-
抗生素不仅可以治疗人类疾病,还可以作为生长促进剂促进动物生长,因此被广泛应用于人类医疗和动物蓄养方面[1-2]。抗生素进入动物和人体后,不能被充分吸收。未被吸收的抗生素通过尿液和粪便排出体外,可能直接排入周边环境,并造成环境污染[3],也可能随生活污水一起进入传统污水处理厂(WWTPs)中。由于WWTPs缺少针对抗生素类污染物的去除单元,故抗生素类污染物不能被完全去除,残留的抗生素随WWTPs尾水排放进入水体及周边环境中[4-5]。尽管抗生素类污染物质在水环境中检测出的残留质量浓度极低(ng·L−1或µg·L−1级别),但环境中持续低浓度的抗生素残留不仅可以选择性抑杀一些环境微生物,而且能够诱导一些耐药菌群或抗性基因(antibiotic resistance genes, ARGs)的产生,且ARGs可以通过水平基因转移在微生物之间转移,一旦转移到人类致病菌中,将会导致抗生素疗效降低甚至治疗失效,严重威胁人类健康[6]。城市在抗生素使用量和集中性上占据主要地位,因此,WWTPs尾水直接排放入河流或作为农田灌溉用水已成为环境抗生素及抗性基因污染的一个主要来源[7-10]。
将WWTPs尾水回用于农业灌溉是缓解城市水资源短缺的一项重要措施[11-13]。尾水中残留的抗生素在再生水灌溉时会转移至土壤中,并诱导抗生素抗性细菌的产生,而ARGs通过基因的垂直转移和水平转移使ARGs在土壤中传播和扩散,从而对生态环境和人类健康造成威胁[14-15]。然而,目前污水灌溉对土壤造成的不利影响研究多集中于重金属、好氧有机物、pH、TSS及微生物等污染方面,WWTPs尾水中频繁检出的抗生素类新兴有机污染物却被忽略,关于污水厂尾水用于稻田灌溉时尾水中抗生素在稻田作物及土壤中的残留还未见有相关的报道。
目前,对于环境中抗生素残留量的检测方法有生物检测法、理化检测法和联用技术[16],相对来说,生物检测法、灵敏性低、准确性差、不够稳定,所以运用较少。理化检测法中应用较多的为超高效液相色谱法(UPLC)和气相色谱法(GC),而联用技术相对来说灵敏度高、重复性好、检出限低,是目前应用最多的检测方法。其中,液相色谱串联质谱(LC-MS/MS)法能够定性、定量的检测环境中的抗生素,并具有较低的检出限(ng·L−1)和较高的回收率,故得到广泛应用[17]。QuEChERS法具有快速、简单、便宜、有效、耐用和安全等优点,是一种可以提取食品中农药和药物残留等化学污染物的新型前处理技术[18-19]。本研究选取被广泛使用及在WWTPs尾水中检出频率较高的抗生素(磺胺甲恶唑、氧氟沙星、罗红霉素、四环素)为研究对象,采用QuEChERS的前处理净化手段,结合HPLC-MS/MS法相结合的方法,建立了稻田模拟系统中土壤及水稻中残留抗生素的测定方法。该方法操作简单、灵敏度高、重现性好,可满足稻田系统中土壤及作物中抗生素残留的检测分析,从而为保障再生水灌溉安全风险监测提供有效手段。
-
TSQ Quantum Access Max三重四极杆质谱仪(配备电喷雾离子源)、Poroshell 120 EC-C18柱(2.1×150 mm,2.7 µm)(美国Agilent)、METTLER TOLEDO电子分析天平、高速匀浆机(德国IKA)、台式离心机(美国Thermo Fisher)、KQ-300TDE超声仪(昆山市超声仪器有限公司)、超纯水仪(美国Millipore)
-
磺胺甲恶唑(Sulfamethoxazole,SMZ)、氧氟沙星(Ofloxacin,OFX)、罗红霉素(Roxithromycin,ROX)、四环素(Tetracycline,TET)、西玛通(Simatone,SMT)、诺氟沙星(Norfloxacin-D5,NOR-D5)、磺胺甲嘧啶(sulfamethazine-D4,SM-D4)标准品:纯度≥99%,均购买自上海麦克林(Macklin)公司,其中SMT、NOR-D5、SM-D4为内标物。甲醇、乙腈均为色谱级,购买自德国Merck公司,甲酸(色谱纯)购买自美国Tedia公司。无水硫酸镁(分析纯)、氯化钠(分析纯)购买自美国Sigma-Aldrich公司。其他有机试剂均为高效色谱级、无机化学品均为分析纯。
-
1)标准溶液的配制。将所有的标准品溶解于甲醇中,OFX、SMZ、ROX和TET母液质量浓度分别为500、1 000、1 000及1 000 mg·L−1;内标物SMT、NOR-D5和SD-D4母液质量浓度分别为10、100及100 mg·L−1。制备好的母液装入50 mL玻璃试管中,于−20 ℃条件下保存。上机前用甲醇将母液稀释至1 mg·L−1,再用初始流动相(乙腈∶水=10∶90)稀释配制工作溶液,所配浓度梯度为0.1、0.2、0.5、1、2、5、10、20、50、100 µg·L−1,稀释后的溶液于4 ℃条件下保存,尽快使用。内标溶液同样用甲醇稀释至10 mg·L−1,保存于4 ℃冰箱中,配制工作溶液时最后加入内标物,使内标物质量浓度均为20 µg·L−1,每15 d更换新鲜溶液。
2)稻田模拟系统的建立。2019年11月,在浙江省杭州市中国水稻研究所某人工气候室中建造稻田模拟系统,稻田系统灌溉水模拟污水处理厂二级出水的水质(COD=60 mg·L−1;氨氮=8 mg·L−1;TN=20 mg·L−1;TP=1 mg·L−1),同时添加4种目标抗生素的混和物(OFX、SMZ、ROX与TET),添加质量浓度分别为0、10、30、100和500 µg·L−1。模拟灌溉水用自来水配制,所用配方参照文献[20]。根据灌溉水中抗生素浓度的不同,将模拟箱进行编号,其中未添加抗生素的对照组编号为P(0),添加质量浓度为10、30、100和500 µg·L−1的目标抗生素处理组分别编号为P(10)、P(30)、P(100)和P(500),每个处理组设置2个平行。所有的稻田模拟装置为PVC塑料方箱,尺寸为60 cm×40 cm×15 cm,系统内的填充土壤取自中国水稻研究所实验田基地同一块田中的稻田土。提前取好实验用土,晒干并称取相同质量的土壤放入模拟箱中。为模拟WWTPs水力停留时间,每隔24 h对模拟箱中的水进行更换,根据水稻不同生长周期需水量差异,每次补充水量为1~3 L。实验期间总共种植了2轮水稻,选用“中浙优8号”优质水稻种子。水稻种子用水培法育苗,幼苗长至2~3叶时,移入模拟箱,进行水稻栽培实验。
3)样品前处理。稻田土壤:在第2轮水稻成熟期,用梅花5点取样法取每个模拟箱中土样,混匀后的样品装入密封盒中,立即送回实验室,进行冷冻干燥。将冷冻干燥后的土壤研磨过筛,准确称取5.00 g过筛土样于250 mL离心管中,加入20 mL超纯水充分浸泡,加入5%甲酸-乙腈25 mL,匀浆1 min(10 000 r·min−1),加入1.00 g NaCl和10.00 g MgSO4作为萃取剂,继续匀浆1 min(10 000 r·min−1),4 000 r·min−1离心5 min,取1 mL上清液过0.22 µm滤膜。将滤液上机测定。
稻米:取完土壤样品后,随即剪下成熟稻穗,将稻谷摘下自然风干,经过脱壳、磨精、磨粉过筛,保存于4 ℃冰箱中备用。准确称取5.00 g米粉于250 mL离心管中,提取方法同稻田土壤。水稻植株(地上部分):第2轮水稻收割完稻穗后,取水稻茎、叶样品,用自来水洗净,吸取表面水分,立即送回实验室,-80 ℃条件下保存。取出冷冻的茎叶,用吸水纸吸取表面水分,剪碎后准确称取5.00 g(鲜质量)于250 mL离心管中,提取方法同稻田土壤。水稻植株(地下部分):将收割完茎叶的根拔出,用自来水清洗干净,吸干表面水分后,于-80 ℃条件下保存。取出水稻根,用吸水纸吸干表面水分,准确称取5.00 g(鲜质量)于250 mL离心管中,提取方法同稻田土壤。
4)色谱条件。Agilent Poroshell 120 EC-C18柱(2.1×150 mm,2.7 µm)为色谱柱,柱前接预柱。流动相为A:0.1%的甲酸水溶液,B:乙腈,流速设置为200 µL·min−1,进样量为2.0 µL,柱温30 ℃。梯度洗脱程序:0~10 min为10%~90%B;10~12 min为90%B;12~12.1 min为10%~90%B;12.1~17 min为10%B。
5)质谱条件。根据4种目标化合物和3种内标物质的化学性质,均采用电喷雾正离子源(ESI+)扫描,动态多反应监测(MRM)。离子传输管温度为300 ℃,气化温度为350 ℃,鞘气压力为35 Arb,辅助气压力为15 Arb,喷雾电压为3 500 V。其他质谱优化结果见表1。
6)加标回收实验。不同基质对目标化合物具有不同的基质效应,因此,在测定回收率时,本实验分别选取空白水稻植株、稻田土壤及稻米作为3类基质,对4种目标抗生素进行加标回收实验。采用1.3.3节中的前处理方法进行样品处理,并按照优化后的色谱及质谱条件进行测定。不同基质条件下的每组实验均进行6次平行实验,计算4种目标物质(OFX、ROX、SMZ和TET)的平均回收率及相对标准偏差值。
-
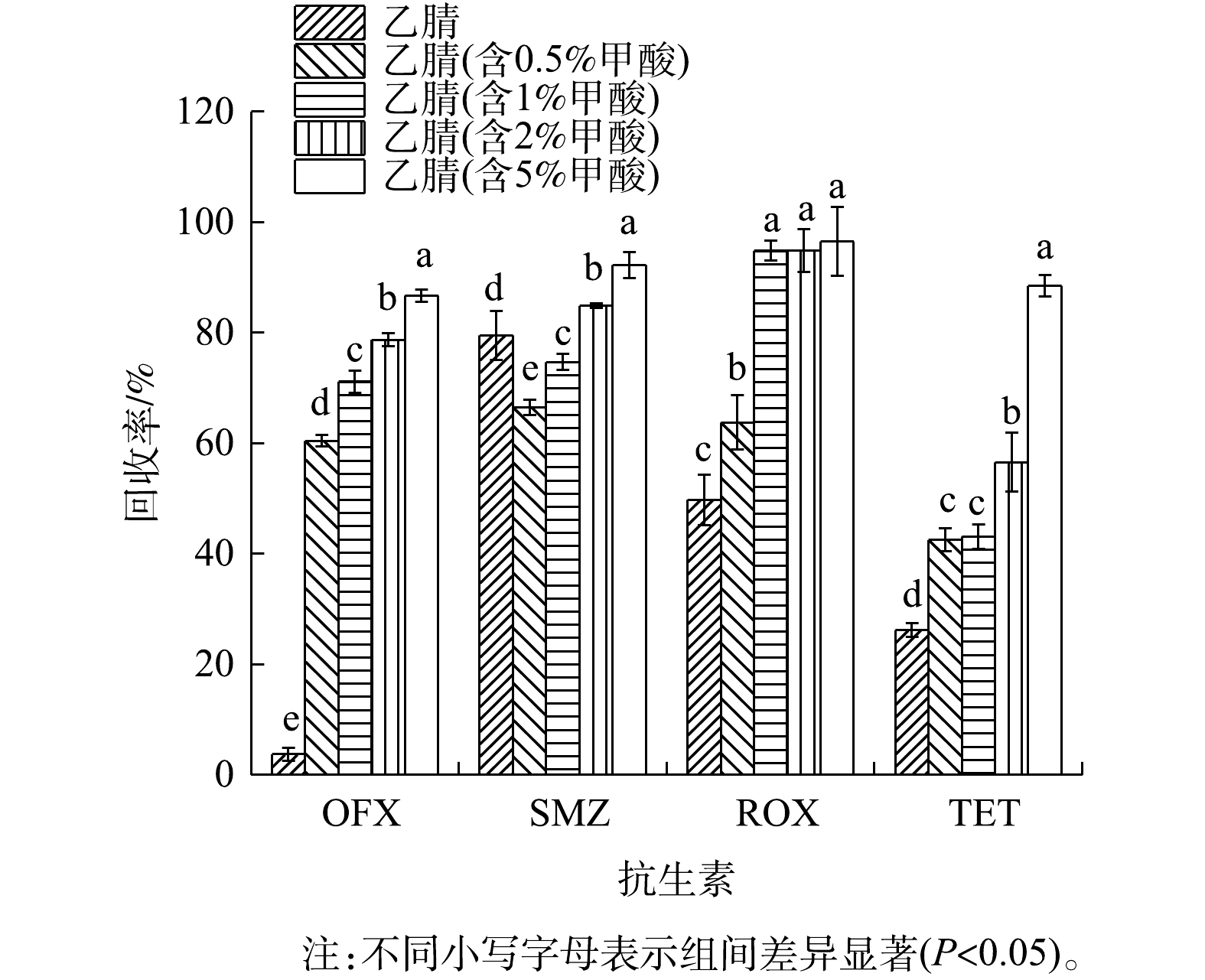
本实验选取稻田土壤为基质,在100 µg·L−1的加标水平下且在体积分数不同的甲酸-乙腈(乙腈、0.5%甲酸-乙腈、1%甲酸-乙腈、2%甲酸-乙腈和5%甲酸-乙腈)条件下,比较了4种目标抗生素的回收率,以确定最佳提取溶剂。结果表明,甲酸的加入可以有效提高4种目标抗生素在稻田土壤中的提取效率。图1为不同提取溶剂条件下稻田土壤中4种目标抗生素的回收率。由图1可以看出,甲酸的存在对OFX、ROX和TET这3种抗生素的提取影响较大,纯乙腈条件下,3种抗生素的回收率均<60%。加入甲酸后,提取效率明显增加。其中ROX的回收率在1%甲酸-乙腈之后趋于平缓,在甲酸含量为1%、2%及5%时,其回收率分别为94.86%、94.84%及96.56%。OFX、SMZ和TET的回收率随甲酸含量的增加而升高,在甲酸体积分数为5%时达到最高,分别为86.7%、92.3%和88.5%。综合考虑,由于TET在低浓度甲酸下,回收率较低(<60%),为同时满足4种目标物的提取需求,最终选择5%甲酸-乙腈作为前处理提取剂。
-
将4种目标化合物的1 mg·L−1标准溶液,按梯度稀释,梯度浓度分别为0.1、0.2、0.5、1、2、5、10、20、50、100 µg·L−1。采用内标法进行定量,用已优化好的色谱条件和质谱条件进行测定,分别以4种目标物质的质量浓度(µg·L−1)为横坐标,以定量离子的峰面积为纵坐标,绘制标准曲线。所得各物质的标准曲线及检出限(LOD)如表2所示。R2均大于0.998,检出限为0.05~0.20 µg·L−1。
-
基质效应主要是由于样品中存在非目标成分,在运用ESI+离子源时,这些其他物质会增强或减弱目标化合物的电离结果,进而影响仪器测定结果的准确性[21]。由于内标物在色谱和质谱扫描过程中的行为与目标化合物一致,因此,本实验采用内标法补偿基质效应。采用空白基质(稻米、水稻植株、稻田土壤)进行前处理后配制相同质量浓度的标准溶液,对4种目标抗生素进行基质效应(Me)评价。用空白基质中目标分析物的峰面积A和乙腈中目标分析物的峰面积B的比值考察基质效应的大小,根据式(1)计算样品的基质效应[22]。
式中:Me为基质效应,%;B为基质配制标准溶液的响应值;A为目标物在纯净溶剂(乙腈)中的响应值。结果表明,4种目标化合物的基质效应在89.7%~106.8%,基质效应的影响可以忽略不计。
-
选取稻米、水稻植株(地上部分)、水稻植株(地下部分)和稻田土壤为基质进行加标回收实验。准确称取不同基质5.00 g于离心管中,加标水平为100 µg·L−1,加入对应的3种内标物,加20 mL超纯水后浸泡30 min,加入5%甲酸-乙腈25 mL,匀浆1 min,加入1.00 g NaCl和10.00 g MgSO4,继续匀浆1 min,4 000 r·min−1离心5 min。取1 mL上清液过0.22 µm滤膜,上机测定。图2为4种目标抗生素在4种基质中的平均回收率。由图2可见,4种化合物的回收率为67.7%~103.5%,RSD值为0.3%~13.8%,符合实验要求。
-
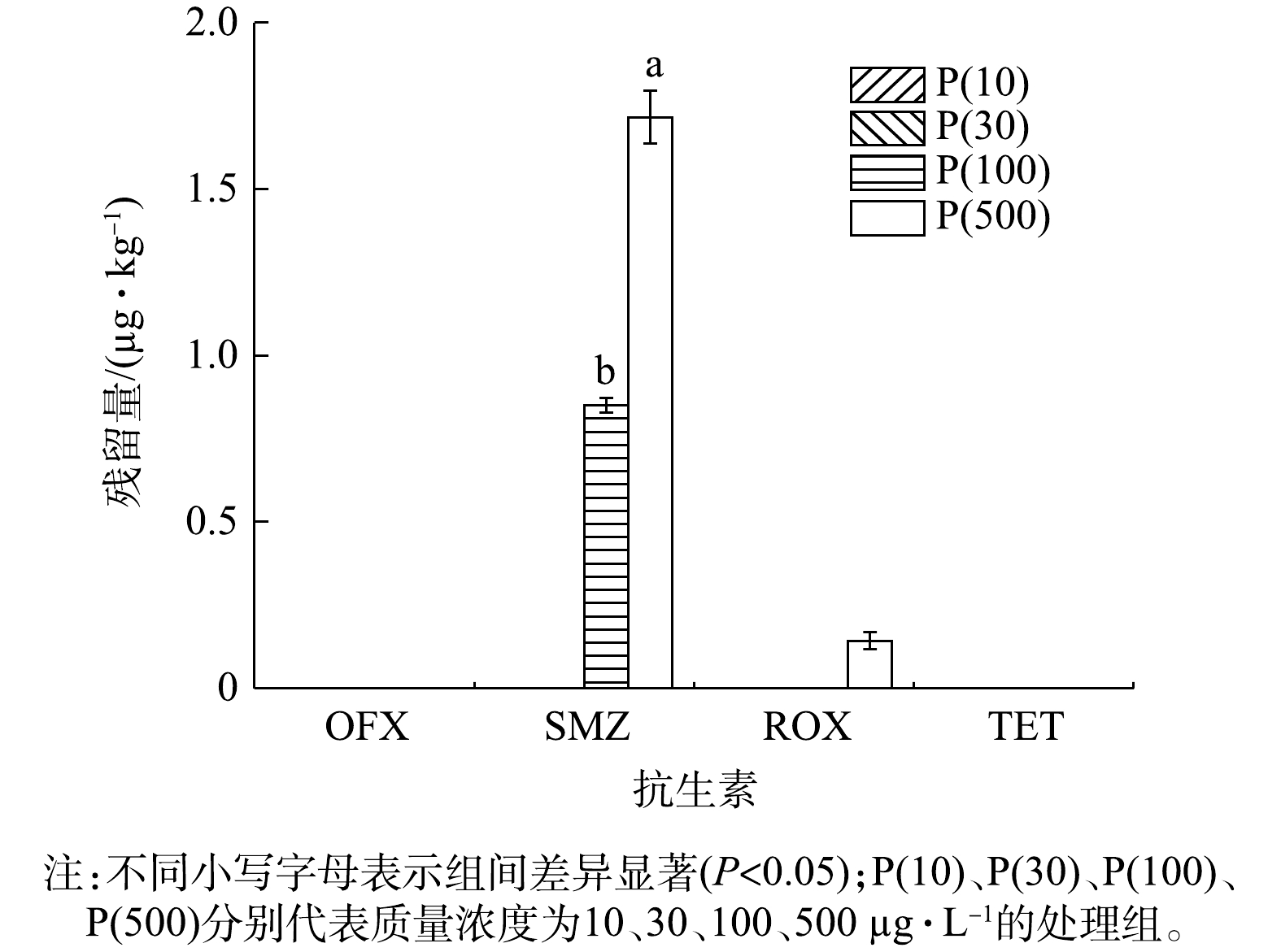
本实验利用优化好的QuEChERS-LC-MS/MS方法,以含抗生素的模拟污水长期灌溉的稻田系统为研究对象,测定了稻米、水稻植株(地上部分和地下部分)和稻田土壤中4种目标抗生素的残留情况,其结果如图3、图4、图5、图6所示。由图3可以看出,在稻米样品中,4种目标抗生素在低浓度处理组(P(10)和P(30))中均未检测到(<LOQ)。SMZ在高浓度处理组(P(100)和P(500))中可被检出,检出质量浓度分别为0.851 µg·kg−1和1.716 µg·kg−1。ROX仅在P(500)处理组中被检出,检出质量浓度为0.142 µg·kg−1。有研究[23]表明,抗生素在植物中的积累与抗生素各自的辛醇/水分配系数(logKOW)密切相关。SMZ的logKOW<1,因此,土壤基质对SMZ的吸附率较低,长期存在于稻田系统中的SMZ通过水稻根部吸收进入水稻体内,进而迁移到稻米中。在本实验结果中,稻米样品中均未检测到OFX和TET,SMZ和ROX虽被检测到,但只在高浓度抗生素条件下才被检出,这与YAN等[24]的研究结果类似。有研究[25]表明,人类每年通过食用作物接触抗生素的量为1.10~7 950 µg·a−1,通过风险评估分析,食用这些作物所带来的风险可以忽略不计。在实际污水处理厂出水中,常见的抗生素残留质量浓度低于100 µg·L−1,这表明低浓度抗生素存在,对稻米安全并没有影响,WWTPs尾水回灌稻田具有推广应用的价值。
分别考察了4种目标抗生素在水稻植株地上部分及地下部分中的残留情况,结果如图4和图5所示。由图4可以看出,SMZ在所有处理组的水稻茎叶中均被检测到,而其余3种目标物只在高浓度处理组(P(100)和P(500))中被检出。SMZ在低浓度抗生素条件下(10 µg·L−1和30 µg·L−1)的检出质量浓度分别为0.514 µg·kg−1和2.303 µg·kg−1。图5为植株地下部分中目标物的残留情况。可以看出,4种目标化合物在所有处理组中均可被检测到,且检出浓度随添加抗生素浓度的增加而升高。对比图4和图5可以看出,在相同处理条件下,4种目标物在水稻植株地下部分的检出浓度均高于地上部分。OFX在水稻地下部分中整体检出浓度较高。在P(500)处理组中,TET在水稻地上部分及地下部分中的检出浓度均为最高,分别为9.709 µg·kg−1和26.047 µg·kg−1。以上研究结果表明,目标抗生素进入稻田环境中后被水稻植株根际所吸收,大部分在根际累积,少部分迁移到水稻的茎叶中。
图6显示了4种目标抗生素在稻田土壤中的检出质量浓度。在所有处理组中,4种抗生素均被检测到。其中OFX和ROX在稻田土壤中的检出质量浓度显著高于SMZ和TET,最大值分别为272.354 µg·kg−1和250.908 µg·kg−1,而TET和SMZ的最高检出质量浓度分别为31.339 µg·kg−1和27.747 µg·kg−1。有研究[26]表明,抗生素在土壤中的残留量与他们的分子结构、半衰期、抗性及吸附性有关,不同的物理化学性质,导致不同种类的抗生素在土壤中的吸附率存在差异。在土壤基质中,在相同处理条件下,OFX的残留量均为最大。虽然OFX具有较低的logKow(<2.5),但氟喹诺酮类抗生素在固体基质中吸附性较高[27-29]。氟喹诺酮类抗生素本身含有羰基、氨基和羧基等化学键,具有两性特征,土壤的pH对其在土壤中的吸附影响较大,使得这类抗生素较容易被土壤吸附,在土壤中的吸附率可以达到接近100%[30]。本研究结果显示,尽管在低浓度处理组(P(10)和P(30))中,4种目标物的检出浓度均相对较低,但在土壤基质中的残留量普遍高于其他3种基质。
综上所述,OFX的检出质量浓度在稻田土壤及水稻根部中较高,分别为8.633~272.354 µg·kg−1和1.766~24.152 µg·kg−1。在水稻植株成熟茎叶中部分可检出,检出质量浓度为0~0.921 µg·kg−1,在稻米样品中未检出(<LOQ)。灌溉水中的抗生素大部分被稻田土壤基质所吸附,其次在水稻根际积累,进而往水稻茎叶中迁移,稻米中残留量最低。从本实验研究结果可以看出,如果长期用含高浓度抗生素污水灌溉稻田,OFX和ROX在稻田土壤中的检出浓度较高,超过生态毒性效应触发值(100 µg·kg−1)[31]。而SMZ和TET 2种目标物在稻田土壤中的残留量相对较少。GU等[31]的研究表明,在菜地环境中TET较容易被降解,检出浓度低于检出限,这和本研究结果相似。因此,从我们获得的实验结果可知,污水尾水回用于农业灌溉时,其残留的低浓度抗生素不会在可食部分中积累,所产出的大米的在日常消费不会构成人体健康威胁。
-
1)通过优化QuEChERS方法的提取溶剂,建立了基于LC-MS/MS法快速定性定量分析稻田模拟系统中土壤及植株中4类常用抗生素残留的方法,该方法操作方便、灵敏度高、重复性好。
2)用5%甲酸-乙腈作为提取溶剂,样品提取回收率达到67.7%~103.5%,相对标准偏差为0.3%~13.8%。4种目标化合物及内标物在同一色谱条件下得到较好的分离,各物质在0.10~100 µg·L−1内呈良好线性关系,R2>0.998。
3)在测定的实际样品中,4种抗生素在各基质中的残留量大小为稻田土壤>水稻植株(地下部分)>水稻植株(地上部分)>稻米,检出浓度为0~272.354 µg·kg−1。由于污水处理厂出水中实际抗生素的质量浓度远低于100 µg·L−1,因此,我们可以推断污水处理厂出水回用于稻田生态系统的灌溉,可以在实现污水资源化的同时实现尾水的无害化。
QuEChERS-液相色谱-串联质谱法测定稻田中土壤及水稻中抗生素的残留量
Determination of antibiotic residues in soil and rice of the simulated paddy field by QUECHERS-LC-MS/MS
-
摘要: 采用QuEChERS-LC-MS/MS法建立了稻田模拟系统中残留的4种目标抗生素的检测方法。样品经5%甲酸-乙腈提取,用内标法进行定量。结果表明,4种目标抗生素在0.10~100 µg·L−1内具有良好的线性关系(R2>0.998)。OFX、SMZ、ROX和TET的检出限分别为0.10、0.10、0.05、0.20 µg·L−1。4种目标物质在稻田水、稻米、水稻植株(地上部分和地下部分)和稻田土壤中的回收率分别为82.8%~166.1%、78.1%~103.5%、94.5%~97.8%、67.7%~100.2%、86.7%~93.9%,相对标准偏差分别为0.3%~8.3%、1.4%~3.8%、1.1%~6.8%和1.1%~2.4%。通过建立稻田模拟系统,长期用含相应质量浓度抗生素的污水灌溉,对稻田模拟系统中抗生素的残留进行了检测。检测结果表明,SMT和ROX在各基质中均有检出,检出质量浓度为未检出~250.908 µg·kg−1。OFX和TET在稻米样品中未检出,但在其余基质中均有检出,检出质量浓度为未检出~272.354 µg·kg−1。
-
关键词:
- QuEChERS-LC-MS/MS /
- 抗生素 /
- 水稻 /
- 残留
Abstract: A method was developed for the determination of four target antibiotics residual in the rice field simulation system by liquid chromatography-tandem mass spectrometry (LC-MS/MS) coupled with QuEChERS. By optimizing the pretreatment conditions, 5% formic acid-acetonitrile was used to extract the antibiotics in each matrix, and the internal standard method was used to quantization detection. The results show that the four target antibiotics had a good linear relationship in the range of 0.1—100 µg·L−1 (R2>0.998). The limits of detection (LOD) of ofloxacin (OFX), sulfamethoxazole (SMZ), roxithromycin (ROX) and tetracycline (TET) were 0.10, 0.10, 0.05 and 0.20 µg·L−1, respectively. The average recoveries of the four target compounds in paddy water, rice flour, rice plants (aboveground and underground parts) and paddy soil were 82.8%—166.1%, 78.1%—103.5%, 94.5%—97.8%, 67.7%—100.2%, 86.7%—93.9%, respectively, with relative standard deviations (RSD, n=6) of 0.3%—8.3%, 1.4%—13.8%, 1.1%—6.8% and 1.1%—2.4%, respectively. A paddy field simulation system was established and irrigated for a long-term by the simulated wastewater containing antibiotics with the corresponding mass concentration. The antibiotic residues in the paddy field simulation system were detected. SMT and ROX were detected in each matrix, and the detected mass concentration was not detected-250.908 µg·L−1. OFX and TET were not detected in rice flour, but were detected in all other matrices, and the detected mass concentration was not detected —272.354 µg·L−1.-
Key words:
- QuECHERS-LC-MS/MS /
- antibiotics /
- rice /
- residues
-

-
表 1 化合物的质谱检测条件
Table 1. Mass spectrometric detection conditions of the compounds
化合物 内标物 母离子 子离子 电离模式 碰撞能量/V SMZ SM-D4 254 155.9a,108.1b + 13,22 TET SMT 445 410a,154b + 17,25 ROX SMT 837.4 679.2a,157.9b + 18,30 OFX NOR-D5 362 261a,318b + 18,25 SMT 198 100.1a,68.2b + 25,35 NOR-D5 325 307a,281b + 20,16 SM-D4 283 185.9a,124.1b + 17,26 注:a 定量离子,b 定性离子。 表 2 目标化合物的线性范围、线性关系、相关系数及检出限
Table 2. Linear ranges, linear relationship, correlation coefficient and limit of detection (LOD) of the target compounds
化合物 浓度范围/(µg·L-1) 线性方程 R2 LOD/(µg·kg-1) 稻米 地上部分 地下部分 稻田土壤 SMZ 0.20~100 Y=1 438.74x-326.703 0.999 8 0.20 0.25 0.30 0.50 OFX 0.20~100 Y=8 377.53x-10 546.8 0.998 1 0.20 0.25 0.35 0.35 ROX 0.10~100 Y=4 707.46x+2 387.1 0.999 1 0.10 0.20 0.25 0.35 TET 0.50~100 Y=2 524.58x-3 041.32 0.998 6 0.50 0.50 0.80 0.85 -
[1] CARVALHO I T, SANTOS L. Antibiotics in the aquatic environments: A review of the European scenario[J]. Environment International, 2016, 94: 736-757. doi: 10.1016/j.envint.2016.06.025 [2] LIU X, GUO X, LIU Y, et al. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: Performance and microbial response[J]. Environmental Pollution, 2019, 254: 112996. doi: 10.1016/j.envpol.2019.112996 [3] GUAN Y, WANG B, GAO Y, et al. Occurrence and fate of antibiotics in the aqueous environment and their removal by constructed wetlands in China: A review[J]. Pedosphere, 2017, 27(1): 42-51. doi: 10.1016/S1002-0160(17)60295-9 [4] HIJOSA-VALSERO M, Fink G, Schlusener M P, et al. Removal of antibiotics from urban wastewater by constructed wetland optimization[J]. Chemosphere, 2011, 83(5): 713-719. doi: 10.1016/j.chemosphere.2011.02.004 [5] BERGLUND B, KHAN G A, WEISNER S E, et al. Efficient removal of antibiotics in surface-flow constructed wetlands, with no observed impact on antibiotic resistance genes[J]. Science of the Total Environment, 2014, 476-477: 29-37. doi: 10.1016/j.scitotenv.2013.12.128 [6] ZHU Y G, JOHNSON T A, SUU J Q, et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(9): 3435-3440. doi: 10.1073/pnas.1222743110 [7] ALINE A G, FABIO K, PAULO A Z P. Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment: A review[J]. Chemosphere, 2015, 138: 281-291. doi: 10.1016/j.chemosphere.2015.06.024 [8] VERLICCHI P, AUKIDY M, ZAMBELLO E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment: A review[J]. Science of the Total Environment, 2012, 429(7): 123-155. [9] JIN L L, MING H W. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China[J]. Environment International, 2013, 59: 208-224. doi: 10.1016/j.envint.2013.06.012 [10] 隋倩雯, 张俊亚, 魏源送, 等. 畜禽养殖废水生物处理与农田利用过程抗生素抗性基因的转归特征研究[J]. 环境科学学报, 2016, 36: 16-26. [11] HAMILTON A J, STAGNITTI F, XIONG X, et al. Wastewater irrigation: The state of play[J]. Vadose Zone Journal, 2007, 6(4): 823-840. doi: 10.2136/vzj2007.0026 [12] TOZE S. Reuse of effluent water-benefits and risks[J]. Agricultural Water Management, 2006, 80: 147-159. doi: 10.1016/j.agwat.2005.07.010 [13] FATTA K D, KALAVROUZIOTIS I K, KOUKOULAKIS P H, et al. The risks associated with wastewater reuse and xenobiotics in the agroecological environment[J]. Science of the Total Environment, 2011, 409(19): 3555-3563. doi: 10.1016/j.scitotenv.2010.03.036 [14] BAI X, MA X, XU F, et al. The drinking water treatment process as a potential source of affecting the bacterial antibiotic resistance[J]. Science of The Total Environment, 2015, 533: 24-31. doi: 10.1016/j.scitotenv.2015.06.082 [15] CHEN C, LI J, CHEN P, et al. Occurrence of antibiotics and antibiotic resistances in soils from wastewater irrigation areas in Beijing and Tianjin, China[J]. Environmental pollution, 2014, 193: 94-101. doi: 10.1016/j.envpol.2014.06.005 [16] 黄璐, 杨成雄, 刘生鹏. 动物源性食品中抗生素残留的危害及检测方法[J]. 广州化工, 2021, 49(5): 44-47. doi: 10.3969/j.issn.1001-9677.2021.05.016 [17] LI Y, TAGGART M A, MCKENZIE C, et al. A SPE-HPLC-MS/MS method for the simultaneous determination of prioritised pharmaceuticals and EDCs with high environmental risk potential in freshwater[J]. Journal of Environmental Sciences, 2021, 100: 18-27. doi: 10.1016/j.jes.2020.07.013 [18] CHEN J, HE L X, CHENG Y X, et al. Trace analysis of 28 antibiotics in plant tissues (root, stem, leaf and seed) by optimized QuEChERS pretreatment with UHPLC-MS/MS detection[J]. Journal of Chromatography B, 2020, 1161: 122450. doi: 10.1016/j.jchromb.2020.122450 [19] ZHANG C Y, DENG Y C, ZHENG J F, et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review[J]. TrAC Trends in Analytical Chemistry, 2019, 118: 517-537. doi: 10.1016/j.trac.2019.06.012 [20] 严清. 典型PhACs在城市水系统中的迁移分布规律及其在人工湿地中的去除研究[D]. 重庆: 重庆大学, 2014. [21] LIU X, LIU Y, XU J R, et al. Tracking aquaculture-derived fluoroquinolones in a mangrove wetland, South China[J]. Environmental Pollution, 2016, 219: 916-923. doi: 10.1016/j.envpol.2016.09.011 [22] MATUSZEWSKI B K, CONSTANZER M L, CHAVEZ-ENG C M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS[J]. Analytical Chemistry, 2003, 75(13): 3019-3030. doi: 10.1021/ac020361s [23] LIU L, LIU Y H, LIU C X, et al. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions[J]. Ecological Engineering, 2013, 53: 138-143. doi: 10.1016/j.ecoleng.2012.12.033 [24] YAN Q, XU Y, CHEN L, et al. Irrigation with secondary municipal-treated wastewater: Potential effects, accumulation of typical antibiotics and grain quality responses in rice (Oryza sativa L.)[J]. Journal of Hazardous Materials, 2020: 124655. [25] PAN M, WONG C K, CHU L M. Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the Pearl River Delta, southern China[J]. Journal of Agricultural & Food Chemistry, 2014, 62(46): 11062-9. [26] SUN Y M, GUO Y J, SHI M M, et al. Effect of antibiotic type and vegetable species on antibiotic accumulation in soil-vegetable system, soil microbiota, and resistance genes[J]. Chemosphere, 2020, 263: 128099. [27] RIAZ L, MAHMOOD T, KHALID A, et al. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil[J]. Chemosphere, 2018, 191: 704-720. doi: 10.1016/j.chemosphere.2017.10.092 [28] VIENO N, TUHKANEN T, KRONBERG L. Elimination of pharmaceuticals in sewage treatment plants in Finland[J]. Water Research, 2007, 41(5): 1001-1012. doi: 10.1016/j.watres.2006.12.017 [29] PICO Y, ANDREU V. Fluoroquinolones in soil-risks and challenges[J]. Analytical & Bioanalytical Chemistry, 2007, 387(4): 1287-1299. [30] 陈磊, 吴赟琦, 赵志勇, 等. QuEChERS/超高效液相色谱-串联质谱法快速测定土壤中19种氟喹诺酮类抗生素残留[J]. 分析测试学报, 2019, 38(2): 194-200. doi: 10.3969/j.issn.1004-4957.2019.02.011 [31] GU J, CHEN C, HUANG X, et al. Occurrence and risk assessment of tetracycline antibiotics in soils and vegetables from vegetable fields in Pearl River Delta, South China[J]. Science of the Total Environment, 2021, 776: 145959. doi: 10.1016/j.scitotenv.2021.145959 -



 下载:
下载: