-
为了保障钻井液的性能稳定,以实现在高温、高压、高盐的地层环境进行快速钻井及后续作业,通常在钻井过程中会大量使用具有高色度、难降解的磺化聚合物泥浆体系[1],从而导致钻井废水具备高色度、高COD、难降解的特点[2-3]。上述钻井废水若直接排放,会严重污染土壤和地下水。因此,钻井废水的达标处理对油气田环境保护具有重大意义。
近年来,三维电解法在废水处理中特别是难降解有机污染物的处理领域备受关注[4-8]。三维电极系统中常规粒子电极主要通过羟基自由基等强氧化性中间物质降解有机物,而钻井废水中的氯离子对羟基自由基具有较强抑制作用[9-10]。钻井废水电解过程中生成的活性氯(Cl2、ClO−、HClO)具有强氧化性,能进一步促进有机物的降解[11]。杨蕴哲等[12]研究发现活性氯能有效降解活性艳蓝KN-R。吉庆华[13]研究证明活性氯可强化溶解性有机碳、三氯甲烷和氯酸烷等的混凝去除作用。刘咏等[14]研究发现Cl−含量越大,苯酚被完全氧化所需的时间越短。基于二氧化锰对电解活性氯具有良好催化活性的特点,而软锰矿的主要成分为MnO2且在我国分布广泛,因而采用软锰矿、石墨为主要成分制备新型粒子电极。将自制软锰矿粒子电极应用于三维电极系统中处理高含氯的钻井废水,通过软锰矿的催化活性和石墨良好的导电性,可有效提高电解过程中活性氯生成量和COD去除率。
磺化聚合物泥浆体系以磺化酚醛树脂(SMP)为主[15],其相对分子质量10 kDa左右[16]。本研究以SMP为目标污染物,通过自制软锰矿粒子电极催化电解产生更多的活性氯,进一步提高SMP的处理效率。以活性氯浓度、COD为主要检测指标,研究并优化了软锰矿粒子电极的制备过程参数;并与常规活性炭粒子电极进行了对比研究,采用电化学分析及紫外分光光度等方法,初步探索软锰矿粒子电极对SMP的电催化降解机理。
-
氯化钠(NaCl)、重铬酸钾(K2Cr2O7)、聚四氟乙烯浓缩分散液(PTFE,质量分数为60%)、石墨粉、浓硫酸(H2SO4)、硫酸银(Ag2SO4)、七水合硫酸亚铁(FeSO4·7H2O)、无水乙醇(C2H5OH)、N,N-二乙基-1,4-苯二胺硫酸盐(NH2-C6H4-N(C2H5)2·H2SO4)均为分析纯,软锰矿系四川青川县产。
X射线衍射仪(X Pert PRO MPD,荷兰帕纳科公司);全自动表面积和孔径分布仪(ChemBET TPR/TPD,美国康塔);原子吸收光谱仪(AA-7020,北京东西分析仪器有限公司);电化学工作站(CHI604D,上海展华仪器有限公司);双光束紫外分光光度计(UV-1800,日本岛津有限公司)。
-
称取石墨粉、软锰矿粉按照一定质量比例混匀,加入适量无水乙醇,在70 ℃恒温水浴下搅拌使其分散均匀;搅拌过程中缓慢滴加PTFE浓缩分散液,继续恒温搅拌直至膏状;碾轧混合后在80 °C下烘干12 h,压制成柱状颗粒;将成型粒子电极放入马弗炉,在预定温度下灼烧一定时间,得到新型软锰矿粒子电极。
-
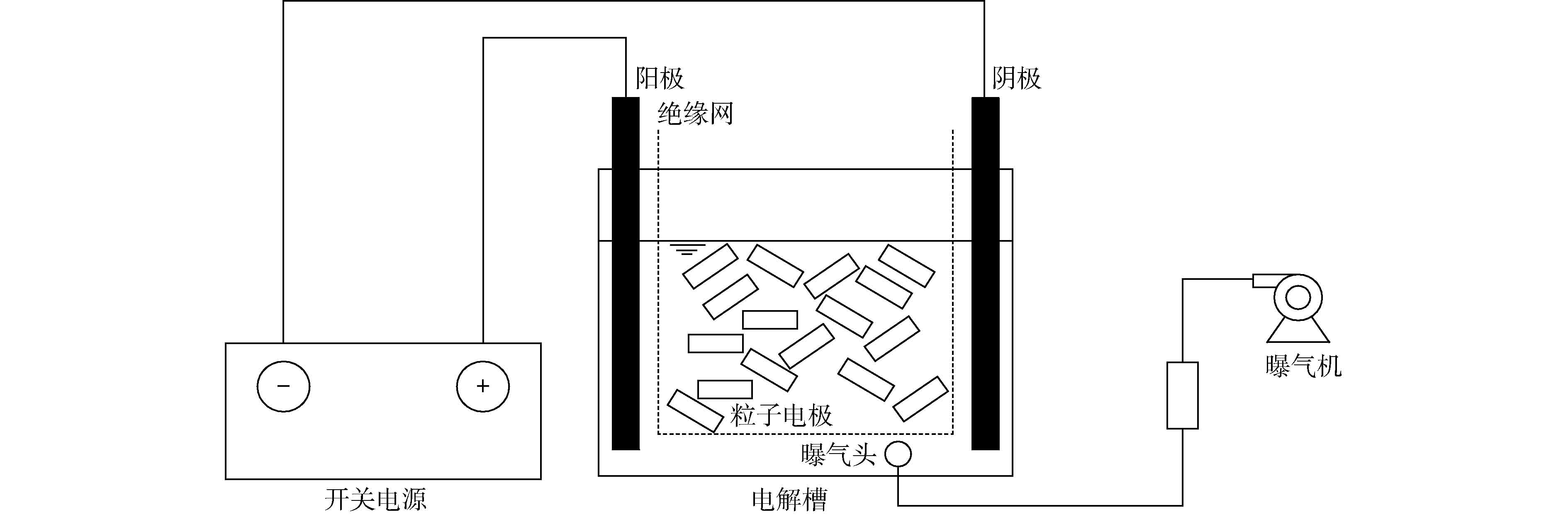
三维电极反应装置如图1所示。电解槽(有机玻璃)大小为7 cm×7 cm×8 cm,主电极为石墨板,板间距6 cm;粒子电极与主极板采用绝缘网隔离,避免粒子电极与极板接触;反应器底部放置曝气头进行曝气。
电解实验条件:SMP模拟废水COD为500 mg·L−1左右、电导率为1 930 μS·cm−1、电流强度0.5 A、粒子电极投加量10 g、溶液pH 7.0、电解水量200 mL、曝气量600 mL·min−1、电解时间40 min,间隔10 min取样测量COD值和活性氯浓度。为了避免SMP的氧化降解对活性氯浓度的影响,本研究通过电解氯化钠溶液考察活性氯浓度变化趋势。
粒子电极预处理:电解实验前将制备的粒子电极在待处理溶液中浸泡,直至吸附饱和,避免吸附作用对实验结果的影响。
-
采用N,N-二乙基-1,4-苯二胺分光光度法(HJ 586-2010)[17]测定活性氯,油气田高氯废水的COD测定方法[18]测定COD值。
-
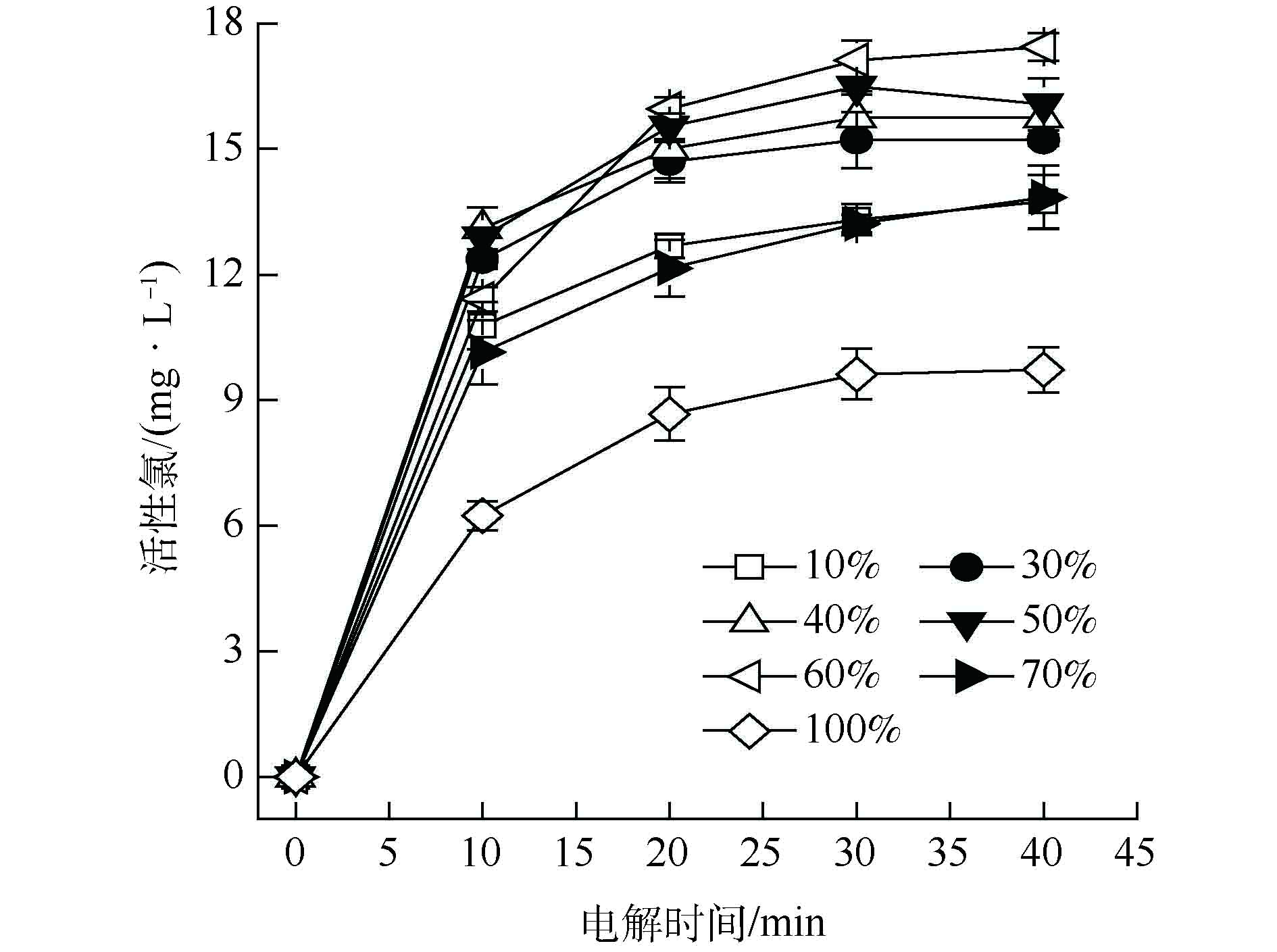
为了考察粒子电极中软锰矿和石墨材料配比对粒子电极催化活性和导电性的影响,将软锰矿和石墨材料分别按不同质量分数比例混合制备粒子电极,得到不同配比对活性氯浓度的影响趋势,如图2所示。由图2可见,随着粒子电极中软锰矿材料所占比例的增加,粒子电极中催化活性物质含量增大,电解产生的活性氯浓度随之增大。当软锰矿质量分数为60%时,活性氯浓度最高达17.44 mg·L−1;继续增大软锰矿材料比例,活性氯浓度反而降低,因为石墨材料过少使粒子电极导电性差,导致电解能力降低。因此,软锰矿与活性炭材料的最佳配比为6∶4。
-
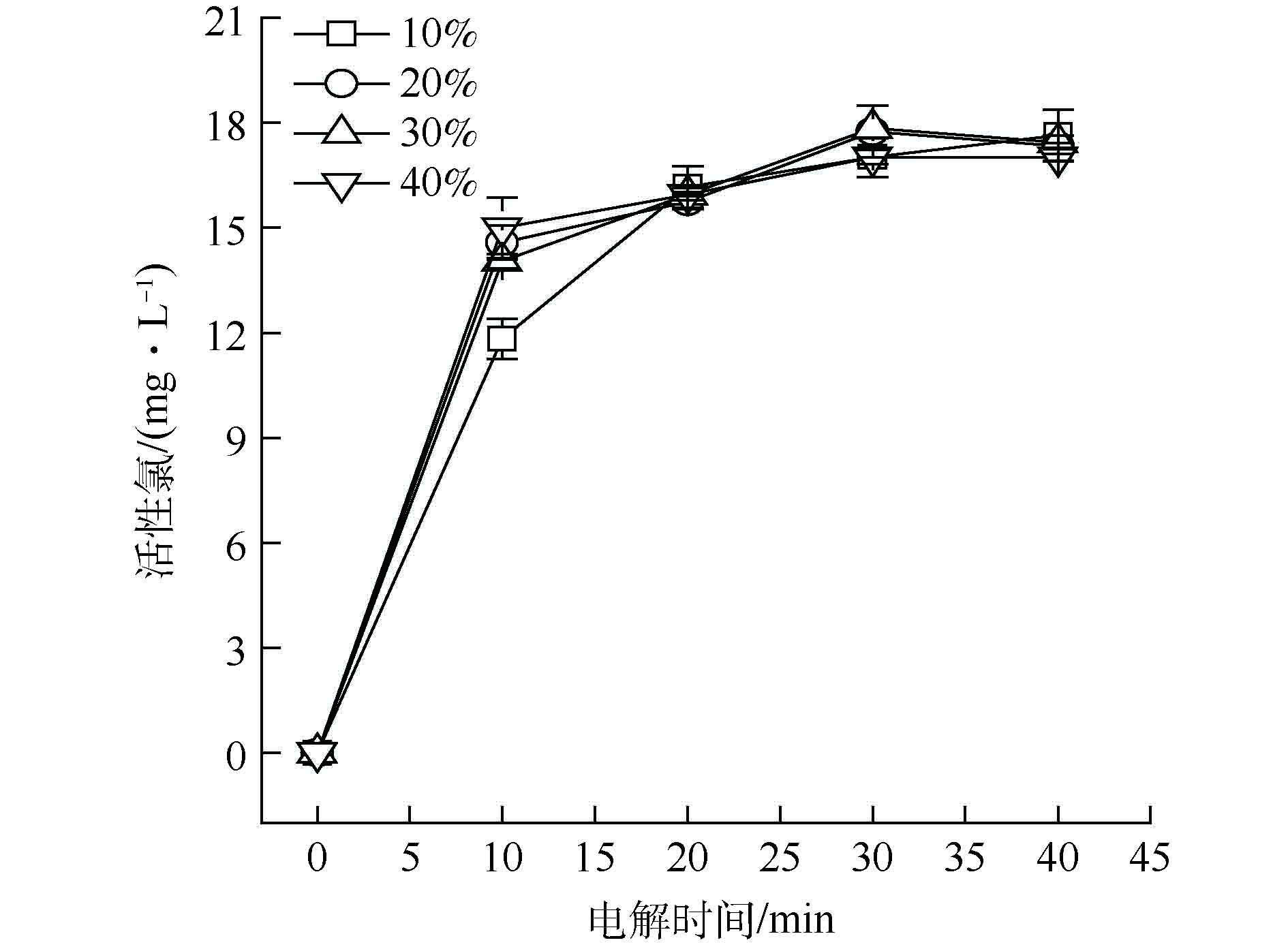
本研究采用PTFE浓缩分散液作为黏结剂,使粉状的软锰矿和石墨材料黏接形成固体颗粒,将PTFE分别以10%、20%、30%和40%的质量分数制备软锰矿粒子电极,得到PTFE加入量对活性氯产生量的影响趋势,如图3所示;采用全自动表面积和孔径分布仪检测不同PTFE加量所得粒子电极的表面积和孔体积,结果如表1所示。
由表1可见,PTFE加入量为10%时,粒子电极的孔容和比表面积最大;继续增大加入量,粒子电极的比表面积与孔体积却随之下降。综合经济性考虑,PTFE加入量10%为最佳。由图3可见,随着PTFE的加入量的增加,活性氯浓度的变化不明显。在电解实验过程中还发现,当PTFE加入量小于10%时,粒子电极表面易脱落,使用寿命短。
-
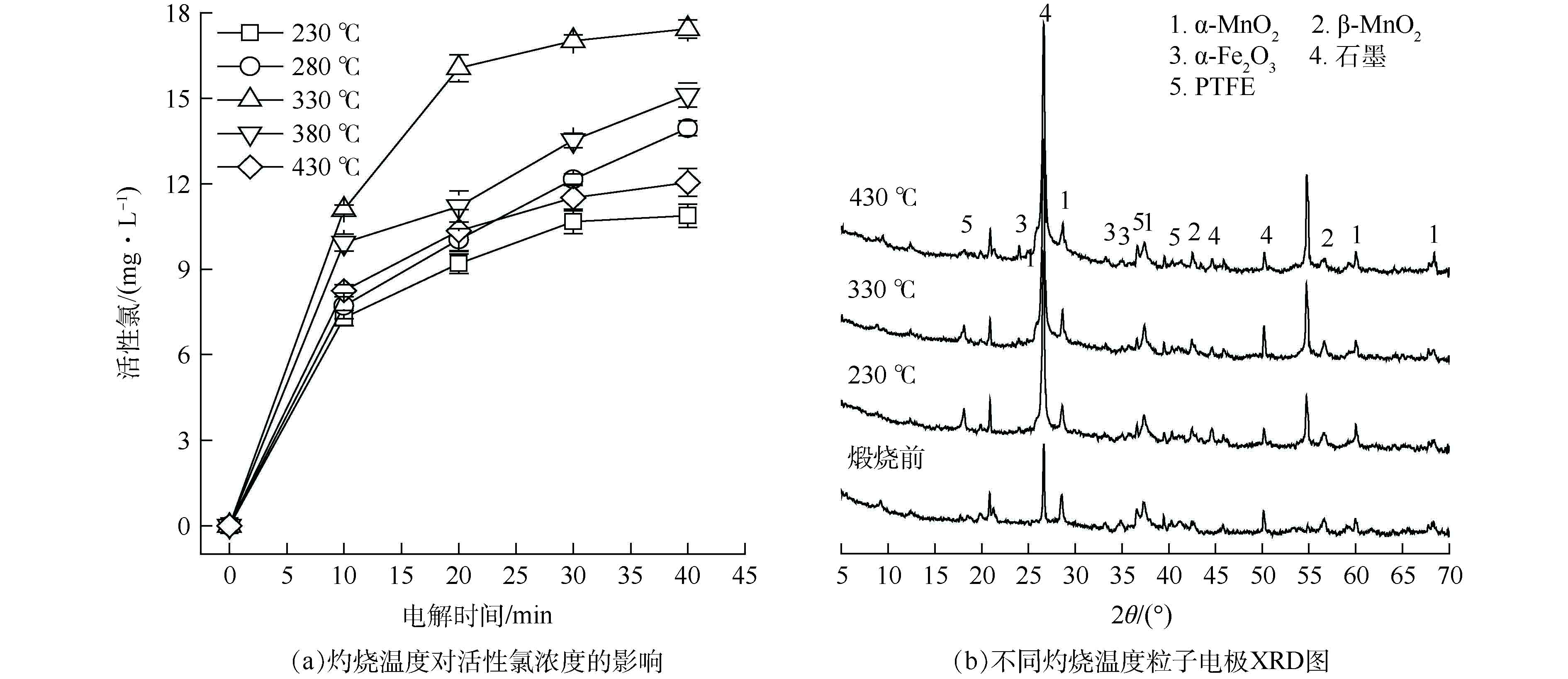
不同灼烧温度对粒子电极性能的影响如图4所示。由图4(a)可见,灼烧温度为330 °C时,粒子电极产生的活性氯浓度最大,继续增大灼烧温度,活性氯浓度反而下降。由图4(b)可见,230、330 °C灼烧温度下所得粒子电极在2θ=18.084°处均存在较明显的PTFE特征衍射峰。实验过程中发现,230 °C灼烧所得粒子电极在电解过程中表面易脱落,因PTFE熔点为330 °C左右[19],230 °C灼烧温度下PTFE尚未完全融化,粒子电极内部粘接剂分布不均,使粒子电极结构内部材料结合牢度不够高,易脱落。在330 °C温度下灼烧能使PTFE融化彻底,PTFE在粒子电极结构内部分布更均匀,有利于粒子电极内部各部分材料的结合更稳定,同时有助于其内部形成纤维状立体结构,增加粒子电极的比表面积,即增大污染物与粒子电极的接触面积,进一步强化传质速率[20-22]。430 °C下所得粒子电极在2θ=18.084°处的衍射峰几乎消失,因过高的灼烧温度导致PTFE分解[23],黏接剂含量减少使其内部材料的粘结牢度变差。综合以上分析,选取330 °C为软锰矿粒子电极最佳灼烧温度。
-
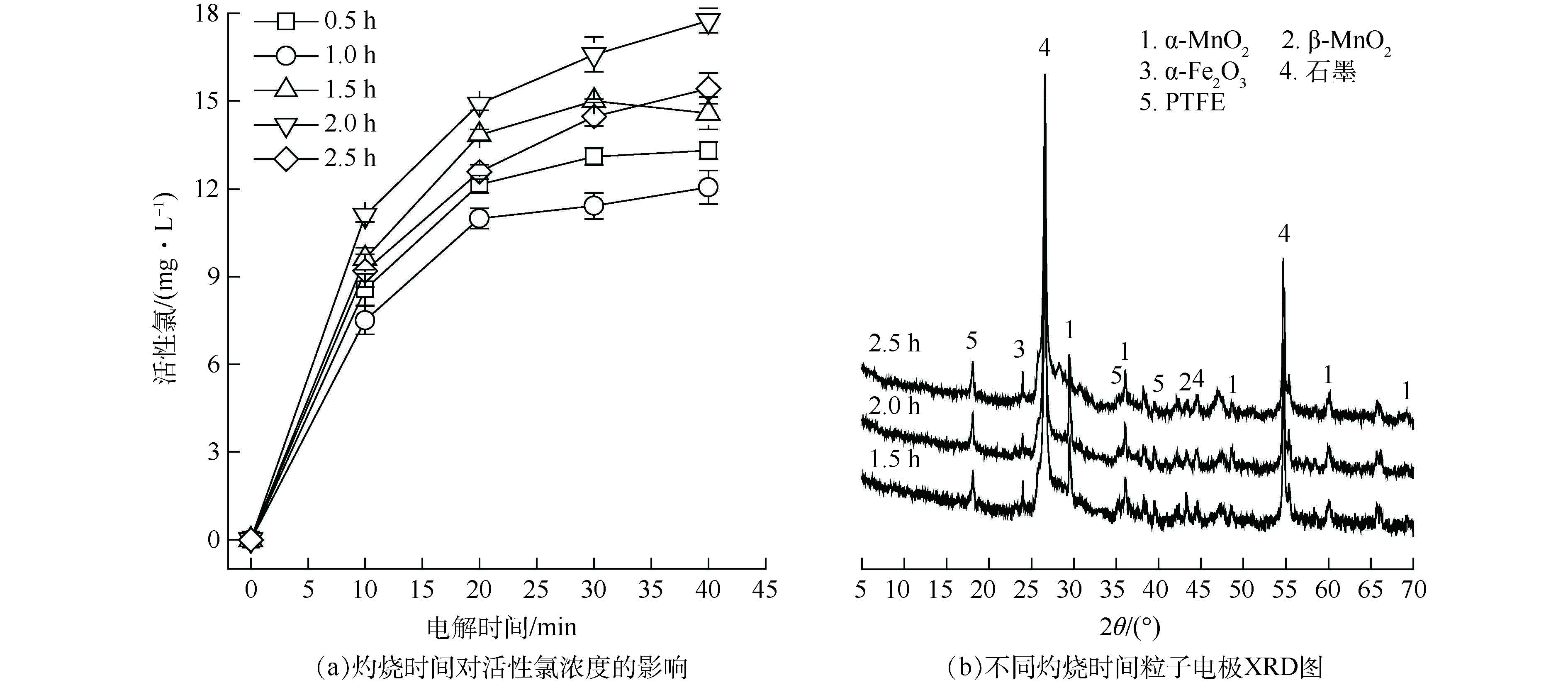
灼烧时间对软锰矿粒子电极电催化活性影响如图5所示。由图5(a)可见,不同灼烧时间所得软锰矿粒子电极的催化活性存在明显差距。灼烧2 h所得粒子电极的电解活性氯浓度最高达17.43 mg·L−1;继续延长灼烧时间,活性氯产生量反而下降。LIANG等[24]、芦佳[25]发现,锰氧化物中α-MnO2晶型的催化氧化性能最好。SELVAKUMAR等[26]发现α-MnO2具有较强的催化氧化活性,其中α-MnO2 (310)晶面对水中污染物的吸附性能和催化性能最强。因此,α-MnO2含量的增加有助于强化SMP的降解。由图5(b)可见,2θ=28.841°处α-MnO2(310)衍射峰强度在灼烧2 h时达到最大,即灼烧时间为2 h时α-MnO2含量最高,该灼烧温度下制备所得的粒子电极在电解过程中活性氯产生量最多。综上所述,2 h为软锰矿粒子电极最佳灼烧时间。
-
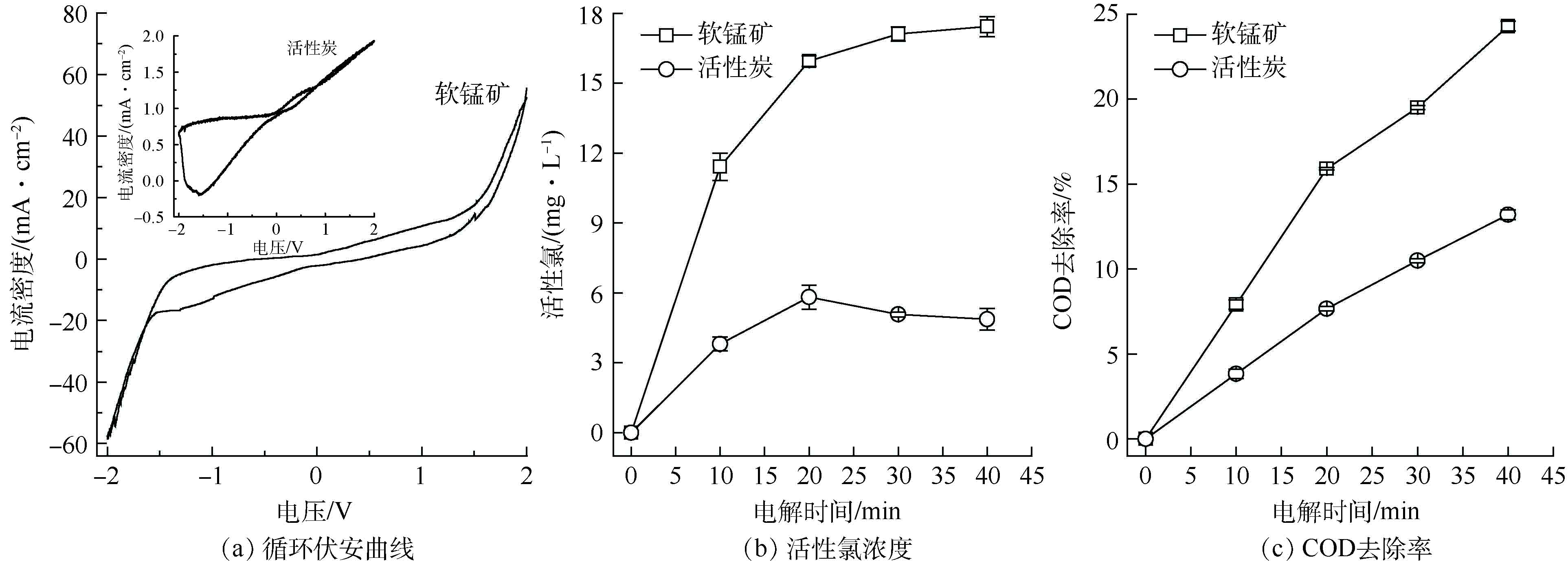
以粒子电极作为工作电极,饱和甘汞电极(SCE)为参比电极,Pt电极为辅助电极。在−2~2 V范围内,扫描速率为10 mV·s−1,在0.5 mol·L−1 NaCl电解溶液中测量软锰矿、活性炭粒子电极体系的循环伏安曲线。由图6(a)可知,软锰矿粒子电极循环伏安曲线对称性良好,且在相同电位下,阳极和阴极过程电流密度相当,说明电极具有良好的可逆性[27-28]。同时,软锰矿粒子电极的析氯电位为1.23 V,图6(a)中的插入图显示活性炭粒子电极在循环伏安测试过程中表现出较高的阻抗,在0~2 V的测试中没有出现明显的氧化还原峰,仅发生电极充放电过程。因此,在相同的电解条件下,软锰矿粒子电极在电解过程中可以产生更多的活性氯,能够有效促进有机物的降解去除 [29-31]。
将自制的软锰矿粒子电极、常规活性炭粒子电极(GAC)分别应用于三维电极系统中电解氯化钠溶液和SMP模拟废水。由图6(b)可见,氯化钠溶液电解过程中,软锰矿粒子电极的电解活性氯浓度显著高于活性炭粒子电极;电解20 min后,活性炭粒子电极电解活性氯产生量趋于平稳,软锰矿粒子电极呈现继续上升的趋势。相比活性炭,二氧化锰析氯电位较低[32],软锰矿粒子电极在电解含氯废水过程中产生了更多的活性氯,这与循环伏安曲线分析一致。由图6(c)可见,SMP模拟废水电解过程中,软锰矿粒子电极的COD去除率明显高于活性炭粒子电极,COD去除率的变化规律和活性氯浓度变化一致。这证明了软锰矿粒子电极通过增大活性氯的生成量而显著提高了难降解有机污染物的处理效果[33]。
-
HUANG等[34]、LIANG等[35]研究发现,NH4+能与活性氯反应生成活性较低、更稳定的氯胺,且与羟基自由基的反应性较差。为了鉴定三维电极反应体系中活性氯的作用,通过向溶液中加入12.5 mmol·L−1 (NH4)2SO4以淬灭活性氯,研究活性氯在SMP降解率中的贡献。由图7可见,加入
${\rm{NH}}_4^{+} $ 后SMP的降解效率显著降低,去除率下降为对照组的63%。该结果表明,自制软锰矿粒子电极三维体系在降解SMP的过程中,电催化产生的活性氯对降解效率确实起着重要作用。由此可知,在高含Cl-条件下,活性氯是提高SMP降解效率的主要氧化剂。 -
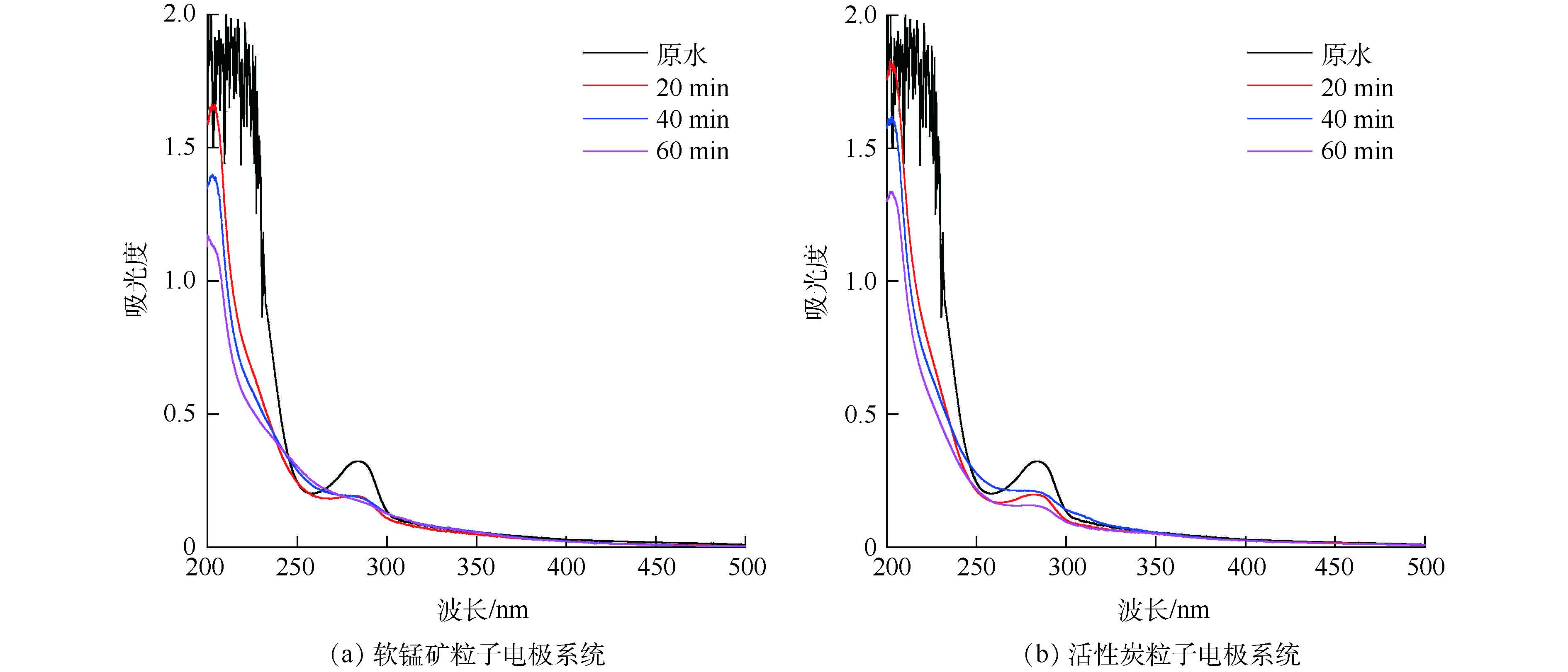
分别采用活性炭粒子电极、软锰矿粒子电极电解处理SMP模拟废水,利用紫外-可见光谱检测分析电解前、后SMP模拟废水,其降解过程的变化如图8所示。
由图8可见,电解前SMP模拟废水在228 nm处为磺甲基的特征吸收峰,283 nm处为苯环的特征吸收峰[2]。电解20 min后,2个反应系统处理后的废水在283 nm处特征吸收峰明显减弱,228 nm处特征吸收峰消失,而在近200 nm处均出现明显的新吸收峰,这是由于电解过程中SMP化学键断裂产生了苯类氧化中间体[36]。电解40 min后,软锰矿粒子电极处理后废水在283 nm处特征吸收峰消失,且近200 nm处特征吸收峰强度显著低于GAC粒子电极。由图8(b)可见,电解60 min后,GAC粒子电极处理后废水在近200 nm处仍存在明显特征吸收峰;由图8(a)可见,软锰矿粒子电极处理后废水在近200 nm处吸收峰消失。这说明软锰矿粒子电极对苯类氧化中间体的降解能力显著强于GAC粒子电极。综上所述可知:含氯废水电解过程中,因软锰矿粒子电极对电解活性氯具备更高的催化活性,更多活性氯的产生进一步强化了SMP的氧化降解。
-
在相同实验条件下,重复电解SMP模拟废水6次,分析软锰矿粒子电极性能稳定性,以及对SMP降解效率的影响。由图9可知,软锰矿粒子电极重复使用5次后,SMP的降解效率没有出现大幅降低,但电解6次之后COD去除率开始下降。经测定,随着电解次数的增加,软锰矿粒子电极中的活性组分锰氧化物呈现不同程度的溶出,在电解6次时Mn浸出浓度达到最高为0.38 mg·L−1,且此时粒子电极出现一定程度的腐蚀,导致电解性能下降。总体上,软锰矿粒子电极活性组分流失量较小,保持了较高的电催化活性和稳定性。
-
1)软锰矿粒子电极最佳制备条件为:软锰矿与石墨材料质量比6∶4,PTFE加入量10%,灼烧温度330 ℃,灼烧时间2 h。330 ℃灼烧温度下能保证PTFE充分融化,使其在粒子电极内部分布更均匀,稳定性最佳;灼烧2 h所得粒子电极活性物质α-MnO2含量最大,在电解时具有更高的催化活性。
2)循环伏安曲线表明,相比常规活性炭粒子电极,软锰矿粒子电极析氯电位较低,对活性氯的生成具备更高的催化活性,在电解过程中催化产生了更多的活性氯。软锰矿粒子电极催化降解SMP的效率优于活性炭粒子电极。通过
${\rm{NH}}_4^{+ } $ 清除实验表明,活性氯是提高SMP氧化降解效率的主要氧化剂。3)粒子电极稳定性实验表明,软锰矿粒子电极具有良好的电催化活性和稳定性,且活性组分溶出率较低,重复使用6次之后仍具有较高的COD降解效率。
软锰矿粒子电极的制备及其对SMP的催化降解
Preparation of pyrolusite particle electrode and its catalytic degradation of SMP
-
摘要: 针对二氧化锰对废水中氯离子电解生成活性氯具有较好催化活性的特点,以软锰矿为原料制备一种新型粒子电极,将其用于三维电极系统处理油气田高含氯废水,利用活性氯的强氧化性进一步强化废水中难降解有机物SMP的处理;并考察软锰矿粒子电极制备条件对电极性能和电解效果的影响。结果表明:软锰矿与石墨质量比6∶4、PTFE分散液加入量10%、330 °C下灼烧2 h为软锰矿粒子电极最优制备条件;与常规活性炭粒子电极相比,软锰矿粒子电极电解活性氯的产生量、COD去除率均显著优于活性炭粒子电极;且软锰矿粒子电极在重复使用多次后,活性组分流失量较小,对SMP的降解效率仍较高,保持了稳定的电催化性能。Abstract: The electrolysis of chloride ions in wastewater by manganese dioxide can produce active chlorine with a good catalytic activity. Thus, a new type of particle electrode was prepared with pyrolusite, which was used in a three-dimensional electrode system to treat high-chlorine wastewater in oil and gas fields. Then the active chlorine with strong oxidation ability was produced to strengthen the treatment of refractory organic matter SMP in wastewater. The effects of preparation conditions of pyrolusite particle electrode on electrode properties and electrolysis performance were investigated. The particle electrode was characterized by specific surface area analyzer and X-ray diffraction. The results show that the optimal preparation conditions of pyrolusite electrode were determined as follows: mass ratio of pyrolusite to graphite of 6∶4, PTFE dispersion dosage of 10%, and 2 h sintering at 330 ℃. Compared with the conventional activated carbon particle electrode, the production of activated chlorine and COD removal rate of the pyrolusite particle electrode were significantly higher. Moreover, after several recycling of the pyrolusite particle electrode, its active component loss was low, the recycled pyrolusite particle electrode still showed high SMP degradation efficiency and maintained a stable electro-catalysis performance accordingly.
-

-
表 1 PTFE加入量对粒子电极比表面积和孔容的影响
Table 1. Effect of PTFE addition on the specific surface area and the pore volume of particle electrode
PTFE加入量/% BET比表面积/(m3·g−1) 孔容/(cm3·g−1) 平均孔径/nm 10 13.885 4 0.057 2 16.489 7 20 11.365 9 0.050 5 17.819 4 30 9.924 2 0.055 2 21.169 3 40 8.249 1 0.051 5 24.679 4 -
[1] 蒋学彬. 川渝地区油气田钻井废水处理研究[D]. 成都: 西南交通大学, 2008. [2] 彭娟华, 莫正平, 李旭东. Fenton试剂预处理提高钻井废水可生化性[J]. 应用与环境生物学报, 2008, 14(2): 265-269. doi: 10.3321/j.issn:1006-687X.2008.02.025 [3] 张红岩, 吕荣湖, 郭绍辉. 混凝-臭氧氧化法处理三磺泥浆体系钻井废水[J]. 过程工程学报, 2007, 7(4): 718-722. doi: 10.3321/j.issn:1009-606x.2007.04.016 [4] HUSSAIN S N, DE LAS HERAS N, ASGHAR H M A, et al. Disinfection of water by adsorption combined with electrochemical treatment[J]. Water Research, 2014, 54: 170-178. doi: 10.1016/j.watres.2014.01.043 [5] CAN W, YAO-KUN H, QING Z, et al. Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects[J]. Chemical Engineering Journal, 2014, 243: 1-6. doi: 10.1016/j.cej.2013.12.044 [6] YAVUZ Y, KOPARAL A S, ÖĞÜTVEREN Ü B. Treatment of petroleum refinery wastewater by electrochemical methods[J]. Desalination, 2010, 258(1/2/3): 201-205. [7] GEDAM N, NETI N R. Carbon attrition during continuous electrolysis in carbon bed based three-phase three-dimensional electrode reactor: Treatment of recalcitrant chemical industry wastewater[J]. Journal of Environmental Chemical Engineering, 2014, 2(3): 1527-1532. doi: 10.1016/j.jece.2014.06.025 [8] 梁宏, 任阳民, 邱阳, 等. 三维电极法处理含氯废水过程中电解活性氯的研究[J]. 工业水处理, 2017, 37(9): 37-40. [9] LAAT J D, LE T G. Effects of chloride ions on the iron(III)-catalyzed decomposition of hydrogen peroxide and on the efficiency of the Fenton-like oxidation process[J]. Applied Catalysis B: Environmental, 2006, 66(1/2): 137-146. [10] LÓPEZ-GÁLVEZ, F, et al. Electrochemical disinfection: An efficient treatment to inactivate Escherichia coli O157∶H7 in process wash water containing organic matter[J]. Food Microbiology, 2012, 30(1): 146-156. doi: 10.1016/j.fm.2011.09.010 [11] CHEN S, ZHENG Y, WANG S, et al. Ti/RuO2-Sb2O5-SnO2 electrodes for chlorine evolution from seawater[J]. Chemical Engineering Journal, 2011, 172(1): 47-51. doi: 10.1016/j.cej.2011.05.059 [12] 杨蕴哲, 杨卫身. 恒电流下原位电生成活性氯氧化降解蒽醌染料[J]. 大连理工大学学报, 2006, 46(6): 813-818. doi: 10.3321/j.issn:1000-8608.2006.06.007 [13] 吉庆华. 活性氯强化混凝对消毒副产物生成势的控制研究[D]. 北京: 中国科学院研究生院, 2008. [14] 刘咏, 赵仕林, 李启彬, 等. 苯酚在氯离子体系中的电化学氧化研究[J]. 环境科学与技术, 2006, 29(11): 21-22. doi: 10.3969/j.issn.1003-6504.2006.11.009 [15] 王平全, 余冰洋, 王波, 等. 常用磺化酚醛树脂性能评价及分析[J]. 钻井液与完井液, 2015, 32(2): 29-33. doi: 10.3969/j.issn.1001-5620.2015.02.008 [16] 樊世忠, 鄢捷年, 周大晨. 钻井液完井液及保护油气层技术[M]. 东营: 石油大学出版社, 1996: 122. [17] 中华人民共和国环境保护部. 环境保护行业标准: HJ 586-2010[S]. 北京: 中国环境科学出版社, 2012. [18] 陈集, 尹忠, 冯英, 等. 油气田高Cl−废水的COD测定[J]. 西南石油学院学报, 1992, 14(4): 94-101. [19] DONG H, YU H, WANG X, et al. A novel structure of scalable air-cathode without nafion and Pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells[J]. Water Research, 2012, 46(17): 5777-5787. doi: 10.1016/j.watres.2012.08.005 [20] 肖惠文, 梁宏, 庞凯, 等. 软锰矿粒子电极处理SMP模拟废水实验研究[J]. 四川理工学院学报, 2016, 29(1): 12-16. [21] CHENG S A, WU J C. Air-cathode preparation with activated carbon as catalyst, PTFE as binder and nickel foam as current collector for microbial fuel cells[J]. Bioelectrochemistry, 2013, 92(2): 22-26. [22] CHEN J Y, LI N, ZHAO L. Three-dimensional electrode microbial fuel cell for hydrogen peroxide synthesis coupled to wastewater treatment[J]. Journal of Power Sources, 2014, 254: 316-322. doi: 10.1016/j.jpowsour.2013.12.114 [23] 柏栋予, 白红伟, 傅强. 高分子材料烧结成型研究进展[J]. 高分子通报, 2017(10): 13-22. [24] LIANG S, TENG F, BULGAN G, et al. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation[J]. Journal of Physical Chemistry C, 2010, 112(14): 5307-5315. [25] 芦佳. α-MnO2纳米颗粒的可控制备及催化性能研究[D]. 石家庄: 河北师范大学, 2010. [26] SELVAKUMAR K, KUMAR S M S, THANGAMUTHU R, et al. Development of shape-engineered α-MnO2, materials as bi-functional catalysts for oxygen evolution reaction and oxygen reduction reaction in alkaline medium[J]. International Journal of Hydrogen Energy, 2014, 39(36): 21024-21036. doi: 10.1016/j.ijhydene.2014.10.088 [27] NI Y L, MENG H M, CHEN D, et al. Preparation of IrO2+MnO2 coating anodes and their application in NaClO production[J]. Journal of University of Science & Technology Beijing, 2008, 15(4): 461-467. [28] LI X, WU Y, ZHU W, et al. Enhanced electrochemical oxidation of synthetic dyeing wastewater using SnO2-Sb-doped TiO2-coated granular activated carbon electrodes with high hydroxyl radical yields[J]. Electrochimica Acta, 2016, 220: 276-284. doi: 10.1016/j.electacta.2016.09.109 [29] ALAOUI A, KACEMI K E, ASS K E, et al. Activity of Pt/MnO2 electrode in the electrochemical degradation of methylene blue in aqueous solution[J]. Separation & Purification Technology, 2015, 154: 281-289. [30] KUAN W H, CHAN Y C. pH-dependent mechanisms of methylene blue reacting with tunneled manganese oxide pyrolusite[J]. Journal of Hazardous Materials, 2012, 239-240: 152-159. doi: 10.1016/j.jhazmat.2012.08.051 [31] SZPYRKOWIC L, RADAELI M, DANIEL S. Electrocatalysis of chlorine evolution on different materials and its influence on the performance of an electrochemical reactor for indirect oxidation of pollutants[J]. Catalysis Today, 2005, 100(3/4): 425-429. [32] ABBAR A H, SALMAN R H, ABBAS A S. Electrochemical incineration of oxalic acid at manganese dioxide rotating cylinder anode: Role of operative parameters in the presence of NaCl[J]. Journal of the Electrochemical Society, 2016, 163(13): E333-E340. doi: 10.1149/2.0551613jes [33] SZPYRKOWICZ L, JUZZOLINO C, KAUL S N. A comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and fenton reagent[J]. Water Research, 2001, 35(9): 2129-2136. doi: 10.1016/S0043-1354(00)00487-5 [34] HUANG T, CHEN J, WANG Z, et al. Excellent performance of cobalt-impregnated activated carbon in peroxymonosulfate activation for acid orange 7 oxidation[J]. Environmental Science and Pollution Research, 2017, 24(10): 9651-9661. doi: 10.1007/s11356-017-8648-7 [35] LIANG H, QIU Y, ZHAO L Y, et al. Investigation of a novel pyrolusite particle electrode effects in the chlorine-containing wastewater[J]. Water Science and Technology, 2018, 78(7): 1427-1437. [36] TIAN S H, TU Y T, CHEN D S, et al. Degradation of acid orange II at neutral pH using Fe2(MoO4)3, as a heterogeneous Fenton-like catalyst[J]. Chemical Engineering Journal, 2011, 169(1): 31-37. -



 下载:
下载: