e [16] GUO J, LIN C, JIANG C, et al. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature[J]. Applied Surface Science, 2019, 475: 237-255. doi: 10.1016/j.apsusc.2018.12.238 [17] OKAL J, ZAWADZKI M. Catalytic combustion of butane on Ru/γ-Al2O3 catalysts[J]. Applied Catalysis B:Environmental, 2009, 89(1-2): 22-32. doi: 10.1016/j.apcatb.2008.11.024 [18] DAI Q, BAI S, WANG J, et al. The effect of TiO2 doping on catalytic performances of Ru/CeO2 catalysts during catalytic combustion of chlorobenzene[J]. Applied Catalysis B:Environmental, 2013, 142-143: 222-233. doi: 10.1016/j.apcatb.2013.05.026 [19] SANTOS V P, CARABINEIRO S A C, TAVARES P B, et al. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts[J]. Applied Catalysis B:Environmental, 2010, 99(1-2): 198-205. doi: 10.1016/j.apcatb.2010.06.020 [20] CARRETTIN S, CONCEPCIÓN P, CORMA A, et al. Nanocrystalline CeO2 Increases the Activity of Au for CO Oxidation by Two Orders of Magnitude[J]. Angewandte Chemie International Edition, 2004, 43(19): 2538-2540. doi: 10.1002/anie.200353570 [21] GÓMEZ D M, GALVITA V V, GATICA J M, et al. TAP study of toluene total oxidation over a Co3O4/La-CeO2 catalyst with an application as a washcoat of cordierite honeycomb monoliths[J]. Physical chemistry chemical physics:PCCP, 2014, 16(23): 11447-11455. doi: 10.1039/C4CP00886C [22] 杨卜源, 佟丽华, 左树锋, 等. 添加铈对锰基催化剂的织构-结构及其氧化还原性能的影响[J]. 中国稀土学报. 2011, 29(4): 433-438. [23] DAI Q, WANG W, WANG X, et al. Sandwich-structured CeO2@ZSM-5 hybrid composites for catalytic oxidation of 1, 2-dichloroethane: An integrated solution to coking and chlorine poisoning deactivation[J]. Applied Catalysis B:Environmental, 2017, 203: 31-42. doi: 10.1016/j.apcatb.2016.10.009 [24] KHAN M E, KHAN M M, CHO M H. Ce3+-ion, surface oxygen vacancy, and visible light-induced photocatalytic dye degradation and photocapacitive performance of CeO2-Graphene nanostructures[J]. Scientific Reports, 2017, 7(1) [25] LIU X, ZENG J, WANG J, et al. Catalytic oxidation of methyl bromide using ruthenium-based catalysts[J]. Catalysis Science & Technology[J], 2016, 6(12): 4337-4344. [26] ZHAO J, XI W, TU C, et al. Catalytic oxidation of chlorinated VOCs over Ru/TixSn1-x catalysts[J]. Applied Catalysis B:Environmental, 2020, 263: 118237. doi: 10.1016/j.apcatb.2019.118237 [27] AOUAD S, ABI-AAD E, ABOUKAÏS A. Simultaneous oxidation of carbon black and volatile organic compounds over Ru/CeO2 catalysts[J]. Applied Catalysis B:Environmental, 2009, 88(3/4): 249-256. doi: 10.1016/j.apcatb.2008.10.002 [28] LIU C, XIAN H, JIANG Z, et al. Insight into the improvement effect of the Ce doping into the SnO2 catalyst for the catalytic combustion of methane[J]. Applied Catalysis B:Environmental, 2015, 176-177: 542-552. doi: 10.1016/j.apcatb.2015.04.042 [29] WANG J, ZHAO H, LIU X, et al. Study on the Catalytic Properties of Ru/TiO2 Catalysts for the Catalytic Oxidation of (Chloro)-Aromatics[J]. Catalysis Letters, 2019, 149(7): 2004-2014. doi: 10.1007/s10562-019-02802-x [30] PENG R, SUN X, LI S, et al. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene[J]. Chemical Engineering Journal, 2016, 306: 1234-1246. doi: 10.1016/j.cej.2016.08.056 [31] SCIRÈ S, MINICÒ S, CRISAFULLI C, et al. Catalytic combustion of volatile organic compounds on gold/cerium oxide catalysts[J]. Applied Catalysis B:Environmental, 2003, 40(1): 43-49. doi: 10.1016/S0926-3373(02)00127-3 -



 下载:
下载: