-
在废水生物处理系统中,微生物是污染物降解的主体。一般而言,活性污泥处理系统中微生物生长繁殖需要的营养物质含量具有一个经验比例,即化学需氧量∶氮∶磷(COD∶N∶P)为100∶5∶1。当处理废水中营养物质含量达不到该比例时,如耗氧有机污染物(以COD计)浓度过低,或者氮、磷浓度比例过低,则该废水被称为贫营养废水。在贫营养水质条件下,系统营养物质比例失调,生物系统中活性污泥微生物由于缺乏某类营养物质而活性降低,甚至大量死亡,导致处理系统失效。WANG等[1]发现,在脱氮除磷系统中,当有机物浓度过低时,微生物细胞内合成和贮存的PHA不足,系统的脱氮除磷效率将大幅降低。PENG等[2]的研究表明,进水底物中缺乏氮或磷会刺激活性污泥中丝状菌的增殖,从而发生丝状污泥膨胀而使系统处理性能降低。为解决贫营养废水处理效率低的难题,目前大多数污水处理厂通常采用投加有机物或氮磷等营养物质的方式调整系统进水营养比例,以满足活性污泥微生物生长需求,保证系统稳定运行。
近年来,随着微生物测序技术的飞速发展,基于16SrRNA高通量测序技术的微生物检测被广泛应用于污水处理厂活性污泥微生物群落结构的分析中,大大提高了人们对污泥微生物群落及其功能的了解。目前,对污水处理系统内主要微生物群落的研究已有大量报道。JIN等[3]的研究表明,在门水平上,活性污泥中的细菌主要集中在变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes)4种。ZHANG等[4]对滁州市污水处理系统5个活性污泥微生物群落结构的研究中发现,在所有活性污泥样品中,除了上述4种菌门,绿弯菌门(Chloroflexi)和酸杆菌门(Acidobacteria)也是优势菌。在这些优势菌门中,变形菌门(Proteobacteria)是活性污泥系统中最常见的原生门类,也是传统废水生物处理过程中的关键细菌群[5]。有研究[6]表明,该菌门与污水生物处理过程中的有机物降解和脱氮除磷密切相关,而隶属于该门的Zoogloea属是构成活性污泥菌胶团的重要组成菌属,在多种有机物的降解过程中发挥着重要作用[7]。拟杆菌门(Bacteroidetes)是异养微生物的另一类重要门类,能参与有机碳和蛋白类物质的循环[8]。该门同时也是厌氧消化过程中常见的产酸菌[9],其部分菌属能降解长链脂肪酸,并可水解发酵多糖类有机化合物[10-11]。有研究[12-13]表明,拟杆菌门中的拟杆菌属(Bacteroidales)能够利用纤维素和多糖,并可将纤维素、二糖、葡萄糖和甘露糖等有机物降解为醋酸酯或琥珀酸。绿弯菌门(Chloroflexi)作为污水处理厂常见的菌群,具有较高的有机负荷承受能力[14],并且对糖类有较好的降解能力,可产生乙酸[15]。厚壁菌门(Firmicutes)广泛分布在厌氧环境中,包含梭菌纲(Clostridium)、芽孢杆菌纲(Bacillibacteria)等多种功能菌纲,其对有机物的水解和酸化有重要作用[16]。此外,酸杆菌门(Acidobacteria)和放线菌门(Actinobacteria)也是污水处理厂厌氧过程中重要的功能菌群,是厌氧消化过程中的主要产酸菌,起水解酸化作用[17-18]。以上这些污水处理厂常见主要菌群的研究结论,大多是基于普通的污水生物处理过程,即微生物生长所需营养物质能够得到满足的条件下得到的结果。然而,当污水处理系统中氮、磷等营养受限时,微生物群落的结构变化和种群分布规律还有待进一步研究。
云南省昆明市某制药厂废水处理系统中,进水耗氧有机污染物(以COD计)和悬浮物浓度高,而氮磷浓度极低,进水营养物质含量比例达不到常规微生物生长需求,COD∶N∶P仅为100∶0.4∶0.07。然而系统长期运行状况表明,在氮磷营养贫乏的条件下,该系统运行稳定,污水处理效果良好,出水水质均达到了国家《污水综合排放标准》(GB 8978-1996)的一级标准。为此,本研究对该贫营养废水处理系统中关键环节的污染物降解情况进行了监测,并对其活性污泥中微生物进行了高通量测序分析。本研究旨在从系统运行及污泥微生物的角度解释该制药厂贫营养废水处理系统长期稳定运行的原因,为营养不均衡的高浓度有机废水的处理及系统维护提供有益指导。
-
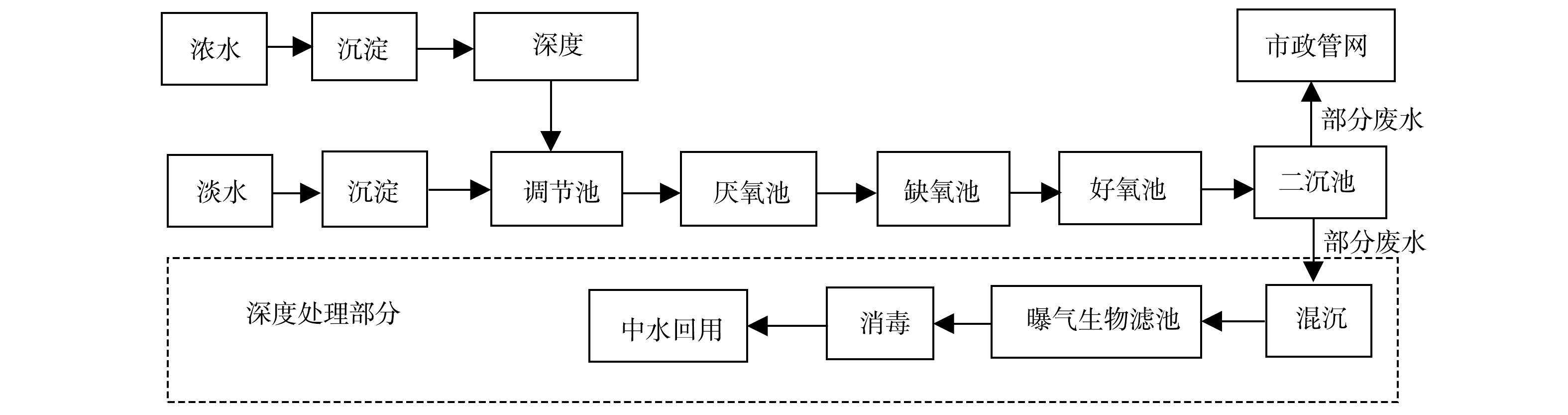
云南省昆明市某制药厂污水处理站总处理水量约为1 000 m3·d−1,进水分别来源于生产废水和生活污水。生产废水主要来自车间洗泡蒸煮药材、冲洗、制剂等生产过程,该废水的特点为:废水COD值高、波动范围较大,耗氧有机污染物(以COD计)质量浓度通常在1 000~2 000 mg·L−1波动;废水中氮磷等营养元素含量较低(TN<9 mg·L−1、TP<1 mg·L−1),加大了生物处理的难度。工艺好氧段曝气池MLSS为3 000~4 000 mg·L−1,MLVSS为1 500~2 000 mg·L−1,SVI为100~150 mL·g−1。废水经污水处理站处理后,部分废水排入市政管网,部分经过深度处理后作为该厂中水进行回用。
-
系统处理工艺为A2O工艺,厌氧过程采用IC工艺,好氧过程采用活性污泥完全混合处理工艺。为了适应不同的进水水质,在生化处理工艺的前端分别设置了2组预处理工艺。污染物浓度较高的生产废水(浓废水),首先经过沉淀作用去除水中部分悬浮物,再进入浓废厌氧池进行部分有机污染物的降解,而后流入调节池调节水质水量。经过这样预处理后,浓废水才能进入后续的主体生化系统进行处理;污染物浓度较低的生活污水(淡废水),经沉淀后直接流入调节池进行后续的生化处理。混合后的调节池出水从厌氧污泥池底部进入,与堆积在池子底部的污泥混合接触,经污泥中微生物对废水中的有机物进行充分降解后,出水自厌氧池上部出水口流入缺氧池进行反硝化脱氮。缺氧池出水继而流入好氧池,在大量好氧异养菌的作用下,有效地去除剩余有机污染物。好氧池出水经过二沉池泥水分离后,部分出水排入市政管网,剩下的部分废水经过混凝沉淀、曝气生物滤池、消毒等深度处理工艺处理后作为中水回用。系统详细工艺流程图如图1所示。
-
分别取调节池和生化处理单元各反应池末端的出水进行水质检测。采用标准方法[19]对COD、氨氮、硝态氮、TN、TP、SS、SVI、MLSS、MLVSS等指标进行测定;DO、pH、水温等用雷磁多参数水质分析仪测定。
-
在工艺系统运行过程中,分别收集厌氧池、曝气池的污泥微生物样品。采用CTAB或SDS方法对污泥样品的基因组DNA进行提取,提取样品总DNA后,根据全长引物序列合成带有Barcode的特异引物,进行PCR扩增并对其产物进行纯化、定量和均一化,形成测序文库(SMRT Bell),建好的文库先进行文库质检,质检合格的文库用PacBio Sequel进行上机测序。使用USEARCH(10.0版)在相似性97%(默认)的水平上对有效的标签序列进行聚类,默认以测序所有序列数的0.005%作为阈值过滤OTUs。以silva为参考数据库,使用朴素贝叶斯分类器对OTUs代表序列进行分类学注释,得到物种分类学信息,进而统计各个样品在门、纲、目、科、属和种水平上的群落组成。利用QIIME软件计算各样品在97%相似度水平下的ACE、Chao、Shannon及Simpson指数。将数据批量导入QIIME软件,对OTUs表进行组间差异性分析,生成不同分类水平上的物种丰度表,再利用R语言工具绘制成样品各分类学水平下的群落结构图。
-
系统在近10年的运行过程中,处理效果稳定,出水有机污染物及氮、磷浓度低,达到国家《污水综合排放标准》(GB 8978-1996)和《污水排入城市下水道水质标准》(CJ 3082-1999)。其中,COD和BOD的去除率均高达90%以上;氮磷去除率也达到70%左右。表1显示了近期系统出水指标的具体监测结果。
-
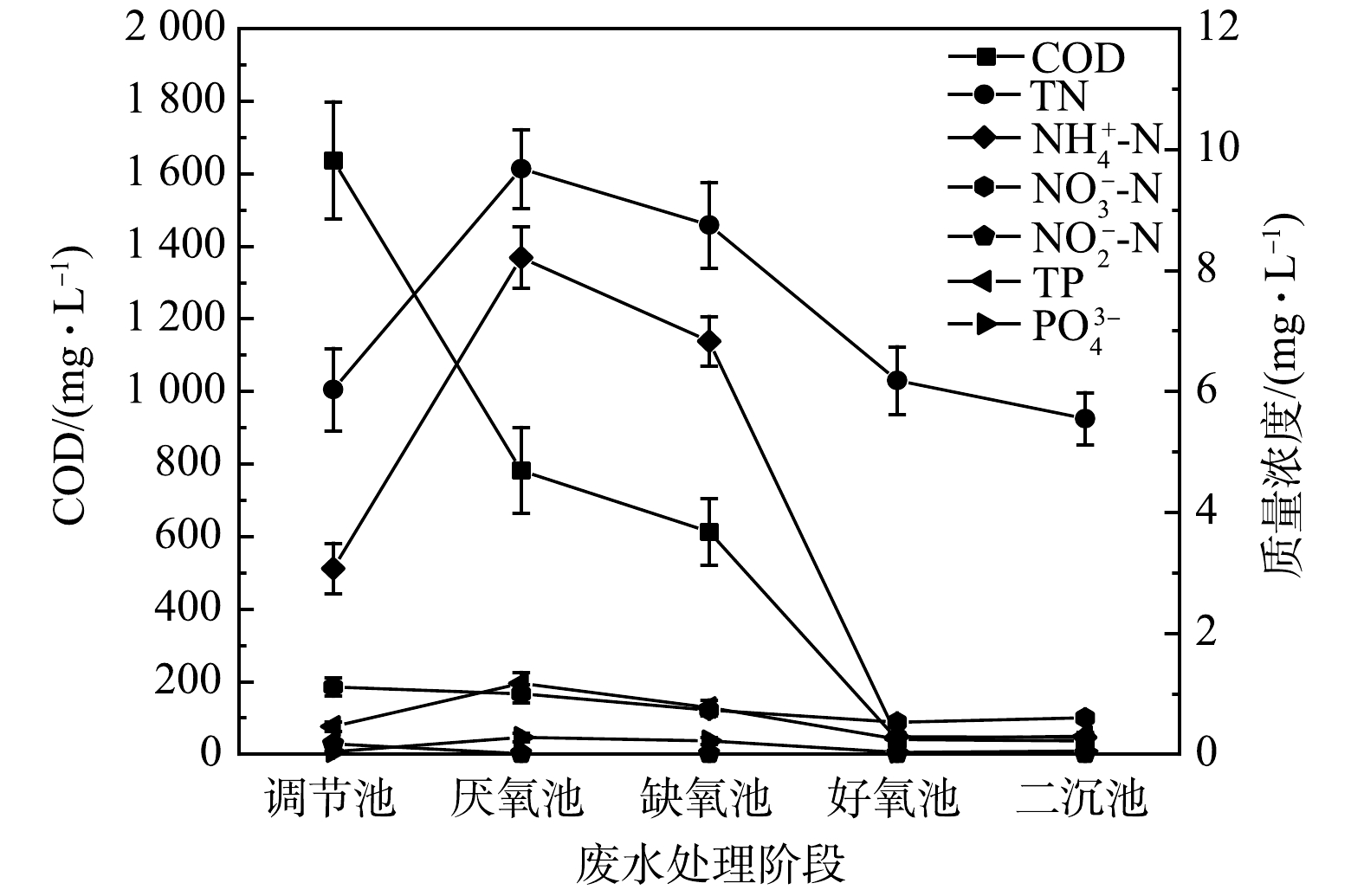
为探查系统在贫营养条件下各阶段的处理性能,本实验对系统进水及各反应器末端出水水质进行了取样检测。系统中各处理构筑物末端出水污染物平均浓度变化如图2所示。水质检测结果表明,贫营养水质并未对系统各处理阶段的处理性能产生明显影响;有机物在系统厌氧、缺氧和好氧过程中都得到了很好的降解,氮、磷在厌氧过程中略有积累,而在缺氧和好氧过程中得到很好的降解。厌氧池的COD去除率为52%左右,好氧池的COD去除率为93%,系统COD总去除率高达98%,这表明系统具有良好的有机物降解效果。TN和氨氮的降解主要在缺氧段和好氧段,缺氧段的去除率为9.61%和16.81%,好氧段为29.37%和95.75%。厌氧过程中氮磷浓度略有上升,这可能与其过长的SRT有关。有研究表明,厌氧条件下氮磷的释放受SRT影响[20],过长的SRT会导致氮磷的释放量增加[21]。本系统中厌氧池污泥长时间停留在反应器内,其SRT长达78 d左右,有可能导致其氮磷浓度增加。此外,有研究[22]表明,当活性污泥系统同时缺乏氮磷时,部分污泥将发生解体。因此,本研究中由于氮磷缺乏而导致的部分微生物死亡解体,从而释放氮磷,也可能是造成厌氧池氮磷浓度升高的原因。
-
在污水生物处理系统中,微生物是污染物去除的主要执行者,系统中微生物的种类、数量及分布等状况,从根本上决定着系统处理性能及效果。为揭示贫营养水质条件对系统优势菌群的影响,本研究对该污水处理系统中的厌氧池和好氧曝气池的污泥样品进行了高通量测序,考察两生化反应池与正常营养水平废水处理系统中微生物群落的差异。
1)高通量测序数据结果表明,该系统中好氧池和厌氧池的有效序列数分别为4 625和4 875;在97%的相似度水平上对有效序列归类OTUs,好氧池和厌氧池的OUTs数量分别为253和78。好氧池的OUT数量较高,表明好氧池具有较多的微生物种类。好氧池的细菌群落具有19门、24纲、55目、62科、70属;厌氧池细菌群落具有18门、27纲、35目、44科、51属。
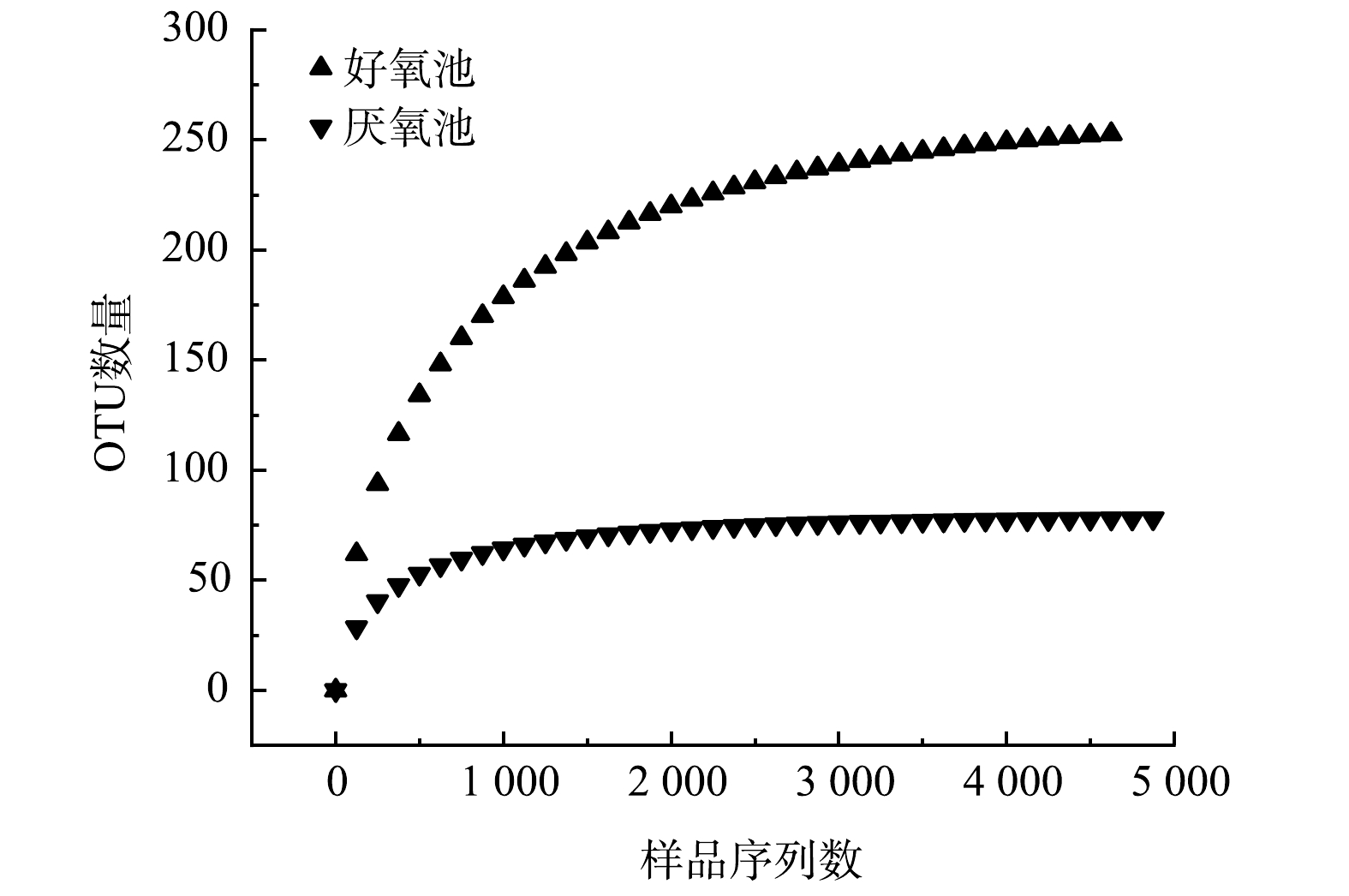
OUTs稀释曲线能够验证测序数据量是否足以反映样品中的物种多样性,并间接反映样品中物种的丰富程度。图3所示的OUTs稀释曲线反映了在持续抽样下系统新物种出现的速率。结果表明,在97%的相似度水平上,当好氧池、厌氧池的样品序列数分别在0~1 500和0~1 000时,曲线表现为急剧上升的趋势,表明群落中有大量物种被发现;而当样品序列数分别大于4 500和3 625后,曲线趋于平缓,表明此环境中的物种并不会随测序数量的增加而显著增多,基本达到饱和。该曲线说明该测序结果能够反映2生化池微生物群落的多样性。
Alpha多样性(Alpha diversity)反映的是单个样品的物种丰度及物种多样性,一般有多种衡量指标,如Chao、ACE、Shannon和Simpson。Chao和ACE指数用于衡量物种丰度即物种数量的多少;Shannon和Simpson指数用于衡量物种多样性,受样品群落中物种丰度和物种均匀度(community evenness)的影响。在相同物种丰度的情况下,群落中各物种均匀度越大,群落多样性越高;Shannon和Simpson指数值越大,样品的物种多样性越高。厌氧池、好氧池所取污泥微生物样品的Alpha多样性分析结果如表2所示。
由表2可知,好氧池的OTUs、Chao指数和ACE指数明显高于厌氧池,说明好氧池的细菌群落更加丰富。同时,好氧池的Shannon指数和Simpson指数也略高于厌氧池,表明好氧池比厌氧池具有更高的群落多样性。测序实验还统计了样品覆盖率(coverage指数),以鉴定测试结果是否代表样品微生物的真实情况。该指数越高,则样本中物种被测出的概率越高。2个反应池的样品覆盖率均大于0.99,说明测序结果能够反映样品微生物的真实情况。
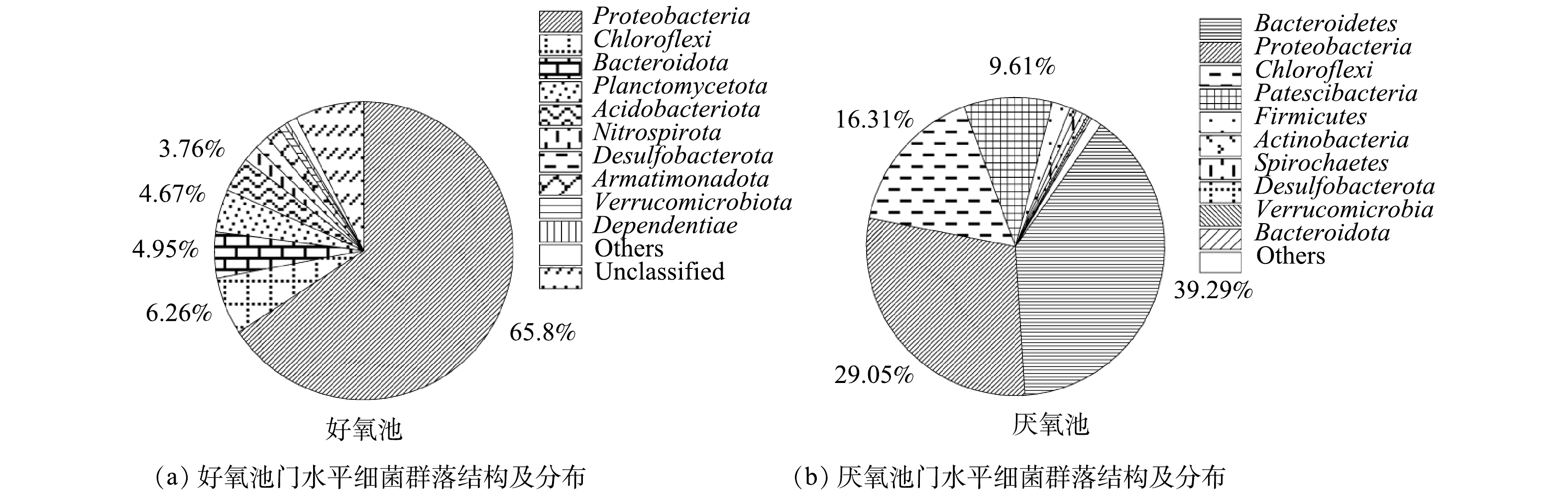
2)好氧池、厌氧池在门水平上的细菌群落分布如图4所示。从门水平上的差异可看出,好氧池的优势菌群为变形菌门(Proteobacteria,65.8%)、绿弯菌门(Chloroflexi,6.26%)、拟杆菌门(Bacteroidota,4.95%)、浮霉菌门(Planctomycetota,4.67%)以及酸杆菌门(Acidobacteriota,3.76%)。这些检测结果与WANG等[23]对中国14个污水处理系统细菌多样性的焦磷酸测序分析结果基本一致。在好氧池还检测到了硝化螺旋菌门(Nitrospirota),相对丰度占1.76%,表明好氧池具有良好的硝化作用。此外,脱硫杆菌门(Desulfobacterota)和Armatimonadota门分别占1.61%和1.57%。ANTUNES等[24]的研究表明,Armatimonadota门与废水中耗氧有机物(以COD计)、TOC、TDS和SS浓度呈显著的正相关。因此,系统中的高有机浓度有助于Armatimonadota门的生长。此外,在其他研究[3]中常发现占优势的厚壁菌门(Firmicutes)和放线菌门(Actinobacteria),在本研究中的数量却较少,存在这一差异的原因有待进一步探究。
相比之下,厌氧池丰度最高的优势菌门是污水处理系统中常见的拟杆菌门(Bacteroidetes),占总群落的39.29%,其次是变形菌门(Proteobacteria,29.05%)和绿弯菌门(Chloroflexi,16.31%)。拟杆菌门在厌氧池中占主导地位,说明厌氧池中发生了良好的水解和酸化作用。另外,Patescibacteria门在厌氧池中占9.61%,厚壁菌门(Firmicutes)占1.98%。除Patescibacteria外,以上几种优势菌门与AHRING[25]所报道的厌氧系统中常见菌门一致。在以往的研究中,关于厌氧系统中Patescibacteria的研究报道还比较少。Patescibacteria门是新定义的超级门,其普遍存在于含水层环境中,如营养物质较少、DO浓度较低的地下水中[26]。由于这一门具有超小的细胞体积,使其具有较高的表容比,能最大限度地利用营养物质[27-28]。由此可推测,该门在本系统厌氧池中的分布与贫营养水体环境的选择作用存在一定的关联。
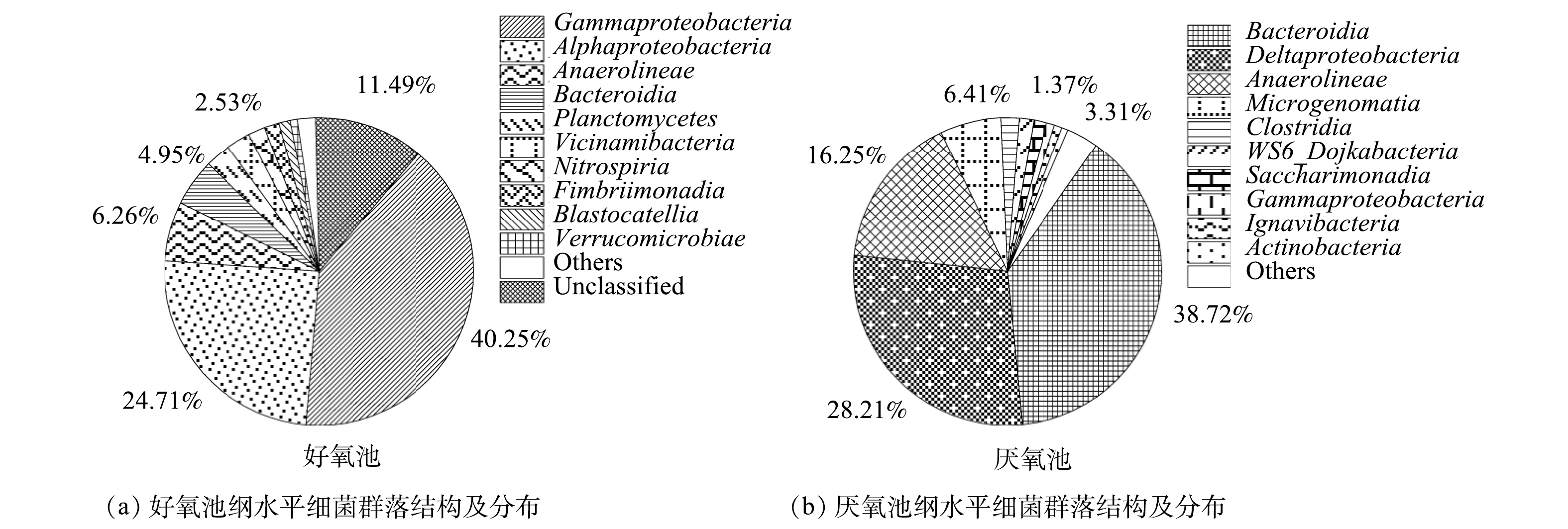
3)从2生化池细菌群落在纲水平上的分布来看(图5),好氧池的优势菌纲主要包括γ-变形菌纲(Gammaproteobacteria,40.25%)、α-变形菌纲(Alphaproteobacteria,24.71%)、厌氧绳菌纲(Anaerolineae,6.26%)、拟杆菌纲(Bacteroidia,4.95%)、浮霉菌纲(Planctomycetes,2.68%)以及Vicinamibacteria菌纲(2.53%)。此外,还发现了硝化螺旋菌纲(Nitrospiria,1.76%)以及不常见的Fimbriimonadia菌纲(1.55%)和Blastocatellia菌纲(1.19%)。与通常的研究结果不同[4],好氧池的优势菌纲中出现了厌氧绳菌纲(Anaerolineae),该菌纲是发酵分解糖类的厌氧细菌纲[29]。其能够在好氧池中富集的原因可能是Anaerolineae纲的一些菌种是半共营养型微生物[30],可与氢营养型产甲烷菌等细菌协同降解糖类[31]。另外,隶属于酸杆菌门(Acidobacteriota,3.76%)的Vicinamibacteria菌纲和Blastocatellia菌纲相对丰度之和为3.72%,表明二者是酸杆菌门的主要贡献者。Vicinamibacteria为好氧有机异养菌,能耐受广泛的pH范围,对不同的糖类、复杂的蛋白质化合物具有良好的代谢效果[32];而Blastocatellia菌纲的细菌偏好缺乏营养的生长条件[33],能够在干旱和营养限制的条件下生存[34]。Fimbriimonadia是一类严格需氧的非运动性杆状细菌,这类细菌大多生长在低营养介质中,绝大部分有机底物都不能作为碳源而被其利用[35]。
厌氧池的优势菌纲则主要为拟杆菌纲(Bacteroidia)、δ-变形菌纲(Deltaproteobacteria)以及厌氧绳菌纲(Anaerolineae),相对丰度分别为38.72%、28.21%和16.25%。其次,Microgenomatia菌纲占比为6.41%、梭状芽孢杆菌纲(Clostridia)为1.90%,最后是WS6_Dojkabacteria菌纲和Saccharimonadia菌纲,相对丰度分别为1.53%和1.37%。大多数厌氧绳菌纲(Anaerolineae)的物种都能进行发酵代谢,有助于在厌氧条件下糖类和蛋白质的降解,是厌氧系统中一个重要的发酵种群[15]。与AHRING[25]的研究结果不同,本系统的厌氧池中出现了2种不常见的菌群,即Microgenomatia和Saccharimonadia。Microgenomatia和Saccharimonadia均属于Patescibacteria门,他们不仅能够在厌氧条件下进行广泛的有机底物代谢,而且能够在营养物质匮乏的贫营养水体中生长[36]。Microgenomatia菌纲能水解发酵纤维素,Saccharimonadia菌纲中的一些菌属被发现有助于降解烃类物质[37-38]。
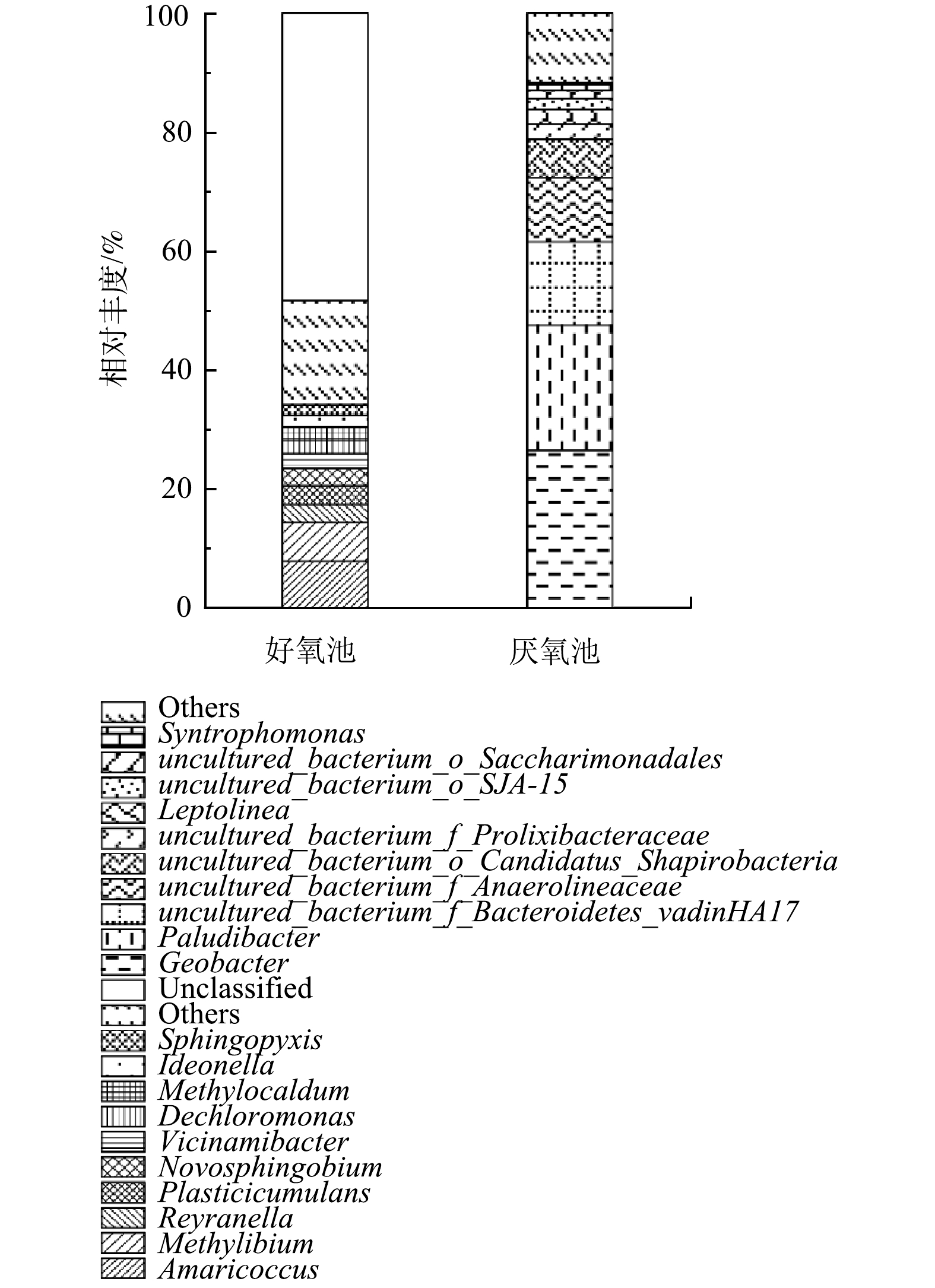
4)图6所示的百分比柱状堆积图反映了好氧池和厌氧池的微生物群落分别在属水平上的分布情况。微生物群落作为生物反应器的主体,决定了生物反应器的性能,而在一个污水生物处理过程中,微生物群落的形成又受当地气候条件、工艺类型和被处理废水的性质等因素的影响。本研究中,高有机物浓度、低氮磷的贫营养条件无疑是一个最重要的影响因素,此条件极有可能为系统中微生物的生长施加选择压。实际结果也表明,本系统的好氧池微生物菌属与常规的好氧活性污泥系统菌属有很大差别。在一般的生活污水处理厂中,活性污泥优势菌属主要为动胶菌属(Zoogloea)、不动杆菌属(Acinetobacter)、丛毛单胞菌属(Comamonas)和黄杆菌属(Flavobacterium)以及硝化螺旋菌属(Nitrospira)等[39];而在本研究中,好氧池的优势菌属包括Amaricoccus(8.03%)、Methylibium(6.50%)、Reyranella(3.1%)、Plasticicumulans(3.06%)、新鞘氨醇杆菌属(Novosphingobium,2.91%)、Vicinamibacter(2.46%)、脱氯单胞菌属(Dechloromonas,2.31%)、甲基暖菌属(Methylocaldum,2.17%)、Ideonella(1.97%)和Sphingopyxis(1.78%)。其中,丰度最高的属Amaricoccus是一种贫营养(氮、磷营养不足)增殖的好氧细菌,属于变形菌门(Proteobacteria),α-变形菌纲(Alphaproteobacteria),革兰氏阴性,能以多糖为底物降解多糖[40]。在氮、磷等营养元素受限制的情况下,他们能大量地储存胞内PHB,并合成胞外荚膜物质[41],因此,能在N、P受限制的条件下增殖[42]。Plasticicumulans属是专性好氧异养菌,能够利用多种有机化合物作为碳源和能源,如醋酸盐、乙醇和乳酸盐等[43]。该属能在超过85%的细胞干重中积累92%的PHB,并利用氨、硝酸盐和复合有机氮作为氮源[44]。通常认为,PHB是微生物在营养不均衡的条件下,如碳源过剩、而其他如氮、磷、硫等营养限制,积累在体内作为其营养和能量储存物质,并参与细胞代谢的天然产物[45]。Plasticicumulans积累PHB的性质使其得以在该系统的贫营养环境中生长。以上这些菌属在好氧池中占主导地位,表明在好氧池中出现了适应贫营养条件的优势菌属,这些菌属能够有效地降解含有大量生物难降解有机物并缺乏氮磷营养物质的中药废水,达到良好的去除效果,以此来保证系统稳定运行。
厌氧池的优势菌属主要集中在地杆菌属(Geobacter,26.62%)、帕卢迪杆菌属(Paludibacter,20.98%)、uncultured_bacterium_f_Bacteroidetes_vadinHA17(14%)、未培养的厌氧菌属(uncultured_bacterium_f_Anaerolineaceae,10.82%)等4种。此外,还发现了uncultured_bacterium_o_Candidatus_Shapirobacteria(6.41%)、uncultured_bacterium_f_Prolixibacteraceae(2.51%)和纤绳菌属(Leptolinea,2.49%)。最后是uncultured_bacterium_o_Saccharimonadales和互营单胞菌属(Syntrophomonas),相对丰度分别占1.37%和1.31%。
地杆菌属(Geobacter)在厌氧池丰度最高,占26.62%。地杆菌属(Geobacter)为变形菌门、δ-变形菌纲;革兰氏阴性、杆状、严格厌氧[46]。这类菌群显著的代谢特征是能将有机物的氧化与Fe(Ⅲ)的还原结合起来,在厌氧条件下以乙酸盐、芳香烃、有机氯化物等多种有机污染物作为电子供体和碳源,还原可溶性或不溶性的Fe(Ⅲ)[47-48]。地杆菌属(Geobacter)对生长环境的适应能力比较强,因而在被石油污染的含水层、被垃圾渗滤液污染的地下水、生活污水和各种废水等环境中均能发现其存在[49]。据以往的研究,地杆菌属(Geobacter)在参与厌氧消化方面有很大的潜力,可以广泛地利用有机物作为底物,如挥发性脂肪酸(VFAs)、醇、酚和苯等[50]。不仅如此,地杆菌属(Geobacter)还可以与其他物种(如产甲烷菌等)建立直接电子连接,即直接种间电子转移,例如,金属还原地杆菌(Geobacter metallireducens)可与甲烷丝菌属(Methanosaeta)或甲烷八叠球菌属(Methanosarcina)等产甲烷菌共培养[51-52]。地杆菌属(Geobacter)在本废水处理系统厌氧池占主导地位,说明厌氧池发生了良好的水解酸化作用,同时也有利于厌氧消化中的产甲烷过程。同时,其它的优势菌属,如帕卢迪杆菌属(Paludibacter)是β-拟杆菌纲(Betabacteroidetes)、紫单胞菌科(Porphyromonadaceae)下的一种严格厌氧菌,该菌能发酵多种单糖和二糖产生乙酸、丙酸和少量丁酸,是一种产酸菌[53-54]。
纤绳菌属(Leptolinea)在厌氧条件下能将多糖等有机物降解转化为有机酸和脂肪酸[55],有利于糖类等有机物的水解酸化。值得注意的是,纤绳菌属(Leptolinea)属于营养匮乏的绿弯菌亚门I(Chloroflexi subphylum I)中的厌氧菌纲,需要与其他微生物结合才能有效生长[30]。uncultured_bacterium_o_Saccharimonadales(1.37%)系超级门Patescibacteria门,具有非常小的基因组和细胞大小,通常与其他微生物共存、依赖共代谢生长[56];一些该细菌被证明能够吸收油酸并具有脂肪酶和其他外酶活性[57];可在发酵途径中将丙酮酸转化为乳酸。互营单胞菌属(Syntrophomonas)是厌氧发酵中常见的微生物,可以降解丁酸,是厌氧活性污泥的功能菌属之一[58]。Syntrophomonas可与氢化产甲烷菌共同消耗长链和短链脂肪酸,是厌氧消化中重要的共营养细菌,具有产生氢或甲酸作为甲烷菌电子载体的功能[59]。Syntrophomonas属在厌氧反应池中的富集,可能也有助于提高COD去除率和产甲烷量。
由上述分析可知,贫营养进水条件下,厌氧池中出现了适应该环境条件并能降解各种复杂有机物的优势菌属Gobacter和Paludibacter,Gobacter主要参与厌氧过程的水解发酵,Paludibacter参与产酸过程。其次,氮磷营养元素的限制为起水解酸化作用的贫营养菌属Leptolinea的生长提供了有利条件。以Leptolinea和Syntrophomonas为代表的优势菌属与其他功能菌属的共代谢生长有利于厌氧消化过程的顺利进行。其中,Syntrophomonas与产甲烷菌的共营养生长不仅促进了产氢产乙酸过程,还有利于厌氧产甲烷。总之,以上这些优势菌属在厌氧池的稳定生长及分工合作是厌氧池能够长期稳定运行的有力保障。
-
1)该贫营养系统运行状况表明,系统能在TN、TP分别低至约10 mg·L−1和1 mg·L−1的条件下长期稳定运行,且系统处理效果良好。
2)中药废水的特殊性质(高COD、低N、P营养)对微生物群落的生长施加了选择压,即大量有机物的存在为具有高有机物降解能力的异养菌提供了微生物选择。其中,厌氧池中表现为Geobacter(26.62%),好氧池中表现为Amaricoccus(8.03%)。
3)贫营养环境和工艺运行条件促进了贫营养需求菌的优势增长,如厌氧池中的纤绳菌属(Leptolinea)、好氧池中的Amaricoccus属和Plasticicumulans属,这些优势菌本身具有很强的有机物降解能力,并在处理统系统中各个阶段分工合作,使系统能够长期稳定运行,达到良好的处理效果。
4)一些优势菌与其他菌种的共营养生存,使系统在污染物降解的各个阶段能够有序地进行,保障了该系统在长期处理过程中的稳定运行。其中厌氧绳菌纲(Anaerolineae)、地杆菌属(Geobacter)、纤绳菌属(Leptolinea)及互营单胞菌属(Syntrophomonas)是典型的代表。
贫营养中药废水处理系统稳定性析因
Cause analysis of the stability of a Chinese medicine wastewater treatment system with deficient nutrition
-
摘要: 云南某制药厂生产废水有机物浓度高、氮磷含量低(贫营养),但废水处理系统长期运行稳定,且处理效果好,COD和BOD的去除率均能达到90%以上。为揭示该贫营养污水处理系统长期高效稳定运行原因,分别从工艺运行和微生物学角度对该系统进行了全面分析。结果表明,该系统厌氧和好氧段氮磷含量低,TN、TP的质量浓度分别为9.68 mg·L−1、1.17 mg·L−1和6.18 mg·L−1、0.25 mg·L−1;该系统好氧池和厌氧池中均发现了以降解有机物为主的优势菌属,好氧池比厌氧池具有更高的微生物丰富度和多样性。好氧池中主要菌属为Amaricoccus、Methylibium、Reyranella和Plasticicumulans4种。其中,丰度最高的Amaricoccus占比为8.03%,该菌属能在氮、磷营养受限的环境中增殖。厌氧池中的优势菌属为Geobacter、Paludibacter、Leptolinea和Syntrophomonas。其中,占比2.49%的Leptolinea为贫营养菌属。贫营养环境条件对微生物群落的生长施加了选择压,促进了贫营养菌的优势增长。这些优势菌在废水处理系统降解污染物的各个阶段分工合作,保障了该系统的稳定运行和良好的处理效果。Abstract: The wastewater discharged from a pharmaceutical factory in Yunnan Province is characterized by high organic concentration and extremely low nitrogen and phosphorus content (deficient nutrition). However, the wastewater treatment system has stably run for the long-term with over 90% of COD and BOD removal. In order to elucidate the mechanisms behind, a comprehensive investigation of the system was conducted from both the operational and microbiological aspects. The results showed that the contents of nitrogen and phosphorus in the anaerobic and aerobic phases were very low, and the corresponding TN and TP concentrations were 9.68 mg·L−1, 1.17 mg·L−1 and 6.18 mg·L−1,0.25 mg·L−1, respectively. The microbial colonies in the aerobic phase presented a higher richness and diversity than those in the anaerobic phase, and the dominant genera in both phases were highly efficient organics-degrading ones. In aerobic phases, the dominant bacteria were Amaricoccus, Methylibium, Reyranella and Plasticicumulans, of which Amaricoccus was the most abundant genus with 8.03% of richness, and could grow in nitrogen and phosphorus deficient environment. In anaerobic phases, the dominant bacteria were Geobacter, Paludibacter, Leptolinea and Syntrophomonas, of which Leptolinea was a type of nutrient-deficiency tolerant species with 2.49% of richness. The nutrient-deficient wastewater environment exerted selective pressure for the microbial colonies, and promoted the dominance of low nutrient demanding bacteria. These dominant bacteria cooperated at each stages of the system to degrade pollutants, which guaranteed high removal efficiency and long-term stability of the system.
-

-
表 1 中药废水处理系统出水指标监测结果
Table 1. Status of the effluent from the Chinese medicine wastewater treatment system
检测单元 pH 其他水质参数浓度/(mg·L−1) 溶解氧 SS COD BOD5 氨氮 TN TP 阴离子表面活性剂 调节池 9.05 1.01 47.00 1296.67 931.33 4.05 8.05 0.38 0.95 二沉池出水 7.46 3.67 13.00 81.33 12.67 0.47 2.33 0.12 0.03 去除率/% — — 72 94 99 88 71 68 97 表 2 好氧池、厌氧池的微生物群落丰富度和多样性指数
Table 2. Richness and diversity indexes of microbial community in the aerobic tank and anaerobic tank
检测单元 OTUs Chao ACE Shannon Simpson 覆盖率/% 好氧池 253 259.2432 262.2059 6.4124 0.9751 99.53 厌氧池 78 78.3333 78.5846 3.9206 0.8622 99.96 -
[1] WANG Y Y, PENG Y Z, PENG C Y, et al. Influence of ORP variation, carbon source and nitrate concentration on denitrifying phosphorus removal by DPB sludge from dephanox process[J]. Water Science and Technology, 2004, 50(10): 153-161. doi: 10.2166/wst.2004.0632 [2] PENG Y Z, GAO C D, WANG S Y, et al. Non-filamentous sludge bulking caused by a deficiency of nitrogen in industrial wastewater treatment[J]. Water Science and Technology, 2003, 47(11): 289-295. doi: 10.2166/wst.2003.0617 [3] JIN D C, WANG P, BAI Z H, et al. Analysis of bacterial community in bulking sludge using culture-dependent andindependent approaches[J]. Journal of Environmental Sciences, 2011, 23(11): 1880-1887. doi: 10.1016/S1001-0742(10)60621-3 [4] ZHANG L, SHEN Z, FANG W K, et al. Composition of bacterial communities in municipal wastewater treatment plant[J]. Science of the Total Environment, 2019, 689: 1181-1191. doi: 10.1016/j.scitotenv.2019.06.432 [5] ZHANG T, SHAO M F, YE L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants[J]. The ISME Journal, 2012, 6(6): 1137-1147. doi: 10.1038/ismej.2011.188 [6] YANG C, ZHANG W, LIU R, et al. Phylogenetic diversity and metabolic potential of activated sludge microbial communities in full-scale wastewater treatment plants[J]. Environmental Science & Technology, 2011, 45(17): 7408-7415. [7] FARKAS M, TANCSICS A, KRISZT B, et al. Zoogloea oleivorans sp. nov. a floc-forming, petroleum hydrocarbon-degrading bacterium isolated from biofilm[J]. International Journal of Systematic and Evolutionary Microbiology, 2015, 65(1): 274-279. [8] NG K K, SHI X, ONG S L, et al. Pyrosequencing reveals microbial community profile in anaerobic bio-entrapped membrane reactor for pharmaceutical wastewater treatment[J]. Bioresourse Technology, 2016, 200: 1076-1079. doi: 10.1016/j.biortech.2015.10.100 [9] LIM J W, CHEN C L, HO I J, et al. Study of microbial community and biodegradation efficiency for single-and two-phase anaerobic codigestion of brown water and food waste[J]. Bioresourse Technology, 2013, 147(7): 193-201. [10] SHIGEMATSU T, TANG Y, MIZUNO Y, et al. Microbial diversity of mesophilic methanogenic consortium that can degrade long-chain fatty acids in chemostat cultivation[J]. Journal of Bioscience and Bioengineering, 2006, 102(6): 535-544. doi: 10.1263/jbb.102.535 [11] HERNON F, FORBES C, COLLERAN E. Identification of mesophilic and thermophilic fermentative species in anaerobic granular sludge[J]. Water Science and Technology, 2006, 54(2): 19-24. doi: 10.2166/wst.2006.481 [12] WILSON L P, SHARVELLE S E, DE LONG S K, et al. Enhanced anaerobic digestion performance via combined solids- and leachate-based hydrolysis reactor inoculation[J]. Bioresourse Technology, 2016, 220: 94-103. doi: 10.1016/j.biortech.2016.08.024 [13] SONG Y L, LIU C X, MCTEAGUE M, et al. ‘‘Bacteroides nordii” sp. nov. and “Bacteroides salyersae” sp. nov. isolated from clinical specimens of human intestinal origin[J]. Journal of Clinical Microbiology, 2004, 42(12): 5565-5570. doi: 10.1128/JCM.42.12.5565-5570.2004 [14] RINCON B, PORTILLO M C, GONZALEZ J M. Microbial community dynamics in the two-stage anaerobic digestion process of two-phase olive mill residue[J]. International Journal of Environmental Science and Technology, 2013, 10(4): 635-644. doi: 10.1007/s13762-013-0290-4 [15] RIVIERE D, DESVIGNES V, PELLETIER E, et al. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge[J]. The ISME Journal, 2009, 3(6): 700-714. doi: 10.1038/ismej.2009.2 [16] BERTIN L, BETTINI C, ZANAEOLI G, et al. Acclimation of an anaerobic consortium capable of effective biomethanization of mechanically-sorted organic fraction of municipal solid waste through a semi-continuous enrichment procedure[J]. Journal of Chemical Technology and Biotechnology, 2012, 87(9): 1312-1319. doi: 10.1002/jctb.3809 [17] 王光华, 刘俊杰, 于镇华, 等. 土壤酸杆菌门细菌生态学研究进展[J]. 生物技术通报, 2016, 32(2): 14-20. [18] NELSON M C, MORRISON M, YU Z T. A meta-analysis of the microbial diversity observed in anaerobic digesters[J]. Bioresourse Technology, 2011, 102(4): 3730-3739. doi: 10.1016/j.biortech.2010.11.119 [19] 国家环境保护总局. 水和废水监测分析方法[J]. 4版. 北京:中国环境科学出版社, 2006: 210-280. [20] ZUO N, JI F Y. The influence of SRT on phosphorus removal and sludge characteristics in the HA-A/A-MCO sludge reduction process[J]. China Water & Wastewater, 2013, 10(2): 025601. [21] XIONG H, CHEN J, HUI W, et al. Influences of volatile solid concentration, temperature and solid retention time for the hydrolysis of waste activated sludge to recover volatile fatty acids[J]. Bioresource Technology, 2012, 119: 285-292. [22] 贺雪濛, 丁丽丽, 张璐璐, 等. 氮磷失衡下膨胀污泥性能及膨胀菌群落结构变化[J]. 环境科学, 2018, 39(4): 1782-1793. [23] WANG X, HU M, XIA Y, et al. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China[J]. Applied and Environmental Microbiology, 2012, 78(19): 7042-7047. doi: 10.1128/AEM.01617-12 [24] ANTUNES T C, MARCONATTO L, BORGES L, et al. Analysis of microbial community biodiversity in activated sludge from a petrochemical plant[J]. Revista Ambiente & Água, 2021, 16(3): 1. [25] AHRING B K. Perspectives for anaerobic digestion[J]. Advances in Biochemical Engineering/Biotechnology, 2003, 81: 1-30. [26] TIAN R M, NING D L, HE Z L, et al. Small and mighty: Adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity[J]. Microbiome, 2020, 8(1): 2-15. [27] HERRMANN M, WEGNER C E, TAUBERT M, et al. Predominance of Cand. patescibacteria in groundwater is caused by their preferential mobilization from soils and flourishing under oligotrophic conditions[J]. Frontiers in microbiology, 2019, 10(15): 1407. [28] SOWELL S M, WILHELM L J, NORBECK A D, et al. Transport functions dominate the SAR11 metaproteome at low-nutrient extremes in the Sargasso Sea[J]. The ISME Journal, 2009, 3(1): 93-105. doi: 10.1038/ismej.2008.83 [29] LIANG B, WANG L Y, MBADINGA S M, et al. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation[J]. AMB Express, 2015, 5(1): 1-13. doi: 10.1186/s13568-014-0092-1 [30] NARIHIRO T, TERADA T, OHASHI A, et al. Quantitative detection of previously characterized syntrophic bacteria in anaerobic wastewater treatment systems by sequence-specific rRNA cleavage method[J]. Water Research, 2012, 46(7): 2167-2175. doi: 10.1016/j.watres.2012.01.034 [31] YAMADA T, IMACHI H, OHASHI A, et al. Bellilinea caldifistulae gen. nov., sp nov and Longilinea arvoryzae gen. nov., sp nov., strictly anaerobic, filamentous bacteria of the phylum Chloroflexi isolated from methanogenic propionate-degrading consortia[J]. International Journal of Systematic and Evolutionary Microbiology, 2007, 57(10): 2299-2306. doi: 10.1099/ijs.0.65098-0 [32] VIEIRA S, LUCKNER M, WANNER G, et al. Luteitalea pratensis gen. nov., sp. nov. a new member of subdivision 6 Acidobacteria isolated from temperate grassland soil[J]. International Journal of Systematic and Evolutionary Microbiology, 2017, 67: 1408-1414. doi: 10.1099/ijsem.0.001827 [33] HUBER K J, WÜST P K, ROHDE M, et al. Aridibacter famidurans gen. nov., sp. nov. and Aridibacter kavangonensis sp. nov., two novel members of subdivision 4 of the Acidobacteria isolated from semiarid savannah soil[J]. International Journal of Systematic and Evolutionary Microbiology, 2014, 64(6): 1866-1875. [34] PASCUAL J, WÜST P K, GEPPERT A, et al. Novel isolates double the number of chemotrophic species and allow the first description of higher taxa in Acidobacteria subdivision 4[J]. Systematic and Applied Microbiology, 2015, 38(8): 534-544. doi: 10.1016/j.syapm.2015.08.001 [35] IM W T, HU Z Y, KIM K H, et al. Description of Fimbriimonas ginsengisoli gen. nov., sp. nov. within the Fimbriimonadia class nov., of the phylum Armatimonadetes[J]. Antonie Van Leeuwenhoek, 2012, 102(2): 307-317. doi: 10.1007/s10482-012-9739-6 [36] TIAN R, NING D, HE Z, et al. Small and mighty: Adaptation of superphylum patescibacteria to groundwater environment drives their genome simplicity[J]. Microbiome, 2020, 8(1): 51. doi: 10.1186/s40168-020-00825-w [37] KADNIKOV V V, MARDANOV A V, BELETSKY A V, et al. Complete genome of a member of a new bacterial lineage in the microgenomates group reveals an unusual nucleotide composition disparity between two strands of dna and limited metabolic potential[J]. Microorganisms, 2020, 8(3): 2-15. [38] CHEN H, LI A, CUI D, et al. Evolution of microbial community and key genera in the formation and stability of aerobic granular sludge under a high organic loading rate[J]. Bioresearch Technology Reports, 2019, 7(1): 100280. [39] DIAS F F, BHAT J V. Microbial ecology of activated sludge[J]. Applied Microbiology and Biotechnology, 1964, 12(5): 412-417. doi: 10.1128/am.12.5.412-417.1964 [40] MASZENAN A M, SEVIOUR R J, PATEL B K C, et al. Amaricoccus gen. nov., a Gram-negative coccus occurring in regular packages or tetrads, isolated from activated sludge biomass, and descriptions of Amaricoccus veronensis sp. nov., Amaricoccus tamwothensis sp. nov, Amaricoccus macauensis sp. nov., Amaricoccus kaplicensis sp. nov.[J]. International Journal of Systematic Bacteriology, 1997, 47(3): 727-734. doi: 10.1099/00207713-47-3-727 [41] FALVO A, LEVANTESI C, ROSSETTI S, et al. Synthesis of intracellular storage polymers by Amaricoccus kaplicensis, a tetrad forming bacterium present in activated sludge[J]. Journal of Applied Microbiology, 2001, 91(2): 299-305. doi: 10.1046/j.1365-2672.2001.01384.x [42] MCLLROY S J, SPEIRS L, TUCCI J, et al. In situ profiling of microbial communities in full-scale aerobic sequencing batch reactors treating winery waste in australia[J]. Environmental Science & Technology, 2011, 45(20): 8794-803. [43] YANG J, DIMITRY YU S, ROBBERT K, et al. Plasticicumulans acidivorans gen. nov., sp. nov., a polyhydroxyalkanoate-accumulating gammaproteobacterium from a sequencing-batch bioreactor[J]. International Journal of Systematic and Evolutionary Microbiology, 2011, 61(9): 2314-2319. doi: 10.1099/ijs.0.021410-0 [44] YANG J, SOROKIN D Y, HELENA J, et al. Plasticicumulans lactativorans sp. nov., a polyhydroxybutyrate-accumulating gammaproteobacterium from a sequencing-batch bioreactor fed with lactate[J]. International Journal of Systematic and Evolutionary Microbiology, 2014, 64(Pt1): 33-38. [45] 杨宇, 徐爱玲, 张燕飞, 等. 生物合成材料聚β-羟基丁酸(PHB)的研究进展[J]. 生命科学研究, 2006(S3): 61-67. [46] COATES J D, PHILLIPS E J, LONERGAN D J, et al. Isolation of Geobacter species from a variety of sedimentary environments[J]. Applied and Environmental Microbiology, 1996, 62(5): 1531-1536. doi: 10.1128/aem.62.5.1531-1536.1996 [47] SNOEYENBOS-WEST O L, NEVIN K P, LOVLEY R, et al. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments[J]. Microbial Ecology, 2000, 39(2): 153-167. doi: 10.1007/s002480000018 [48] LOVLEY D R, BAEDECKER M J, LONERGAN D J, et al. Oxidation of aromatic contaminants coupled to microbial ironreduction[J]. Nature, 1989, 339(6222): 297-300. doi: 10.1038/339297a0 [49] DU Q, MU Q H, CHENG T, et al. Real-time imaging revealed that exoelectrogens from wastewater are selected at the center of a gradient electric field[J]. Environmental Science & Technology, 2018, 52(15): 8939-8946. [50] LOVLEY D R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination[J]. Energy and Environmental Science, 2011, 4(12): 4896-4906. doi: 10.1039/c1ee02229f [51] ROTARU A E, SHRESTHA P M, LIU F, et al. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane[J]. Energy and Environmental Science, 2014, 7(1): 408-415. doi: 10.1039/C3EE42189A [52] ROTARU A E, SHRESTHA P M, LIU F, et al. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri[J]. Applied and Environmental Microbiology, 2014, 80(15): 4599-4605. doi: 10.1128/AEM.00895-14 [53] 李慧星, 杜风光, 薛刚. 高通量测序研究酒精废水治理中厌氧活性污泥的微生物菌群[J]. 环境科学学报, 2016, 36(11): 4112-4119. [54] 王学华, 黄俊, 宋吟玲, 等. 高效水解酸化UASB活性污泥的菌群结构分析[J]. 环境科学学报, 2014, 34(11): 2779-2784. [55] MCILROY S J, KIRKEGAARD R H, DUEHOLM M S, et al. Culture-independent analyses reveal novel anaerolineaceae as abundant primary fermenters in anaerobic digesters treating waste activated sludge[J]. Frontiers in Microbiology, 2017, 8: 1134. [56] LEMOS L N, MEDEROS J D, DINI-ANDREOTE F, et al. Genomic signatures and co-occurrence patterns of the ultra-small Saccharimonadia (phylum CPR/Patescibacteria) suggest a symbiotic lifestyle[J]. Molecular Ecology, 2019, 28: 4259-4271. doi: 10.1111/mec.15208 [57] KINDAICHI T, YAMAOKA S, UEHARA R, et al. Phylogenetic diversity and ecophysiology of Candidate phylum Saccharibacteria in activated sludge[J]. FEMS Microbiology Ecology, 2016, 92(6): 1-11. [58] MCINERNEY M J, BRYANT M, HESPELL R, et al. Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium[J]. Applied and Environmental Microbiology, 1981, 41(4): 1029-1039. doi: 10.1128/aem.41.4.1029-1039.1981 [59] JACKSON B E, BHUPATHIRAJU V K, TANNER R S, et al. Syntrophus aciditrophicus sp. nov., a new anaerobic bacterium that degrades fatty acids and benzoate in syntrophic association with hydrogen-using microorganisms[J]. Archives of Microbiology, 1999, 171(2): 107-114. doi: 10.1007/s002030050685 -



 下载:
下载: