-
近年来,随着印染工业的迅猛发展,工业废水的排放量也在逐年增加,其中染料废水约占工业废水总排放量的10%[1]。染料废水具有色度高,成分复杂,化学性质比较稳定,难生物降解等特点[2]。染料废水中含有的大部分有机物能够致癌、致畸和致突变,对人类身体健康和生态系统造成极大的破坏[3]。酸性大红3R(AR3R)是一种常用的偶氮酸性染料,含有对生物呈强抑制作用的苯环,并且为高共轭分子体系,无法采用一般方法将其降解成无机小分子。少量的AR3R物质,即可造成大范围的水污染,达不到废水排放的无色要求。AR3R的降解处理,对于其他含有相似结构的有机物也具有推广意义[4]。
目前,染料的去除方法主要包括生物处理法、化学处理法、电化学絮凝法、膜过滤法、氧化法和吸附法[5]。其中,因吸附法具有操作简单、可供选择的吸附材料丰富和见效快等优点,故已得到广泛应用[6]。碳质材料是一种传统的吸附剂材料,其中,活性炭是应用最广泛的吸附剂。但这些碳质材料大多数具有微孔的结构,从而限制了其在高分子染料去除上的应用[7]。
金属有机骨架(metal-organic framework,MOFs)是由金属离子和有机骨架通过配位键构筑的一类配位聚合物。MOFs具有较高的孔径、较大的比表面积、可调谐的活性位点和可功能化的有机骨架等独特的理化性质[8]。MOFs最大的优点就是具有较简单的合成步骤,可以通过设计调谐不同的金属离子和有机骨架来合成具有不同理化性质的MOFs材料[9]。但传统的MOFs材料由于在水介质中不稳定从而限制了它在水体治理与修复中的应用[5]。本研究以Sn4+为金属中心、对苯二甲酸为有机配体,利用水热法合成出了一种水稳定性较高的金属有机骨架(Sn-MOF),并用XRD、FT-IR、TEM、BET等手段对Sn-MOF进行了表征。以AR3R为目标污染物,系统地研究了AR3R在Sn-MOF表面上的吸附特性及相关的影响因素,为实现MOFs材料在染料废水处理中的应用进行了初步的探索。
-
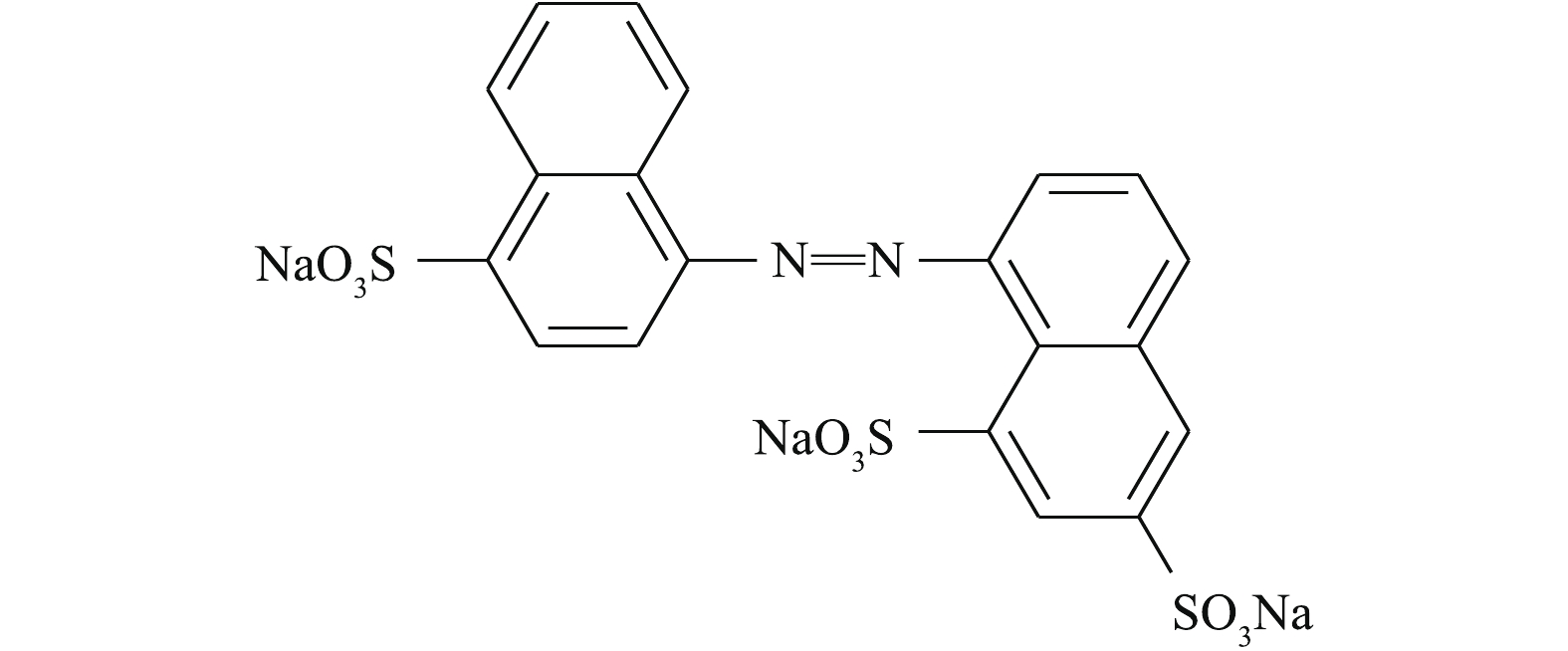
氯化亚锡(SnCl2·3H2O),N,N-二甲基甲酰胺(HCON(CH3)2),无水乙醇(CH3CH2OH),氢氧化钠(NaOH),对苯二甲酸(C8H6O4),酸性大红3R(AR3R)均为分析纯。AR3R的结构图如图1所示。
-
透射电子显微镜(JEM-2100,日本JEOL公司);X射线衍射仪(D/max-2500型,日本Rigaku);傅里叶红外吸收光谱仪(FT-IR650,北京国谱科技有限公司);比表面积及孔隙度自动分析仪(ASIQM000100-6型,美国Quantachrome公司);紫外-可见光分光光度计(UV-vis DRS EVOLUTION 220,瑞轩电子科技有限公司);质谱仪(Agilent7500ce,安捷伦科技公司)。
-
1.163 g的SnCl2·3H2O和0.428 g的对苯二甲酸分别溶入48 mL的N,N-二甲基甲酰胺和12 mL的无水乙醇的混合液中,得到无色透明溶液,将其转移到100 mL带有聚四氟乙烯内衬的不锈钢水热合成釜中,170 ℃反应8 h后,自然冷却到室温。混合物过滤得到白色的固体,用去离子水和无水乙醇交替离心洗涤3次,后在70 ℃下烘干,研磨得到粉末状的Sn-MOF材料。
-
分别称取一定质量经预处理过的Sn-MOF(质量为0.05~1.35 g),投加至100 mL分别含有不同质量浓度的AR3R溶液中(浓度为150~500 mg·L−1),并用0.1 mol·L−1的NaOH或HCl溶液调节溶液的pH至预定值(pH为3~11),保持一定温度(分别为323.15、333.15和343.15 K),置于摇床上150 r·min−1振荡至吸附-解吸平衡。每隔0.5 h取样一次,每次取2 mL过滤,测定溶液中AR3R随时间的变化规律。计算Sn-MOF对AR3R 的平衡吸附量和去除率。
-
图2(a)为Sn-MOF的扫描电镜图,可以看出,Sn-MOF表面呈现出蓬松的结构。图2(b)为Sn-MOF的透射电镜图,可以看出,Sn-MOF具有明显的多孔网状结构。利用N2吸附-脱附实验对Sn-MOF的比表面积和孔径分布情况进行表征,其结果如图2(c)所示。该N2吸附-脱附曲线呈典型的Ⅲ型曲线并且滞后环为H3型,这表明Sn-MOF具有高度的中孔结构,比表面积为48.679 m2·g−1、平均孔径为3.825 nm、孔容为0.808 cm3·g−1。
本研究还将吸附AR3R前后的Sn-MOF进行了红外图谱分析,得到的红外图谱如图2(d)所示。从吸附前的红外图谱可以看出,在3 401.7 cm−1处出现了H2O的H—O—H伸缩振动特征峰;在1 618.3 cm−1和1 342.8 cm−1处出现了COO—的对称伸缩振动特征峰和不对称伸缩特征峰;在1 037.5 cm−1处出现的是C—O—C的伸缩振动特征峰。在545.7 cm−1处出现的是Sn—O的伸缩振动特征峰[10]。对比Sn-MOF吸附AR3R前的FT-IR谱图,可以看出,Sn-MOF吸附AR3R后COO—的对称伸缩振动特征峰有所减弱,并且C—O—C的伸缩振动特征峰变强,这表明AR3R在Sn-MOF上的作用位点集中在COO—上。
-
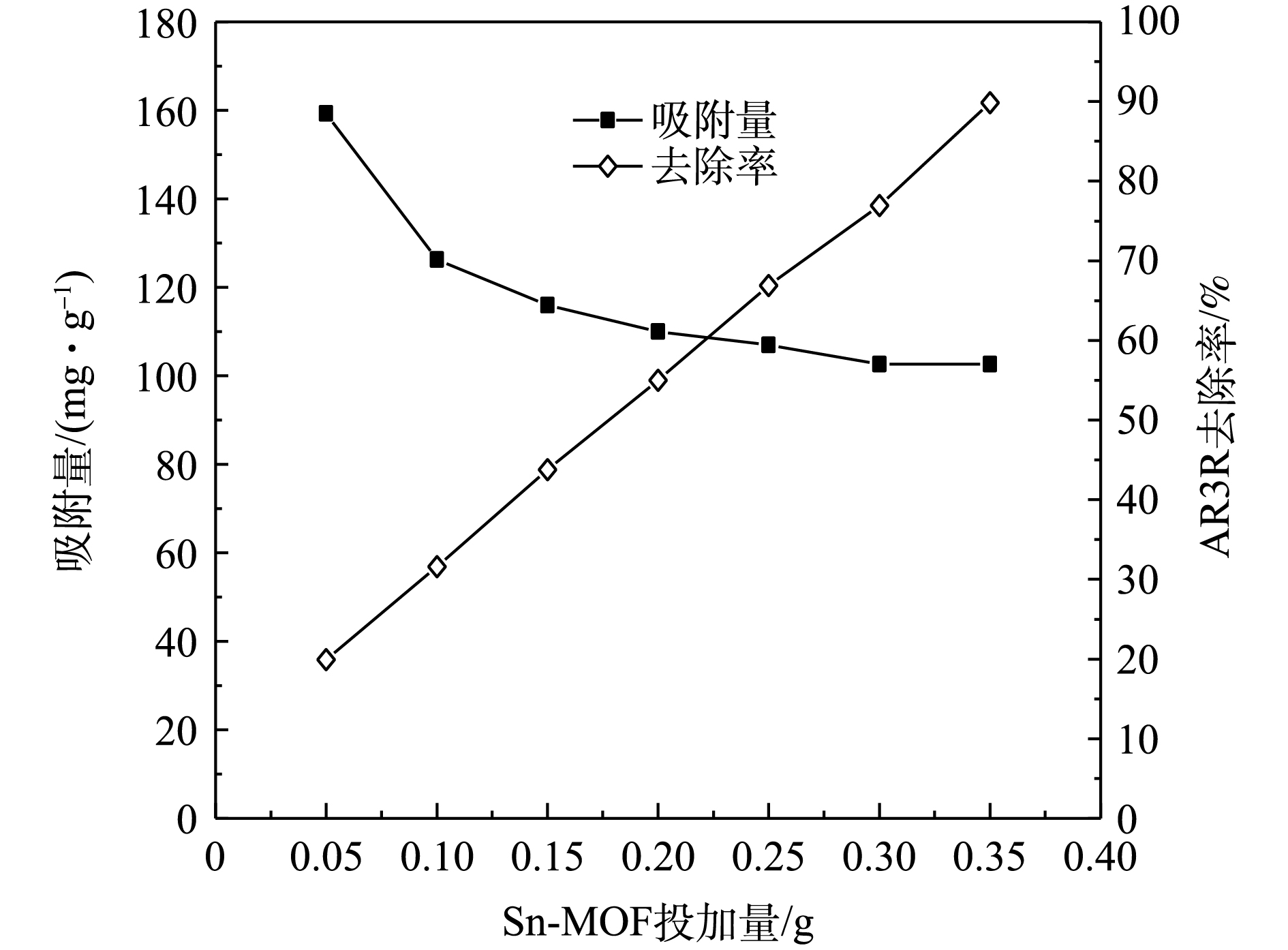
图3为不同Sn-MOF投加量对吸附性能的影响,投加量为0.05~0.35 g,可以看出,随着Sn-MOF投加量逐渐增多,单位吸附量逐渐下降。Sn-MOF从0.05 g增加到0.35 g时,单位吸附量从159.13 mg·g−1下降到102.60 mg·g−1。这主要是因为:随着Sn-MOF投加量的增多,吸附剂表面处于不饱和的活性位点也在逐渐增多,使得吸附活性位点不能够被充分利用,导致单位吸附量逐渐减小[11]。对于去除率而言,随着Sn-MOF投加量的增加,去除率也随之逐渐增加。从吸附量和去除率2个方面综合考虑,选择0.30 g为Sn-MOF的最佳投加量,此时,吸附量达到102.60 mg·g−1,去除率达到了76.95%。
-
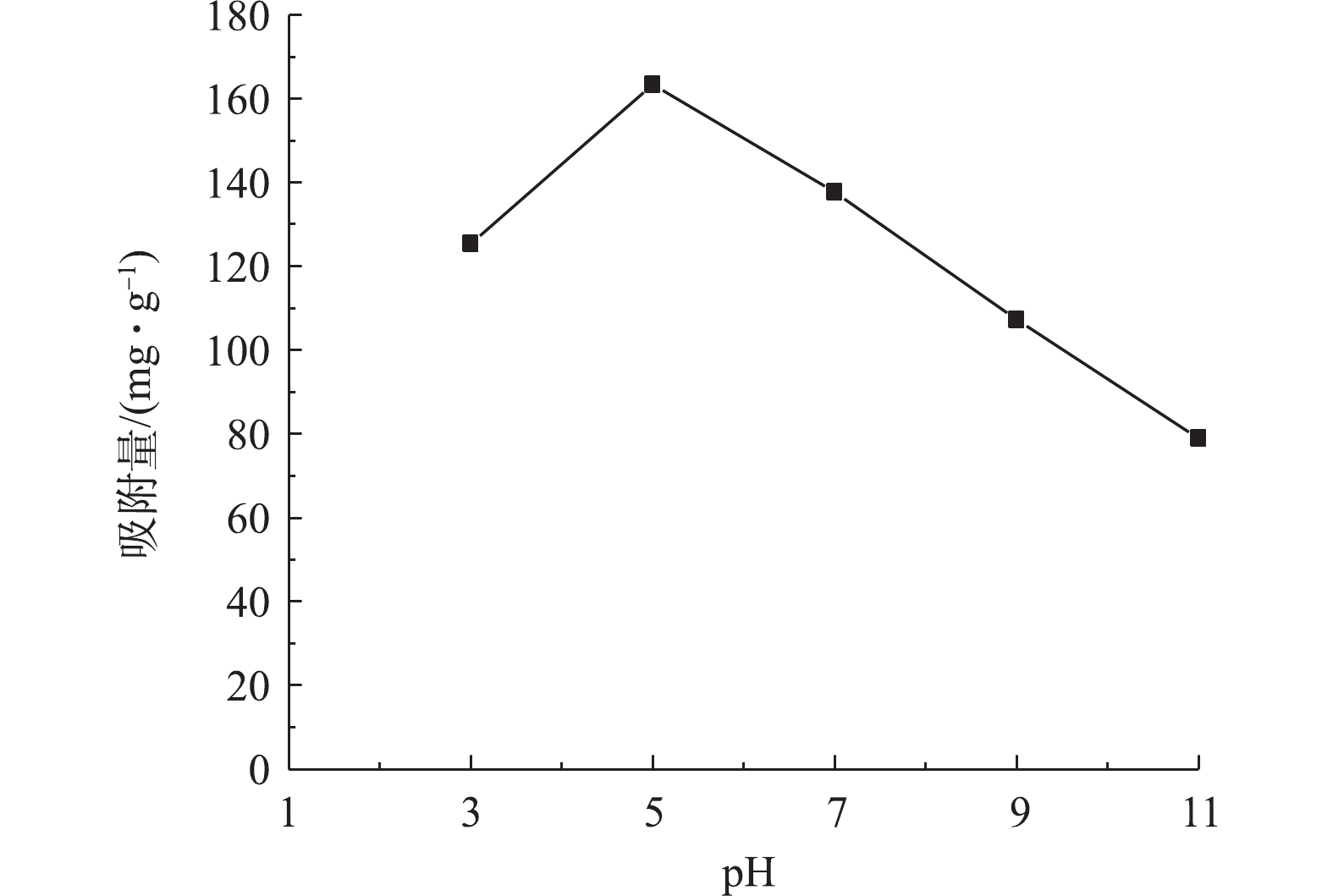
污染物的pH能够影响吸附剂的表面活性位点和水溶液的化学性质,从而影响其吸附过程。图4为pH对Sn-MOF吸附AR3R的影响结果。由图4可知,当pH=3~5时,AR3R吸附量随着pH的升高而增加;当pH=5~11时,AR3R吸附量随着pH的升高而减小;在pH=5时吸附效果最好,AR3R吸附量可达到163.40 mg·g−1。对于MOFs材料来说,pH较小时,粒子表面带正电;pH较大时,粒子表面带负电。当Sn-MOF表面带正电时,溶液中的OH−、Cl−和AR3R负离子靠库仑引力紧密吸附在粒子表面形成双电层。当Sn-MOF表面带负电时,溶液中的Na+、H+和AR3R正离子靠库仑引力紧密吸附在粒子表面形成双电层。双电层产生的ζ电位越大,斥力越大,越不利于AR3R的吸附(4]。当pH=5,颗粒的电位等于0(即等电点)时,粒子之间的库仑斥力将完全消失,此时吸附量达到最大值。同时当pH较大时,溶液中的OH−含量增加会与AR3R阴离子染料竞争Sn-MOF表面的吸附活性位点,OH−浓度越大,竞争则越激烈,导致染料分子的吸附容量越低[12]。
-
为了研究AR3R在Sn-MOF上的吸附机理,分别使用4种动力学方程[13]对所得到的实验数据进行了拟合。动力学方程如式(1)~式(4)所示,拟合结果见图5,拟合得到的各动力学参数如表1和表2所示。
拟一级动力学方程见式(1),拟二级动力学方程见式(2),颗粒内扩散方程见式(3),Elovich方程见式(4)。
式中:qt为时间t时的吸附量,mg·g−1;qe为吸附平衡时的吸附量,mg·g−1;k1为拟一级动力学反应速率常数,h−1;k2为拟二级动力学反应速率常数,g·(mg·h)−1,Kd为颗粒内扩散常数,g·(mg·h1/2)−1;Ci为与边界层的厚度相关的常数,mg·g−1。A和B分别为动力学方程的常数。
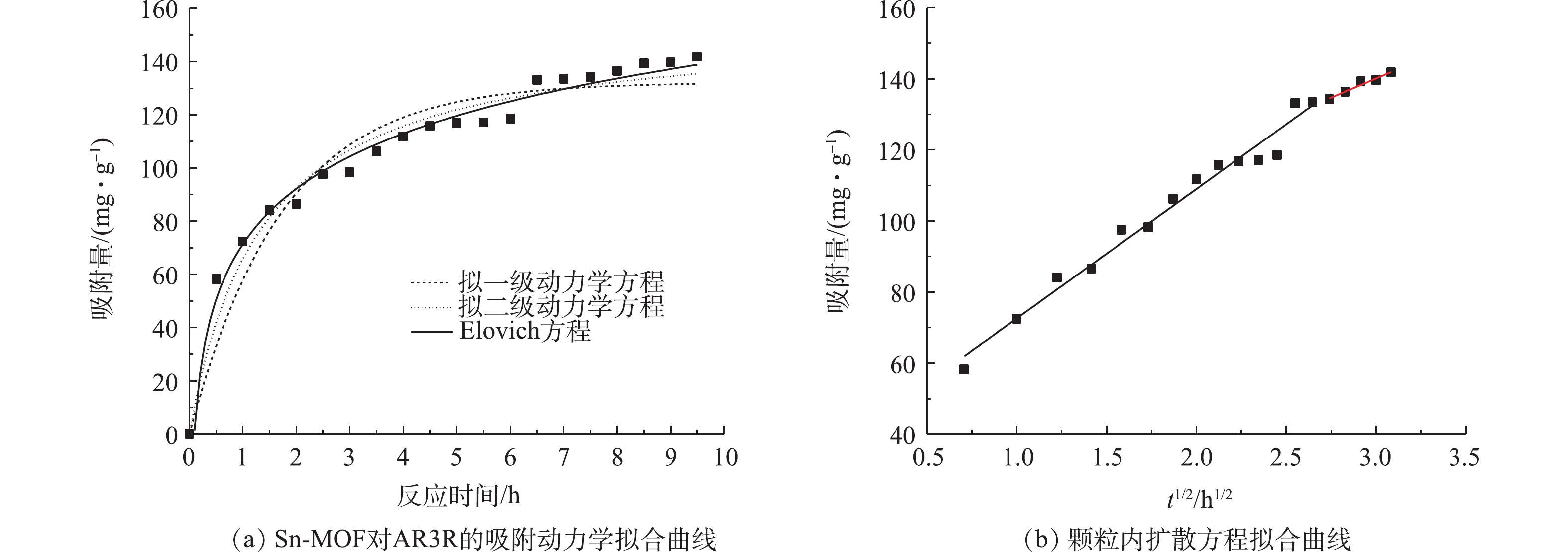
由图5(a)可知,Sn-MOF对AR3R的吸附量随着时间的延长而逐渐增大,在4 h左右,吸附基本达到平衡。如表1所示,与拟一级动力学方程(R2=0.918 9)相比,拟二级动力学方程(R2=0.963 5)和Elovich方程(R2=0.985 8)的可决系数更高,并且由计算得到的吸附量值与实测值比较接近。这表明Sn-MOF对AR3R的吸附属于化学吸附,并且吸附过程可能是通过表面交换反应进行的,直到表面活性位点完全被占据;同时,AR3R分子扩散到Sn-MOF的孔中与吸附剂进行相互作用[14]。
颗粒内扩散方程可以用来描述AR3R的吸附过程,根据颗粒内扩散方程所作的Sn-MOF吸附AR3R的qt对t1/2的关系曲线如图5(b)所示,动力学参数如表2所示。在整个吸附时间内,图形并不是一条直线,而是分为2部分的曲线,表明吸附机理遵循3个不同的阶段。第1阶段,AR3R向Sn-MOF外表面扩散,这一过程称为边界层扩散,第1阶段的高Kd值表明,吸附速率较高。第2阶段,AR3R分子逐渐占据Sn-MOF的外表面,驱动力不再维持外部传质,由于较低的Kd值,颗粒内扩散是限速步骤。第3阶段,吸附剂中几乎所有孔都被AR3R分子占据,外部质量传递急剧减少,并且实际上没有膜或颗粒内扩散。值得注意的是,第2阶段的线条远离原点,这表明吸附过程的限速步骤不受单颗粒内扩散的控制[15]。计算颗粒内扩散模型的值如表2所示,较高的Ci值表明较大的边界层效应,这表明表面吸附也限制了速率步骤[16]。
-
吸附等温线是描述体系中吸附剂表面和溶液中吸附质数量关系的曲线。在室温条件下,将0.30 g的Sn-MOF加入到pH均为5,浓度分别为100、150、200、250、300、350、400、450和500 mg·L−1的染料溶液中,振荡12 h,使其达到吸附平衡,分别利用Langmuir等温方程(式(5))和Freundlich等温方程(式(6))对Sn-MOF吸附AR3R的吸附等温线数据进行拟合,拟合曲线如图6所示,拟合参数如表3所示。Langmuir等温方程和Freundlich等温方程分别见式(5)和式(6)。
式中:ce为吸附达平衡时液相中染料的浓度,mg·L−1;qm为饱和吸附量,mg·g−1;KL是Langmuir常数,L·mg−1;KF是和吸附剂吸附量有关的Freundlich常数,mg·g−1;n则是与吸附强度有关的Freundlich指数。
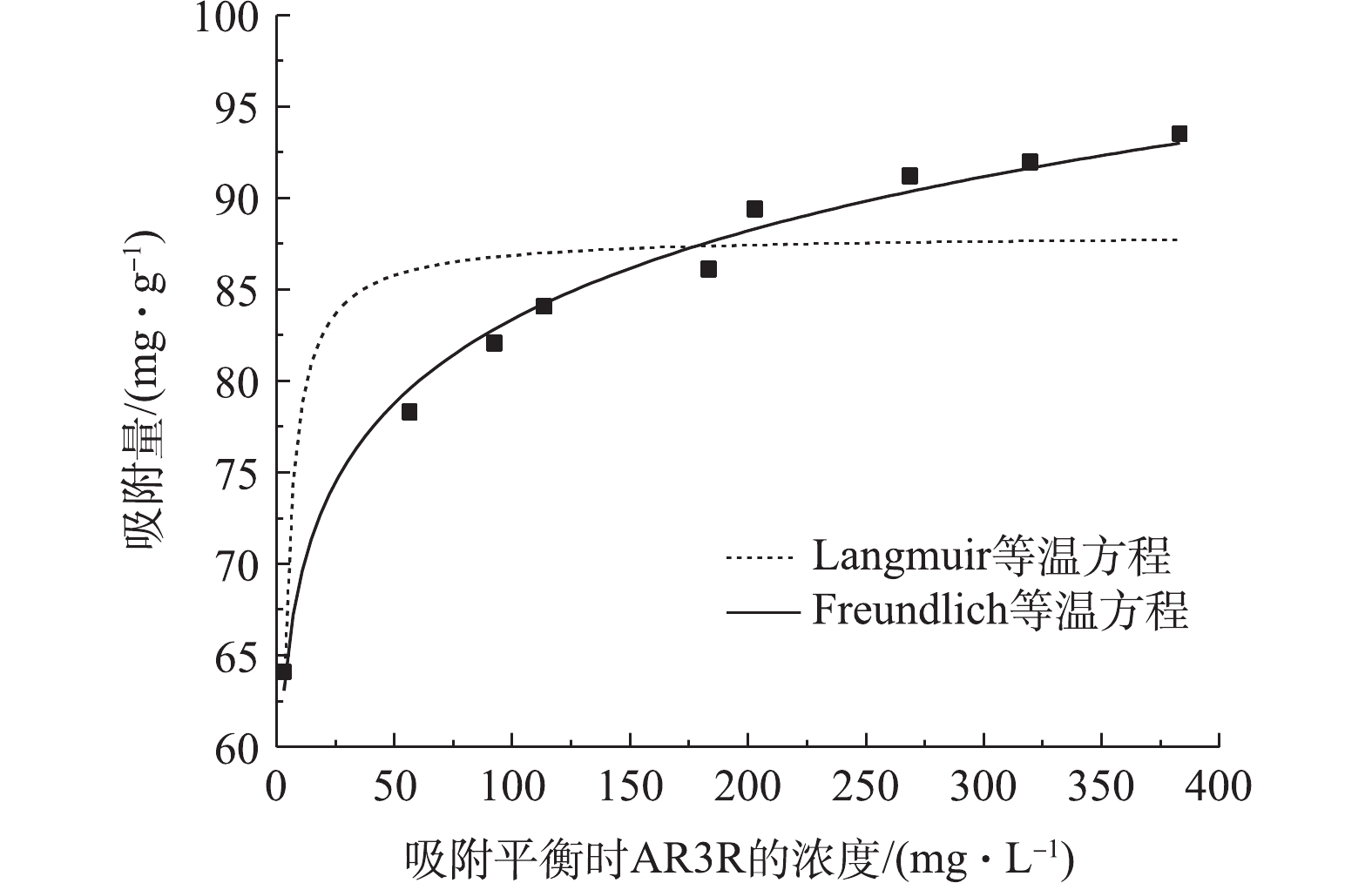
由图6可以看出,Sn-MOF对AR3R的吸附量随着平衡时溶液中AR3R浓度的升高而升高。Freundlich等温方程(R2=0.986 8)对AR3R吸附的拟合要好于Langmuir等温方程(R2=0.721 8),同时n<1表明该吸附为有利的化学吸附[17]。
-
为了确定Sn-MOF对AR3R吸附过程的本质,必须确定反应的活化能(Ea)、焓变(ΔH0)与熵变(ΔS0)及吉布斯自由能变化(ΔG0)。
-
1)活化能的确定。考察了在AR3R的初始浓度均为400 mg·L−1,Sn-MOF添加量为0.30 g的条件下,温度对吸附动力学的影响,结果如图7所示。由图7(a)可知,随着温度的升高吸附量逐渐增加。动力学参数列于表4中,反应速率常数随着温度的升高而增大。
反应速率常数k可用阿伦尼乌斯公式描述,由斜率(Ea/R)可计算出活化能。阿伦尼乌斯公式见式(7)。
式中:k为反应速率常数;A为指前因子;Ea为活化能,kJ·mol−1;R为摩尔气体常数,8.314 5 J·(mol·K)−1;T为热力学温度,K。在扩散控制的过程中,Ea低于20 kJ·mol−1[18]。
根据图7(b)可以计算出活化能Ea的值为51.46 kJ·mol−1。活化能的数量级可以表明吸附的类型,吸附通常包括物理吸附和化学吸附。物理吸附的活化能一般不超过4.20 kJ·mol−1,化学吸附包括活化与非活化2种形式。在活化的化学吸附过程中,活化能为8.40~83.70 kJ·mol−1;在非活化的化学吸附中,活化能为0 kJ·mol−1[19]。以上实验结果表明,Sn-MOF对AR3R的吸附属于活化的化学吸附且吸附过程属于吸热反应。
-
2)吉布斯自由能变化与熵变及焓变的确定。温度的吉布斯自由能变化(ΔG0)、熵变(ΔS0)和焓变(ΔH0)可以通过Eyring方程[20]计算。
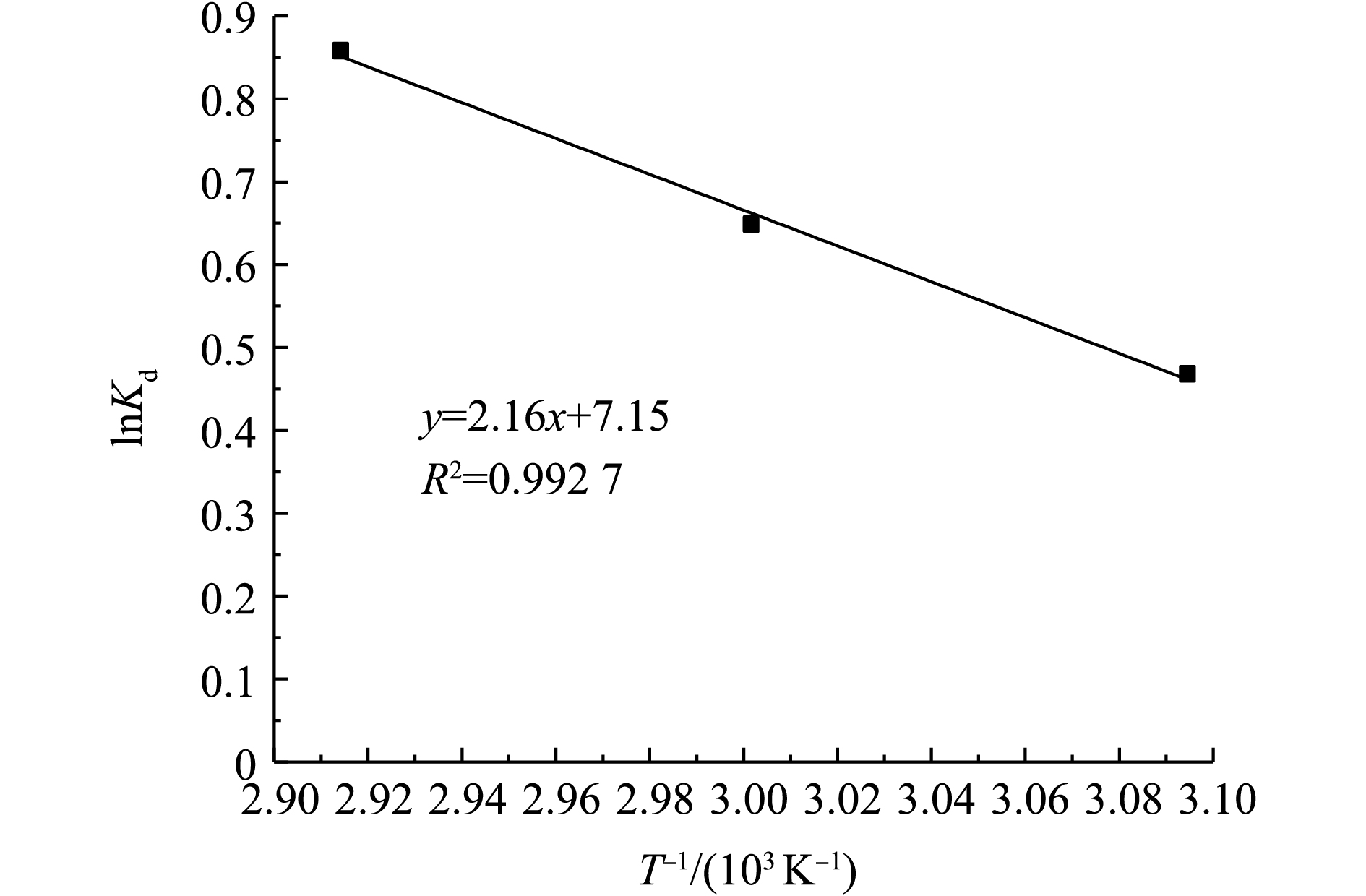
式中:Kd=qe/ce;R为摩尔气体常数;T为热力学温度。以lnKd对T−1作图,可以得到线性回归直线,如图8所示。
根据回归直线的斜率和截距可求得ΔH0和ΔS0,结果如表5所示。ΔH0>0说明Sn-MOF对AR3R的吸附过程是吸热反应,Sn-MOF吸附吉布斯自由能变化(ΔG0)随着温度的增加而减小,ΔG0为负值,说明吸附反应是自发进行的。
-
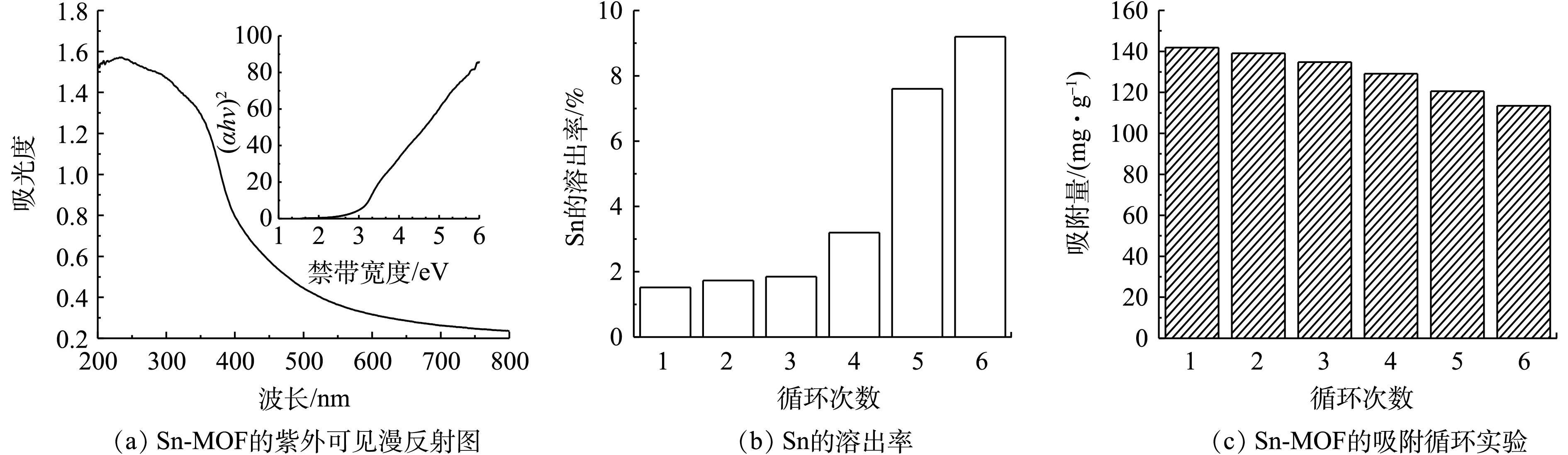
再生性和可重复使用性是吸附剂工业应用的关键问题。通过漫反射光谱(DRS)表征技术对合成的Sn-MOF的吸光性能进行检测。Sn-MOF的吸收波长为447.65 nm(可见光吸收)。使用Taucs公式估算Sn-MOF的禁带宽度,如式(10)所示。
式中:α是吸收系数;hv是光子能量;A为常数;n表示电子跃迁的类型,可以在0.5~3之间取合适的值;Eg为半导体禁带宽度,eV。
图9为Sn-MOF的再生对其吸附性能的影响。根据图9(a)的插图,计算出的制备的Sn-MOF的Eg值为2.77 eV,这表明Sn-MOF具有较长的吸收波范围,且禁带宽度较窄对光的吸收利用率较高,在可见光下具有较好的光催化活性[20]。由于合成的材料具有较好的可见光响应,我们使用光催化反应来处理吸附后的吸附剂。首先,将用过的吸附剂放入50 mL石英玻璃试管中,加入30 mL去离子水。然后用Xe灯照射悬浮液,Xe灯起到光源的作用,并搅拌120 min,以确保吸附物完全降解,之后将吸附剂干燥,用于下一次使用。图9(b)为每次使用后Sn的溶出率,可以看出,当使用6次后,Sn的溶出率仅为9.20%,这表明合成的Sn-MOF具有较好的水稳定性。如图9(c)所示,再生吸附剂在6轮循环后仍保持良好的吸附能力,每个循环的吸附量下降仅为2.01%~6.32%,这可能是在吸附过程的中Sn-MOF丧失了部分的吸附位点所引起的。尽管如此,经过6个连续循环后,颗粒的吸附量仍保持在较高值,为113.50 mg·g−1,这表明Sn-MOF具有较好的可循环利用性。
-
1) Sn-MOF对AR3R模拟染料废水具有较好的脱色效果。在室温下,Sn-MOF最佳投加量为0.30 g时,其对浓度为400 mg·L−1的AR3R去除率可以达到76.95%,吸附量可以达到102.60 mg·g−1;当pH为5时,吸附效果达到最佳,pH过低或过高均不利于吸附。
2)吸附过程符合拟二级动力学方程和Elovich方程,这表明Sn-MOF对AR3R的吸附是由化学吸附进行控制的,并且该吸附反应可能是通过表面交换反应进行的,直到表面活性位点被完全占据。同时,AR3R分子扩散到Sn-MOF的孔中,与吸附剂相互作用。粒子扩散方程表明颗粒内扩散不是控制Sn-MOF吸附过程的唯一步骤,而是由膜扩散和颗粒内扩散联合控制的。
3)吸附规律较好地符合Freundlich吸附等温方程,这表明Sn-MOF吸附是一种有利的化学吸附。热力学实验结果表明,AR3R的去除过程是一种自发进行的吸热过程。
4)再生实验结果表明,Sn-MOF具有较好的水稳定性和重复利用性,经光催化再生处理6个循环后,吸附容量没有明显降低。
Sn-MOF对染料废水中酸性大红3R的吸附特性
Adsorption characteristics of acid red 3R in dyes wastewater by Sn-MOF
-
摘要: 通过水热法合成了一种新型的金属有机骨架Sn-MOF,研究了Sn-MOF对染料废水中酸性大红3R(AR3R)的吸附特性。通过SEM、TEM、比表面积测定和红外光谱等方法对Sn-MOF进行了表征,并探讨了初始pH、吸附剂投加量对吸附效果的影响。用拟一级动力学方程、拟二级动力学方程、Elovich方程、粒子扩散方程对吸附曲线进行了分析,研究了其吸附的动力学机理。在研究的条件范围内,拟二级动力学方程和Elovich方程的拟合度较好。通过吸附等温线拟合发现Freundlich方程(R2=0.986 8)能很好地描述Sn-MOF对AR3R的吸附行为,这说明Sn-MOF对AR3R的吸附是化学吸附。粒子扩散方程表明,粒子内扩散不是控制Sn-MOF吸附过程的唯一步骤,膜扩散也参与了吸附过程。热力学实验结果表明,AR3R的去除过程是一种自发的吸热过程。经光催化再生处理6个循环后,吸附容量没有明显降低。
-
关键词:
- Sn-MOF /
- 酸性大红3R(AR3R) /
- 吸附动力学 /
- 吸附热力学
Abstract: In this study, a novel metal-organic framework of Sn-MOF was synthesized by hydrothermal method, and its adsorption characteristics of acid red 3R (AR3R) in dyes wastewater was tested. The Sn-MOF was characterized by SEM, TEM, BET and FT-IR. The effects of initial pH and Sn-MOF dosage on the adsorption performance were investigated. The adsorption kinetic mechanism was studied by using the first-order kinetic equation, the quasi-second-order kinetic equation, the Elovich equation and the particle diffusion equation. The results showed that the adsorption kinetics could be better fitted by the quasi-second-order kinetic equation and the Elovich equation, which indicated that the adsorption of AR3R by Sn-MOF was chemisorption. The particle diffusion equation showed that the intraparticle diffusion was not the only step to control the adsorption process, and the membrane diffusion also took part in the adsorption process. Through adsorption isotherm fitting, it could be found that Freundlich equation (R2=0.986 8) could well describe the adsorption behavior of AR3R on Sn-MOF, which further indicated that the adsorption of AR3R by Sn-MOF is chemisorption. Thermodynamic experiments showed that the removal process of AR3R was a spontaneous endothermic process. The adsorption capacity of photocatalytic regenerated Sn-MOF did not decrease obviously after six cycles.-
Key words:
- Sn-MOF /
- acid red 3R(AR3R) /
- adsorption kinetics /
- adsorption thermodynamics
-
近年来,随着印染工业的迅猛发展,工业废水的排放量也在逐年增加,其中染料废水约占工业废水总排放量的10%[1]。染料废水具有色度高,成分复杂,化学性质比较稳定,难生物降解等特点[2]。染料废水中含有的大部分有机物能够致癌、致畸和致突变,对人类身体健康和生态系统造成极大的破坏[3]。酸性大红3R(AR3R)是一种常用的偶氮酸性染料,含有对生物呈强抑制作用的苯环,并且为高共轭分子体系,无法采用一般方法将其降解成无机小分子。少量的AR3R物质,即可造成大范围的水污染,达不到废水排放的无色要求。AR3R的降解处理,对于其他含有相似结构的有机物也具有推广意义[4]。
目前,染料的去除方法主要包括生物处理法、化学处理法、电化学絮凝法、膜过滤法、氧化法和吸附法[5]。其中,因吸附法具有操作简单、可供选择的吸附材料丰富和见效快等优点,故已得到广泛应用[6]。碳质材料是一种传统的吸附剂材料,其中,活性炭是应用最广泛的吸附剂。但这些碳质材料大多数具有微孔的结构,从而限制了其在高分子染料去除上的应用[7]。
金属有机骨架(metal-organic framework,MOFs)是由金属离子和有机骨架通过配位键构筑的一类配位聚合物。MOFs具有较高的孔径、较大的比表面积、可调谐的活性位点和可功能化的有机骨架等独特的理化性质[8]。MOFs最大的优点就是具有较简单的合成步骤,可以通过设计调谐不同的金属离子和有机骨架来合成具有不同理化性质的MOFs材料[9]。但传统的MOFs材料由于在水介质中不稳定从而限制了它在水体治理与修复中的应用[5]。本研究以Sn4+为金属中心、对苯二甲酸为有机配体,利用水热法合成出了一种水稳定性较高的金属有机骨架(Sn-MOF),并用XRD、FT-IR、TEM、BET等手段对Sn-MOF进行了表征。以AR3R为目标污染物,系统地研究了AR3R在Sn-MOF表面上的吸附特性及相关的影响因素,为实现MOFs材料在染料废水处理中的应用进行了初步的探索。
1. 材料与方法
1.1 实验药品
氯化亚锡(SnCl2·3H2O),N,N-二甲基甲酰胺(HCON(CH3)2),无水乙醇(CH3CH2OH),氢氧化钠(NaOH),对苯二甲酸(C8H6O4),酸性大红3R(AR3R)均为分析纯。AR3R的结构图如图1所示。
1.2 实验仪器
透射电子显微镜(JEM-2100,日本JEOL公司);X射线衍射仪(D/max-2500型,日本Rigaku);傅里叶红外吸收光谱仪(FT-IR650,北京国谱科技有限公司);比表面积及孔隙度自动分析仪(ASIQM000100-6型,美国Quantachrome公司);紫外-可见光分光光度计(UV-vis DRS EVOLUTION 220,瑞轩电子科技有限公司);质谱仪(Agilent7500ce,安捷伦科技公司)。
1.3 Sn-MOF的制备
1.163 g的SnCl2·3H2O和0.428 g的对苯二甲酸分别溶入48 mL的N,N-二甲基甲酰胺和12 mL的无水乙醇的混合液中,得到无色透明溶液,将其转移到100 mL带有聚四氟乙烯内衬的不锈钢水热合成釜中,170 ℃反应8 h后,自然冷却到室温。混合物过滤得到白色的固体,用去离子水和无水乙醇交替离心洗涤3次,后在70 ℃下烘干,研磨得到粉末状的Sn-MOF材料。
1.4 实验及分析方法
分别称取一定质量经预处理过的Sn-MOF(质量为0.05~1.35 g),投加至100 mL分别含有不同质量浓度的AR3R溶液中(浓度为150~500 mg·L−1),并用0.1 mol·L−1的NaOH或HCl溶液调节溶液的pH至预定值(pH为3~11),保持一定温度(分别为323.15、333.15和343.15 K),置于摇床上150 r·min−1振荡至吸附-解吸平衡。每隔0.5 h取样一次,每次取2 mL过滤,测定溶液中AR3R随时间的变化规律。计算Sn-MOF对AR3R 的平衡吸附量和去除率。
2. 结果与讨论
2.1 Sn-MOF表征
图2(a)为Sn-MOF的扫描电镜图,可以看出,Sn-MOF表面呈现出蓬松的结构。图2(b)为Sn-MOF的透射电镜图,可以看出,Sn-MOF具有明显的多孔网状结构。利用N2吸附-脱附实验对Sn-MOF的比表面积和孔径分布情况进行表征,其结果如图2(c)所示。该N2吸附-脱附曲线呈典型的Ⅲ型曲线并且滞后环为H3型,这表明Sn-MOF具有高度的中孔结构,比表面积为48.679 m2·g−1、平均孔径为3.825 nm、孔容为0.808 cm3·g−1。
本研究还将吸附AR3R前后的Sn-MOF进行了红外图谱分析,得到的红外图谱如图2(d)所示。从吸附前的红外图谱可以看出,在3 401.7 cm−1处出现了H2O的H—O—H伸缩振动特征峰;在1 618.3 cm−1和1 342.8 cm−1处出现了COO—的对称伸缩振动特征峰和不对称伸缩特征峰;在1 037.5 cm−1处出现的是C—O—C的伸缩振动特征峰。在545.7 cm−1处出现的是Sn—O的伸缩振动特征峰[10]。对比Sn-MOF吸附AR3R前的FT-IR谱图,可以看出,Sn-MOF吸附AR3R后COO—的对称伸缩振动特征峰有所减弱,并且C—O—C的伸缩振动特征峰变强,这表明AR3R在Sn-MOF上的作用位点集中在COO—上。
2.2 Sn-MOF投加量对吸附AR3R的影响
图3为不同Sn-MOF投加量对吸附性能的影响,投加量为0.05~0.35 g,可以看出,随着Sn-MOF投加量逐渐增多,单位吸附量逐渐下降。Sn-MOF从0.05 g增加到0.35 g时,单位吸附量从159.13 mg·g−1下降到102.60 mg·g−1。这主要是因为:随着Sn-MOF投加量的增多,吸附剂表面处于不饱和的活性位点也在逐渐增多,使得吸附活性位点不能够被充分利用,导致单位吸附量逐渐减小[11]。对于去除率而言,随着Sn-MOF投加量的增加,去除率也随之逐渐增加。从吸附量和去除率2个方面综合考虑,选择0.30 g为Sn-MOF的最佳投加量,此时,吸附量达到102.60 mg·g−1,去除率达到了76.95%。
2.3 pH对Sn-MOF吸附AR3R的影响
污染物的pH能够影响吸附剂的表面活性位点和水溶液的化学性质,从而影响其吸附过程。图4为pH对Sn-MOF吸附AR3R的影响结果。由图4可知,当pH=3~5时,AR3R吸附量随着pH的升高而增加;当pH=5~11时,AR3R吸附量随着pH的升高而减小;在pH=5时吸附效果最好,AR3R吸附量可达到163.40 mg·g−1。对于MOFs材料来说,pH较小时,粒子表面带正电;pH较大时,粒子表面带负电。当Sn-MOF表面带正电时,溶液中的OH−、Cl−和AR3R负离子靠库仑引力紧密吸附在粒子表面形成双电层。当Sn-MOF表面带负电时,溶液中的Na+、H+和AR3R正离子靠库仑引力紧密吸附在粒子表面形成双电层。双电层产生的ζ电位越大,斥力越大,越不利于AR3R的吸附(4]。当pH=5,颗粒的电位等于0(即等电点)时,粒子之间的库仑斥力将完全消失,此时吸附量达到最大值。同时当pH较大时,溶液中的OH−含量增加会与AR3R阴离子染料竞争Sn-MOF表面的吸附活性位点,OH−浓度越大,竞争则越激烈,导致染料分子的吸附容量越低[12]。
2.4 吸附动力学
为了研究AR3R在Sn-MOF上的吸附机理,分别使用4种动力学方程[13]对所得到的实验数据进行了拟合。动力学方程如式(1)~式(4)所示,拟合结果见图5,拟合得到的各动力学参数如表1和表2所示。
表 1 Sn-MOF对AR3R的吸附动力学参数Table 1. Adsorption kinetic parameters of AR3R by Sn-MOFC0/(mg·L−1) 拟一级动力学方程 拟二级动力学方程 Elovich方程 qe/(mg·g−1) k1/h−1 R2 qe/(mg·g−1) k2/(g·(mg·h)−1) R2 A B R2 400 132.212 0.576 9 0.918 9 154.675 0.004 8 0.963 5 71.459 1 29.943 1 0.985 8 表 2 Sn-MOF对AR3R的颗粒内扩散动力学参数Table 2. Intraparticle diffusion kinetic parameters of AR3R by Sn-MOF第1阶段 第2阶段 K1d/(g·(mg·h1/2)−1) C1/(mg·g−1) R2 K2d/(g·(mg·h1/2)−1) C2/(mg·g−1) R2 75.272 1 21.643 2 0.952 2 36.193 1 36.442 8 0.975 9 拟一级动力学方程见式(1),拟二级动力学方程见式(2),颗粒内扩散方程见式(3),Elovich方程见式(4)。
qt=qe(1−e−k1t) (1) qt=q2ek2t1+qek2t (2) qt=Kdt1/2+Ci (3) qt=A+Blnt (4) 式中:qt为时间t时的吸附量,mg·g−1;qe为吸附平衡时的吸附量,mg·g−1;k1为拟一级动力学反应速率常数,h−1;k2为拟二级动力学反应速率常数,g·(mg·h)−1,Kd为颗粒内扩散常数,g·(mg·h1/2)−1;Ci为与边界层的厚度相关的常数,mg·g−1。A和B分别为动力学方程的常数。
由图5(a)可知,Sn-MOF对AR3R的吸附量随着时间的延长而逐渐增大,在4 h左右,吸附基本达到平衡。如表1所示,与拟一级动力学方程(R2=0.918 9)相比,拟二级动力学方程(R2=0.963 5)和Elovich方程(R2=0.985 8)的可决系数更高,并且由计算得到的吸附量值与实测值比较接近。这表明Sn-MOF对AR3R的吸附属于化学吸附,并且吸附过程可能是通过表面交换反应进行的,直到表面活性位点完全被占据;同时,AR3R分子扩散到Sn-MOF的孔中与吸附剂进行相互作用[14]。
颗粒内扩散方程可以用来描述AR3R的吸附过程,根据颗粒内扩散方程所作的Sn-MOF吸附AR3R的qt对t1/2的关系曲线如图5(b)所示,动力学参数如表2所示。在整个吸附时间内,图形并不是一条直线,而是分为2部分的曲线,表明吸附机理遵循3个不同的阶段。第1阶段,AR3R向Sn-MOF外表面扩散,这一过程称为边界层扩散,第1阶段的高Kd值表明,吸附速率较高。第2阶段,AR3R分子逐渐占据Sn-MOF的外表面,驱动力不再维持外部传质,由于较低的Kd值,颗粒内扩散是限速步骤。第3阶段,吸附剂中几乎所有孔都被AR3R分子占据,外部质量传递急剧减少,并且实际上没有膜或颗粒内扩散。值得注意的是,第2阶段的线条远离原点,这表明吸附过程的限速步骤不受单颗粒内扩散的控制[15]。计算颗粒内扩散模型的值如表2所示,较高的Ci值表明较大的边界层效应,这表明表面吸附也限制了速率步骤[16]。
2.5 吸附等温线
吸附等温线是描述体系中吸附剂表面和溶液中吸附质数量关系的曲线。在室温条件下,将0.30 g的Sn-MOF加入到pH均为5,浓度分别为100、150、200、250、300、350、400、450和500 mg·L−1的染料溶液中,振荡12 h,使其达到吸附平衡,分别利用Langmuir等温方程(式(5))和Freundlich等温方程(式(6))对Sn-MOF吸附AR3R的吸附等温线数据进行拟合,拟合曲线如图6所示,拟合参数如表3所示。Langmuir等温方程和Freundlich等温方程分别见式(5)和式(6)。
表 3 吸附等温线参数和可决系数Table 3. Adsorption isotherm parameters and the correlation coefficientsLangmuir等温方程 Freundlich等温方程 qm/(mg·g−1) KL/(L·mg−1) R2 KF/(mg·g−1) n R2 87.98 0.777 5 0.721 8 57.29 0.081 4 0.986 8 qe=qmKLce1+KLce (5) qe=KFc1/ne (6) 式中:ce为吸附达平衡时液相中染料的浓度,mg·L−1;qm为饱和吸附量,mg·g−1;KL是Langmuir常数,L·mg−1;KF是和吸附剂吸附量有关的Freundlich常数,mg·g−1;n则是与吸附强度有关的Freundlich指数。
由图6可以看出,Sn-MOF对AR3R的吸附量随着平衡时溶液中AR3R浓度的升高而升高。Freundlich等温方程(R2=0.986 8)对AR3R吸附的拟合要好于Langmuir等温方程(R2=0.721 8),同时n<1表明该吸附为有利的化学吸附[17]。
2.6 吸附热力学
为了确定Sn-MOF对AR3R吸附过程的本质,必须确定反应的活化能(Ea)、焓变(ΔH0)与熵变(ΔS0)及吉布斯自由能变化(ΔG0)。
1)活化能的确定。考察了在AR3R的初始浓度均为400 mg·L−1,Sn-MOF添加量为0.30 g的条件下,温度对吸附动力学的影响,结果如图7所示。由图7(a)可知,随着温度的升高吸附量逐渐增加。动力学参数列于表4中,反应速率常数随着温度的升高而增大。
表 4 不同温度下的吸附动力学参数(拟二级动力学方程拟合)Table 4. Adsorption kinetics parameters at different temperatures (pseudo-second-order kinetics model)热力学温度/K qe/(mg·g−1) k2/(g·(mg·h)−1) R2 323.15 150.90 0.002 14 0.999 7 333.15 152.54 0.003 06 0.990 2 343.15 171.19 0.006 52 0.994 5 反应速率常数k可用阿伦尼乌斯公式描述,由斜率(Ea/R)可计算出活化能。阿伦尼乌斯公式见式(7)。
k=Ae−Ea/RT (7) 式中:k为反应速率常数;A为指前因子;Ea为活化能,kJ·mol−1;R为摩尔气体常数,8.314 5 J·(mol·K)−1;T为热力学温度,K。在扩散控制的过程中,Ea低于20 kJ·mol−1[18]。
根据图7(b)可以计算出活化能Ea的值为51.46 kJ·mol−1。活化能的数量级可以表明吸附的类型,吸附通常包括物理吸附和化学吸附。物理吸附的活化能一般不超过4.20 kJ·mol−1,化学吸附包括活化与非活化2种形式。在活化的化学吸附过程中,活化能为8.40~83.70 kJ·mol−1;在非活化的化学吸附中,活化能为0 kJ·mol−1[19]。以上实验结果表明,Sn-MOF对AR3R的吸附属于活化的化学吸附且吸附过程属于吸热反应。
2)吉布斯自由能变化与熵变及焓变的确定。温度的吉布斯自由能变化(ΔG0)、熵变(ΔS0)和焓变(ΔH0)可以通过Eyring方程[20]计算。
lnKd=−ΔH0RT+ΔS0R (8) ΔG0=ΔH0−TΔS0 (9) 式中:Kd=qe/ce;R为摩尔气体常数;T为热力学温度。以lnKd对T−1作图,可以得到线性回归直线,如图8所示。
根据回归直线的斜率和截距可求得ΔH0和ΔS0,结果如表5所示。ΔH0>0说明Sn-MOF对AR3R的吸附过程是吸热反应,Sn-MOF吸附吉布斯自由能变化(ΔG0)随着温度的增加而减小,ΔG0为负值,说明吸附反应是自发进行的。
表 5 活化能及热力学参数Table 5. Parameters of activation energies and thermodynamic热力学温度/K ΔG0/(kJ·mol−1) ΔS0/(kJ·(mol·K)−1) ΔH0/(kJ·mol−1) Ea/(kJ·mol−1) 323.15 −1.106 0.059 17.96 51.46 333.15 −1.696 343.15 −2.286 2.7 Sn-MOF的再生
再生性和可重复使用性是吸附剂工业应用的关键问题。通过漫反射光谱(DRS)表征技术对合成的Sn-MOF的吸光性能进行检测。Sn-MOF的吸收波长为447.65 nm(可见光吸收)。使用Taucs公式估算Sn-MOF的禁带宽度,如式(10)所示。
(αhν)1/n=A(hν−Eg) (10) 式中:α是吸收系数;hv是光子能量;A为常数;n表示电子跃迁的类型,可以在0.5~3之间取合适的值;Eg为半导体禁带宽度,eV。
图9为Sn-MOF的再生对其吸附性能的影响。根据图9(a)的插图,计算出的制备的Sn-MOF的Eg值为2.77 eV,这表明Sn-MOF具有较长的吸收波范围,且禁带宽度较窄对光的吸收利用率较高,在可见光下具有较好的光催化活性[20]。由于合成的材料具有较好的可见光响应,我们使用光催化反应来处理吸附后的吸附剂。首先,将用过的吸附剂放入50 mL石英玻璃试管中,加入30 mL去离子水。然后用Xe灯照射悬浮液,Xe灯起到光源的作用,并搅拌120 min,以确保吸附物完全降解,之后将吸附剂干燥,用于下一次使用。图9(b)为每次使用后Sn的溶出率,可以看出,当使用6次后,Sn的溶出率仅为9.20%,这表明合成的Sn-MOF具有较好的水稳定性。如图9(c)所示,再生吸附剂在6轮循环后仍保持良好的吸附能力,每个循环的吸附量下降仅为2.01%~6.32%,这可能是在吸附过程的中Sn-MOF丧失了部分的吸附位点所引起的。尽管如此,经过6个连续循环后,颗粒的吸附量仍保持在较高值,为113.50 mg·g−1,这表明Sn-MOF具有较好的可循环利用性。
3. 结论
1) Sn-MOF对AR3R模拟染料废水具有较好的脱色效果。在室温下,Sn-MOF最佳投加量为0.30 g时,其对浓度为400 mg·L−1的AR3R去除率可以达到76.95%,吸附量可以达到102.60 mg·g−1;当pH为5时,吸附效果达到最佳,pH过低或过高均不利于吸附。
2)吸附过程符合拟二级动力学方程和Elovich方程,这表明Sn-MOF对AR3R的吸附是由化学吸附进行控制的,并且该吸附反应可能是通过表面交换反应进行的,直到表面活性位点被完全占据。同时,AR3R分子扩散到Sn-MOF的孔中,与吸附剂相互作用。粒子扩散方程表明颗粒内扩散不是控制Sn-MOF吸附过程的唯一步骤,而是由膜扩散和颗粒内扩散联合控制的。
3)吸附规律较好地符合Freundlich吸附等温方程,这表明Sn-MOF吸附是一种有利的化学吸附。热力学实验结果表明,AR3R的去除过程是一种自发进行的吸热过程。
4)再生实验结果表明,Sn-MOF具有较好的水稳定性和重复利用性,经光催化再生处理6个循环后,吸附容量没有明显降低。
-
表 1 Sn-MOF对AR3R的吸附动力学参数
Table 1. Adsorption kinetic parameters of AR3R by Sn-MOF
C0/(mg·L−1) 拟一级动力学方程 拟二级动力学方程 Elovich方程 qe/(mg·g−1) k1/h−1 R2 qe/(mg·g−1) k2/(g·(mg·h)−1) R2 A B R2 400 132.212 0.576 9 0.918 9 154.675 0.004 8 0.963 5 71.459 1 29.943 1 0.985 8 表 2 Sn-MOF对AR3R的颗粒内扩散动力学参数
Table 2. Intraparticle diffusion kinetic parameters of AR3R by Sn-MOF
第1阶段 第2阶段 K1d/(g·(mg·h1/2)−1) C1/(mg·g−1) R2 K2d/(g·(mg·h1/2)−1) C2/(mg·g−1) R2 75.272 1 21.643 2 0.952 2 36.193 1 36.442 8 0.975 9 表 3 吸附等温线参数和可决系数
Table 3. Adsorption isotherm parameters and the correlation coefficients
Langmuir等温方程 Freundlich等温方程 qm/(mg·g−1) KL/(L·mg−1) R2 KF/(mg·g−1) n R2 87.98 0.777 5 0.721 8 57.29 0.081 4 0.986 8 表 4 不同温度下的吸附动力学参数(拟二级动力学方程拟合)
Table 4. Adsorption kinetics parameters at different temperatures (pseudo-second-order kinetics model)
热力学温度/K qe/(mg·g−1) k2/(g·(mg·h)−1) R2 323.15 150.90 0.002 14 0.999 7 333.15 152.54 0.003 06 0.990 2 343.15 171.19 0.006 52 0.994 5 表 5 活化能及热力学参数
Table 5. Parameters of activation energies and thermodynamic
热力学温度/K ΔG0/(kJ·mol−1) ΔS0/(kJ·(mol·K)−1) ΔH0/(kJ·mol−1) Ea/(kJ·mol−1) 323.15 −1.106 0.059 17.96 51.46 333.15 −1.696 343.15 −2.286 -
[1] ROTT U, MINKE R. Overview of wastewater treatment and recycling in the textile processing industry[J]. Water Science & Technology, 1999, 40(1): 137-144. [2] 张波, 戚永洁, 蒋素英, 等. 铁碳微电解-生物膜法-高级氧化工艺处理印染废水中试研究[J]. 环境工程学报, 2018, 36(3): 44-48. [3] 刘斌, 李孟斌, 王红华. 水中染料在活性炭上的动态吸附行为研究[J]. 工业水处理, 2018, 38(5): 21-24. doi: 10.11894/1005-829x.2018.38(5).021 [4] 赵景联, 种法国, 赵靓, 等. 磁场TiO2光催化耦合降解酸性大红3R的研究[J]. 西安交通大学学报, 2006, 40(7): 851-855. doi: 10.3321/j.issn:0253-987X.2006.07.025 [5] JUNG K W, CHOI B H, DAO C M, et al. Aluminum carboxylate-based metal organic frameworks for effective adsorption of anionic azo dyes from aqueous media[J]. Journal of Industrial & Engineering Chemistry, 2017, 59(3): 149-159. [6] AYATI A, SHAHRAK M N, TANHAEI B, et al. Emerging adsorptive removal of azo dye by metal-organic frameworks[J]. Chemosphere, 2016, 160(32): 30-44. [7] HASAN Z, JHUNG S H. Removal of hazardous organics from water using metal-organic frameworks(MOFs): Plausible mechanisms for selective adsorptions[J]. Journal of Hazardous Materials, 2015, 283(8): 329-339. [8] QIU J, YI F, ZHANG X, et al. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms[J]. Journal of Colloid & Interface Science, 2017, 499(7): 151-158. [9] MCDONALD T M, LEE W R, MASON J A, et al. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc)[J]. Journal of the American Chemical Society, 2012, 134(16): 7056-7068. doi: 10.1021/ja300034j [10] BAI S, LIU C, LUO R, et al. Metal organic frameworks-derived sensing material of SnO2/NiO composites for detection of triethylamine[J]. Applied Surface Science, 2017, 437(15): 304-311. [11] 龚正君, 周文波, 陈钰. 活性炭纤维对水中酸性染料的吸附研究[J]. 工业水处理, 2012, 32(9): 24-28. doi: 10.3969/j.issn.1005-829X.2012.09.007 [12] 张聪璐, 胡筱敏. 磁性壳聚糖衍生物对阴离子染料的吸附行为[J]. 环境科学, 2015, 36(1): 55-56. doi: 10.3969/j.issn.1673-288X.2015.01.013 [13] 马锋锋, 赵保卫, 刁静茹, 等. 牛粪生物炭对水中氨氮的吸附特性[J]. 环境科学, 2015, 36(5): 1678-1685. [14] DANESHVAR E, KOUSHA M, SOHRABI M S, et al. Biosorption of three acid dyes by the brown macroalga Stoechospermum marginatum: Isotherm, kinetic and thermodynamic studies[J]. Chemical Engineering Journal, 2012, 74(2): 195-196. [15] MARZBALI M H, ESMAIELI M, ABOLGHASEMI H, et al. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: A batch study[J]. Process Safety & Environmental Protection, 2016, 102(6): 700-709. [16] 成芳芳, 孔庆山, 纪全, 等. 海藻酸纤维对水溶液中Fe3+的吸附动力学研究[J]. 合成纤维工业, 2010, 33(2): 1-4. doi: 10.3969/j.issn.1001-0041.2010.02.001 [17] CANZANO S, IOVINO P, SALVESTRINI S, et al. Comment on “Removal of anionic dye congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design”[J]. Water Research, 2012, 46(13): 4314-4315. doi: 10.1016/j.watres.2012.05.040 [18] 吴盼盼, 方婷婷, 于欢, 等. 有机改性镁铝层状氢氧化物对酸性橙Ⅱ的吸附研究[J]. 环境科学学报, 2013, 33(6): 1576-1584. [19] 白鹭, 吴春英, 魏薇, 等. 稻壳对废水中Cr(Ⅵ)吸附及动力学研究[J]. 粮食与油脂, 2018, 31(2): 45-49. doi: 10.3969/j.issn.1008-9578.2018.02.013 [20] LIN Y, YING Z, WENHUI S, et al. Synthesis of a novel (NH4)3PW11O39Sn/TiO2 heterostructure for efficient photocatalytic degradation and removal of water pollutants[J]. Materials Letters, 2019, 237(32): 137-140. 期刊类型引用(9)
1. 杨智程,岳琳,王梦菲,廉静,罗晓,刘艳芳. Sn基双配体金属有机骨架光催化性能与机理研究. 现代化工. 2025(01): 178-183 .  百度学术
百度学术
2. 李运龙,刘忆贤,刘苗,韩继龙,周理龙,李正杰,甄崇礼,刘润静. 基于静电和金属络合协同作用的MIL-101(Cr)-NH_2高效吸附水中单宁酸. 材料导报. 2024(14): 281-288 .  百度学术
百度学术
3. 易皓,余仪,柳泽伟,丁泽聪,奚红霞,胡勇有. Ni-MOFs对水中全氟烷基醚磺酸盐的吸附性能及机理. 环境工程学报. 2023(09): 2861-2871 .  本站查看
本站查看
4. 陆砚秋,周俊,吴慧芳,吴艳霞. 复合凝胶球对水中结晶紫的吸附特性. 印染助剂. 2022(01): 45-50 .  百度学术
百度学术
5. 范文玉,刘健,谷精华,应志明. SiO_2@CuO-ZnO双壳催化剂对臭氧催化降解印染废水的影响. 安全与环境学报. 2021(02): 780-786 .  百度学术
百度学术
6. 李彦成,刘彦禧,刘叶芳,黄雷,黄瑞敏. MnO_2/CoFe_2O_4活化过一硫酸盐降解酸性大红3R. 中国给水排水. 2021(17): 78-85 .  百度学术
百度学术
7. 岳琳,曹云梦,张迎,王涛,罗晓,廉静,李再兴. 核壳结构Fe_3O_4@Sn-MOF的制备及其光催化性能研究. 现代化工. 2021(12): 125-129 .  百度学术
百度学术
8. 刘永德,马琳,孙旭镯,赵海亮,李波,万东锦,肖书虎. 葫芦[6]脲对印染废水中染料曙红的吸附特性及影响因素. 环境工程技术学报. 2020(05): 837-844 .  百度学术
百度学术
9. 狄婧,刘海霞,郭金鑫,姜永强,赵国虎. 戊二醛/壳聚糖交联复合材料的制备及其对3种染料的吸附性能及其机理. 甘肃农业大学学报. 2020(06): 175-183+192 .  百度学术
百度学术
其他类型引用(8)
-






 下载:
下载: